Abstract
Light microscopy and scanning and transmission electron microscopy were used to examine the micromorphology and structure of the fruit skin as well as the phenols and flavonoids contents were determined in the mature fruits of three Prunus domestica cultivars. The fruits varied in the structure of crystalline wax, the number of microcracks and stomata present on their surface, the content of antioxidants, and the values of various anatomical parameters. The fruits of the “President” were characterized by the highest number of desirable quality characteristics, i.e., the lowest number of microcracks and stomata, and the highest content of antioxidants.
INTRODUCTION
Romania, Yugoslavia, France, Germany, Turkey, and Italy are the largest producers of plums in Europe. Approximately 2000 European cultivars originate from the species Prunus domestica (L.), with Hungarian plums, greengages, and mirabelle plums as the best-known sub-species.[Citation1,Citation2] Fruit producers are interested in highly fertile and frost- and disease-resistant varieties, which begin fruiting early and produce fruits that ripen at a defined period, do not exhibit rain cracking, and are suitable for storage. In turn, consumers look for plums with an attractive appearance and flavor, with a pit separating well from the flesh, and with a relatively long shelf life.[Citation3,Citation4] More attention has been paid to the health-enhancing properties of plums associated with their high nutritional (dietary) value and antioxidant content.[Citation5,Citation6] In particular, varieties with dark fruits are regarded as valuable antioxidants, as they contain phenols, including catechins, and exhibit a high content of unique phytonutrients called neochlorogenic and chlorogenic acids.[Citation7] The major limitations of plums storage and shelf life are related to softening, fungal decay, reduced flavor quality, and less favorable appearance (break freshness, shriveling, or bruising).[Citation8]
P. domestica cultivars differ in many fruit quality traits, e.g., weight and size, firmness, color, flavor, and the content of health-enhancing components, including phenols and anthocyanins.[Citation5,Citation9] Differences between cultivars also involve the amount and form of crystalline wax forming characteristic bloom on the fruit surface[Citation10–Citation12] and susceptibility to cracking, which has a negative repercussion on shelf life, consumer’s appreciation of the product, and its economic value.[Citation13,Citation14] The “Bluefre,” “Sweet Common Prune,” and “President” varieties of P. domestica are commonly cultivated and appreciated by consumers, and their fruits differ in many pomological characteristics ( and reference wherein). Moreover, in the literature in this research area there is no comprehensive and detailed information concerning differences in fruits structure of different cultivars of the same species. For these reasons, the objective of this study was to investigate differences in the micromorphology, histology, and, for the first time, the ultrastructure of the fruit skin of these cultivars with particular emphasis on features associated with fruit quality and attractiveness.
Since mechanical properties of the fruit skin can have a positive or negative effect on disorders, e.g., fruit cracking, pest infestation, and fungal pathogen penetration, special attention was paid to the traits of the epidermis, i.e., the characteristics of the cuticle, presence of epicuticular waxes, number of stomata, and susceptibility to cracking. Additionally, the fruit of the tested cultivars were analyzed for the content of phenolic compounds and flavonoids, which determine the health-enhancing values of the fruits.
MATERIAL AND METHODS
Plums of three cultivars: “Bluefre,” “Sweet Common Prune,” and “President” at harvest maturity were obtained from a commercial orchard in the Lublin region (Southeast Poland), characterized by temperate continental climate. The orchard employs conventional methods involving the use of standard mineral fertilizers and chemical plant protection products. The fruits were picked in 2013 at the beginning of October 10 (“Bluefre”), half of October 19 (“Sweet Common Prune”), and the end of October 29 (“President”). Medium-sized, similarly colored, intact, healthy plums were collected from the central part of randomly chosen trees. Special care was taken to avoid touching the fruit surface area intended for observation while picking, transporting, and preparing the plums for SEM (to avoid rubbing off and degradation of the wax layer). For microscopic analysis, fruit fragments with the skin were sampled from the sun-exposed side in the equatorial region of each cultivar.
Scanning Electron Microscopy (SEM)
Conventional fixation of the plant tissue used for SEM investigations involves dehydration, which can remove or alter lipids forming the wax coating on the plum surface, and critical point drying can shrink and distort tissues.[Citation15] Therefore, samples of the external part of 10 fruits (5 mm × 5 mm × 2 mm) were cut with a razor blade, immediately after fruits had been collected from trees. Samples were not dried. Only the cutting planes were gently wiped before mounting them carefully onto aluminum stubs with double-sided carbon tape. In addition, thin strips of tape were also mounted on the margins of the samples. After coating them with 15 nm thick layer of gold, samples were examined with a TESCAN/VEGA LMU scanning electron microscope at an accelerating voltage of 30 kV. In each varieties the number of stomata within a 1 cm2 area of the epidermis and the length of ten stomatal pores in five fruits was measured using the morphology software combined with SEM.
Light Microscopy (LM)
Using a razor blade, hand-cut cross-sections were made through fresh skin (the specimen was prepared from fresh material-cross sections) of ten fruits of each varieties. Then, the samples were embedded in glycerol gelatine on a glass slide and observed under the Nicon SE 102 light microscope. For each sample, cuticle thickness, height of the epidermal cells (length of its radial axis), number layers of hypodermis, thickness of hypodermis layer, thickness of the peryclinal cell walls of hypodermis and total thickness of skin were determined in five sites. Further, the samples were stained with FeCl3 in order to detect phenolic compounds and with Sudan III (ethanol solution of Sudan III) to visualize lipophilic substances. For each cultivar, semi-thin 0.7-µm transverse sections (perpendicular to the fruit axis) were cut using a Reichert Ultracut-S ultramicrotome and a glass knife and stained with 1% methylene blue and 1% azure II in a 1% aqueous solution of sodium tetraborate. Samples were fixed and embedded in synthetic resin using the standard methods for transmission electron microscopy (TEM; see below). The sections and hand-cut samples were observed with a Nikon Eclipse 90i microscope. Images were captured using a digital camera (Nikon Fi1) and NIS-Elements Br 2 software.
TEM
Small samples (2 mm × 2 mm × 2 mm) of 5 “President” fruits with skin were fixed in 2% paraformaldehyde and 2.5% glutaraldehyde buffered at pH 7.4 in 0.1 M cacodylate buffer. Fixation was performed at room temperature for 2 h, followed by 12 h at 4°C. After rinsing with 0.1 M cacodylate buffer at 4°C for 24 h, samples were treated with 1% OsO4. Subsequently, the samples were transferred to re-distilled water and stained with a 0.5 M aqueous uranyl acetate. After passage through increasing concentrations of propylene oxide in ethanol and finally through pure propylene oxide, the samples were embedded for 12 h in Spurr Low Viscosity resin at 70°C.[Citation16] Ultrathin sections (70 nm thick) were treated with a 8% solution of uranyl acetate in acetic acid and with lead citrate.[Citation17] Samples were observed using the FEI Technai G2 Spirit Bio TWIN transmission electron microscope at an accelerating voltage of 120 kV. Images were captured using a Megaview G2 Olympus Soft Imaging Solutions camera.
Determination of Total Phenolic and Flavonoid Contents
The total content of phenols in the methanol fruit extracts of each variety was determined using Folin-Ciocalteu reagent according to the calorimetric method described by Singleton and Rossi.[Citation18] Caffeic acid was used as a standard. The concentration of total phenols was expressed in equivalents of caffeic acid as g · 100 g–1 of dry extract. The contents of flavonoids in fruit extracts of each variety were determined by the spectrophotometer method according to Farmakopea Polska.[Citation19] Quercetin was used as a standard. The total content of flavonoids was expressed as equivalents of quercetin in g · 100 g–1 of dry extract.
Statistical Analyses
Means (±SD) were calculated for all the parameters measured. Data were analyzed by one-way analysis of variance (ANOVA) and Tukey’s multiple range test for comparison of means, using Statistica 7 software. Differences were considered statistically significant at the level of p < 0.05.
RESULTS AND DISCUSSION
Besides the basic pomological characteristics (color, size, shape, and separation of the pit from the flesh), the fruits of the investigated cultivars differed in the microstructure and values of anatomical parameters. Additionally, differences were found between the contents of phenolic compounds and flavonoids.
Characteristics of the Fruit Surface
Tiny microwrinkles (“Bluefre” and “President”) and small undulations (“Sweet Common Prune”) were shown by SEM on the fruit surface of the examined plum cultivars (–). Collapsed cells formed lines arranged in various directions (“President”) or concentrated around the stomata (“Bluefre”) (–). The fruit surface was covered by an epicuticular wax layer composed of abundant, densely arranged crystalline wax microgranules (–). In the “Bluefre” cultivar, considerably larger, vertical, disorderly arranged and various-shaped wax platelets were visible between the microgranules ( and ). In turn, in the “Sweet Common Prune” cultivar, besides typical confluent wax microgranules, there were vertical platelets with a very regular shape and, frequently, linear arrangement ( and ). In the “President” cultivar, the epicuticular wax coating was homogeneous, but the wax microgranules were more densely arranged at the site of contact of epidermal cell walls ( and ). Wax microgranules were also visible inside the stomata and on the surface of stomatal cells (, ; , , and ). Crystalline wax with a similar closely packed granular structure was also observed by Storey and Price[Citation12] on “d’Agen” variety fruits. Jeffree et al.[Citation20] and Baker[Citation21] report that, similar to plums, fruits of other species are covered by wax bloom that moderates gas exchange and protects the fruits against UV light, insects, and pathogens. Skene[Citation10] and Riederer and Markstadter[Citation21] claim that the differences in the form of epicuticular waxes between different cultivars and species are associated with wax arrangement and ascribe a modifying role to the fruit developmental stage, position on the fruit, and environmental factors. Furthermore, Kolattukudy[Citation22] found that plums show more definite bloom than apples or pears because the fine wax protuberances present on a plum scatter more effectively than the platelets of wax found on apples or pears, and the shape of wax crystals is determined by its chemical composition. In turn, Müller and Riederer[Citation23] report that the bluish coloration of plums is induced by the reflection of part of the visible light spectrum by a dense coverage of the three-dimensional wax structure. The trees of “Bluefre,” “Sweet Common Prune,” and “President” cultivars grew in the same climatic and environmental conditions and their fruits were harvested at the same developmental stage, which implies a major impact of the genetic factor on the formation of different forms of crystalline wax.
FIGURE 1 SEM. Surface of the Prunus fruits; A, D, G, J—“Bluefre;” B, E, H, K—“Sweet Common Prune;” C, F, I, L—“President.” Visible microwrinkles (A, C) and undulations (B) centered around the stomata. D–F—visible wax platelets (E; arrow heads) and stomata (D–F; arrows) surrounded by wrinkles. G–L—visible crystalline wax in the form of vertical plates (arrows; G, H, J, K) and microgranules (I–L), and numerous microcracks in the wax layer (H, K), asterisks—wax microgranules in high density.
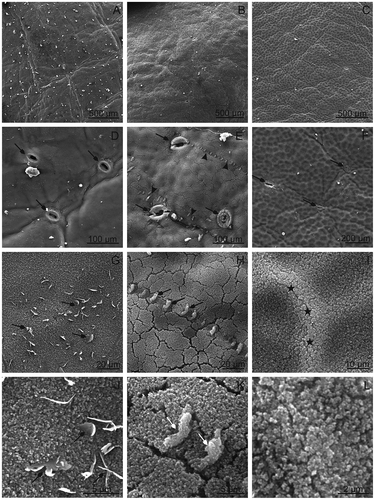
FIGURE 2 SEM. Surface of the Prunus fruits with microcracks and stomata; A–C—“Bluefre;” D–F—“Sweet Common Prune;” G–I—“President.” F, L—LM. Note stomata with wax microgranules visible on the cell surface and in the interior of stomata (A, D, G), and stomata with microcracks (arrows; B, C, E, H, I). F—A microcrack (arrows) in the skin of “Sweet Common Prune.”
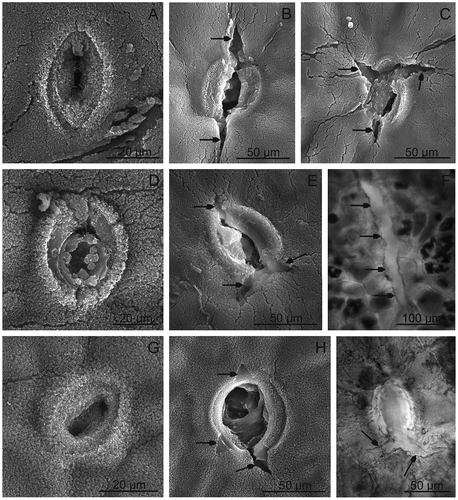
The surface of the fruits of the tested plum cultivars differ significantly in numbers of stomata characterised by a varied size and degree of opening and filling of pores with crystalline wax (, –, –, and –). The stomata usually occurred in groups of two or three and were least abundant in “President” and most numerous in “Bluefre” and “Sweet Common Prune.” In all the cultivars, smaller or larger microcracks were visible; they either were extensions of the long axis of the stomatal guard cell pair or were arranged in different directions (, , , and –). Similar microcracks were also visible beyond the stomata, particularly in the “Sweet Common Prune” cultivar (, , and ). Most frequently, the microcracks were relatively shallow and covered exclusively the layer of epicuticular waxes; however, particularly around the stomata, the microcracks reached deeper layers of the cuticle (, , , , and ). A similar type of microcracks in plums was described by Storey and Price[Citation12] and Mrozek and Burkhardt,[Citation24] who found that they led to local water loss resulting in fruit softening. Belmans et al.[Citation13] and Simon[Citation14] reported that the susceptibility to cracking is largely a genetically determined feature of plum varieties, although such factors as water uptake, fruit characteristics, orchard temperature, and other environmental conditions may also induce cracking. Many researchers claim that biosynthesis of cuticle components as well as their transport and turnover do not coincide with enlargement of the fruit surface area, which leads to deformations and formation of microcracks. Moreover, excess of water during fruit growth causes swelling and enlargement of the skin cell volume, which is accompanied by reduction of the amount of the cuticle per unit area resulting from stretching.[Citation25–Citation27] Knoche and Peschel[Citation28] report that, in plums and cherries, which are characterized by a double-sigmoidal growth pattern, wax deposition continues throughout the period of fruit growth until fruit maturity, whereas cutin deposition ceases much earlier at the stage of fruit growth. According to Gibert et al.,[Citation29] the number of microcracks is directly proportional to fruit fresh weight (and, hence, fruit volume). However, the author of the present study observed that in the “President” characterised by the largest fruits, the microcracks were confined almost exclusively to the area around the stomata, whereas the “Sweet Common Prune” cultivar, which bears the smallest fruits, the microcracks were more numerous and covered the fruit surface beyond the stomata. Probably, in the case of the examined cultivars, the fruit size had a lesser impact on formation of microcracks than thickness of the cuticle or the epicuticular wax layer and number of functional (non-wax-clogged, penetrable) stomata per fruit surface area (not investigated). The important role in modulating the permeability of stomata cuticle was also highlighted by Storey and Price[Citation12] and Kerstiens.[Citation30] In the case of the “Sweet Common Prune” cultivar, the abundance of microcracks and stomata may be the major factor leading to fruit softening and reducing their quality and, hence, to limiting their storage time and shelf life. Similar fruit surface microcracks leading to an important reduction of the commercial yield have been observed in many crops: apples,[Citation31,Citation32] cherries,[Citation14,Citation28] grape berries,[Citation33] Ribes species,[Citation34] nectarines,[Citation35] and pears.[Citation31,Citation36]
TABLE 2 Fruit skin characteristics and antioxidant contents of Prunus fruits
Anatomy and Ultrastructure Characteristics
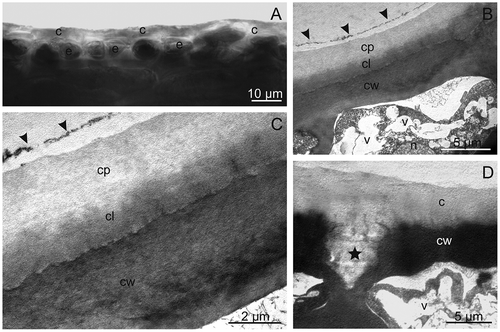
Microscopic observations showed that the pericarp of the investigated plum cultivars is formed of the skin composed of epidermis covered by the cuticle and waxes, multi-layered hypodermis, multi-layered flesh, and an endocarp composed of sclereids forming the pit. A similar arrangement of the successive layers of the pericarp in plums, peaches, and nectarines was presented by Storey and Price,[Citation12] Archibald and Melton,[Citation37] and King et al.[Citation38] The cuticle observed under LM in hand-made cross-sections formed a bright layer with a varied thickness on the epidermis surface (, ), which was stained orange-red by Sudan III (not shown). The thickest cuticle was found in the “President” fruits and this value was significantly different from the thickness of the cuticle of the other varieties. In turn, the cuticle of “President” observed under TEM formed a brighter electron layer, compared with the cell wall (–) and, at some sites, it penetrated between the epidermal cells and filled the intracellular spaces (). Two layers were distinguished in the cuticle: a darker cuticular layer adjacent to the cell wall and a thicker and brighter outer layer forming the cuticle proper ( and ). At some sites on the cuticle surface, an outer boundary layer of epicuticular waxes characterized by discontinuity relative to the cuticle proper was visible ( and ). Storey and Price[Citation12] emphasize that the cuticle in plum fruits exhibits high heterogeneity and comprises four to five discrete layers composed of the cell wall, cuticle layer, and cuticle proper, whereas Schneider and Dargent[Citation39] and Holloway[Citation40] classify the cuticle in Prunus leaves as an amorphous-reticulate or only reticulate type. The author of the present article did not observe a reticulate structure of the cuticle in the “President” fruits; in contrast, the entire cuticle layer seemed to have an amorphous structure. Other researchers also observed differences in the cuticle structure, composition, thickness, and quantity among plants, organs, and growth stages.[Citation41–Citation44]
FIGURE 3 The cuticle on the “President” fruit surface. A—visible a brightly shining layer of the cuticle observed under LM. B–D—The cuticle observed under TEM. B, C—Note two layers of the cuticle: brighter—cuticle proper and darker—cuticular layer and the border of the occurrence of the epicuticular wax layer (arrow heads). D—Visible cuticle penetrating between the cells of the epidermis (asterisk). c: cuticle, cp: cuticle proper, cl: cuticular layer, cw: cell wall, e: epidermis, n: nucleus, v: vacuole.
The author of the present study found that the fruit epidermis in the investigated plum cultivars was single (“Bluefre”) or sometimes double-layered (“Sweet Common Prune,” “President”) and consisted of cells with a similar (“Bluefre” and “Sweet Common Prune”) or different (“President”) height (, –). Similarly, the hypodermal cells formed a layer of a varied thickness and were often tangentially elongated and strongly flattened (“Sweet Common Prune”) as a result of enlargement of the fruit volume during growth. “Bluefre” exhibited the thickest layer of hypodermis and total skin, which was significantly different from the thickness of these layers in the fruits of the other varieties (). The thickest tangential cell walls of hypodermis was observed in the “Sweet Common Prune” fruits (). The cytoplasm of the epidermal and hypodermal cells viewed under TEM contained nuclei, plastids with small starch grains, mitochondria, and endoplasmic reticulum, whereas the vacuoles exhibited fibrous and granular deposits and membranes forming different-shaped compartments indicative of fruit ripening and senescence (–). In turn, the hypodermal and parenchymal cells of the fruits contained numerous chloroplasts (, , and ) and druses (“Sweet Common Prune”). The available literature does not provide information about the ultrastructure of plum skin cells. However, similar electron transparent vesicles with different shapes and sizes in the vacuoles of the mesocarp of ripening grape berries were described by Da-Peng et al.[Citation45]
FIGURE 4 LM. Fragments of sections through the fruit surface layer; A—‘”Bluefre,” B—“Sweet Common Prune,” C—“President.” Visible chloroplasts in many hypodermal and parenchymal cells (arrows). e: epidermis, h: hypodermis, p: parenchyma.
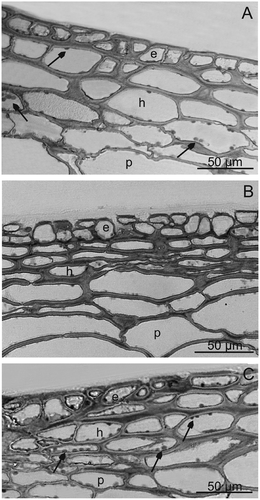
FIGURE 5 TEM. Skin and parenchymal cells of “President” fruits; A—epidermis and hypodermis cells with nuclei, plastids with starch grains and numerous vacuoles with fibrous and granular deposits. B, C—fragments of epidermis cells with plastids containing starch grains, mitochondria, and vacuoles with membranes forming compartments of various shapes (asterisks). D, E—chloroplasts (arrows) with parenchymal cells. c: cuticle, cw: cell wall, n: nucleus, p: plastid with starch grains, m: mitochondrion, v: vacuole.
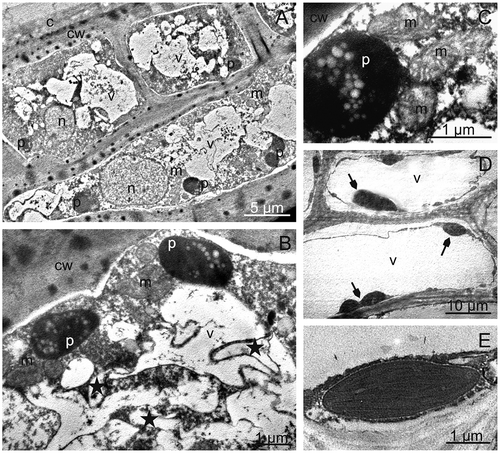
The vacuoles of the epidermal and hypodermal cells in the fruits of all the analyzed cultivars contained anthocyanins, frequently in the form of large, spherical globules (“Bluefre” and “President;” not shown). Bae et al.,[Citation46] who described similar inclusions in apple peel, reported that these were phenol and anthocyanin deposits forming electron dense, membrane-less globules. These might also be complexes of anthocyanins with flavonoids or tannins occurring in the form of three morphological types of deposits: an indeterminate type, a hemispherical type, and a spherical type. Similarly, in the pericarp of Prunus persica fruits, King et al.[Citation38] observed dark-staining vacuolar inclusions as a virtually continuous layer on the inside of the tonoplast, which could probably be tannin deposits.
Content of Phenols and Flavonoids
Significant differences in the content of phenolic compounds and flavonoids were found between the studied cultivars (). Based on the reaction with FeCl3, it was found that, similar to anthocyanins, phenolic compounds occurred in the form of large brown-purple deposits mainly in the epidermal and hypodermal cells, while in the parenchymal cells they were present in smaller quantities as granularities or fibers (not shown). Similar localization of phenolic contents in plum skin at all stages of fruit ripeness was described by Łata et al.[Citation47] and Treutter et al.[Citation48] as well. The highest content of flavonoids and phenols was reported for the “President” fruits and the lowest for “Sweet Common Prune,” which was also confirmed by the results of the microscopic analysis. The “Sweet Common Prune” fruits contained nearly eight-fold lower amounts of phenols than the other cultivars. In turn, the content of flavonoids in the “President” fruits was eight-fold higher than in the “Bluefre” cultivar and 18-fold higher than in “Sweet Common Prune” fruits (). Ertekin et al.[Citation5] and Usenik et al.[Citation9] found that different plum cultivars differed in the content of soluble solids, sugars, organic acids, phenolic compounds, and anthocyanins. In turn, Walkowicz-Tomczak et al.[Citation4] described low oxidative properties of “Sweet Common Prune” fruits. The results obtained in the present study concerning the content of total phenols are in the range reported by other authors for various plum cultivars, in which the total phenol content fluctuated between several and several hundred g 100 g–1 dry or fresh weight.[Citation48–Citation51] The “President” and “Bluefre” are cultivars with the highest content of these antioxidants. According to many researchers, plums have higher contents of phenols and flavonoids than other fruits, e.g., peaches, nectarines, oranges, grapes, blueberries, apples, pears, or strawberries.[Citation52–Citation56] Antioxidants protect humans against free radicals and fruits against pests and diseases.[Citation57,Citation58] Besides flavor, smell, and firmness, the content of antioxidants is regarded as another very important factor determining fruit quality.[Citation59,Citation60]
CONCLUSIONS
The lower number of microcracks and stomata with smaller pores as well as the thicker cuticle on the surface of the “President” fruit can reduce fruit transpiration and contribute to maintenance of their firmness. In turn, the wider hypodermal layer and the thicker skin in “Bluefre” and “Sweet Common Prune” fruits may partially limit the rapid loss of water by the fruits of these varieties, caused by the presence of a greater number of microcracks and stomata. The greatest health-enhancing value related to the presence of phenolic compounds and flavonoids was found in the “President” fruits, whereas the “Sweet Common Prune” were characterized by the lowest value. The analysis of the ultrastructure of the “President” skin cells performed for the first time showed an amorphous structure of the cuticle on the fruit surface and the presence of chloroplasts in the hypodermis cells indicating the photosynthetic activity of ripe fruits.
FUNDING
This work was supported by the Ministry of Science and Higher Education of Poland as part of the statutory activities of the Department of Botany, University of Life Sciences in Lublin.
Additional information
Funding
REFERENCES
- Żurawicz, E. Pomology; Państwowe Wydawnictwa Rolnicze i Leśne: Warszawa, 2003; 101–125 pp.
- Hodun, G. Plum trees; Działkowiec: Warszawa, 2008; 10 p.
- Crisosto, C.H.; Garner, D.; Crisosto, G.M.; Bowerman, E. Increasing “Blackamber” plum (Prunus salicina Lindell) consumer acceptance. Postharvest Biology and Technology 2004, 34, 237–244.
- Walkowiak-Tomczak, D.; Reguła, J.; Łysiak, G. Physico-chemical properties and antioxidant activity of selected plum cultivars fruit. Acta Scientiarum Polonorum Technologia Alimentaria 2008, 7, 15–22.
- Ertekin, C.; Gozlekci, S.; Kabas, O.; Sonmez, S.; Akinci, I. Some physical, pomological, and nutritional properties of two plum (Prunus domestica L.) cultivars. Journal of Food Engineering 2006, 75, 508–514.
- Fijałkowski, D.; Chojnacka-Fijałkowska, E. Medicinal plants in the Lublin region; Lubelskie Towarzystwo Naukowe: Lublin, 2009; 273–274 pp.
- Slimestad, R.; Vangdal, E.; Brede, C. Analysis of phenolic compounds in six Norwegian plum cultivars (Prunus domestica L.). Journal of Agricultural and Food Chemistry 2009, 57, 11370–11375.
- Vangdal, E.; Flatland, S.; Nordbø, R. Fruit quality changes during marketing of new plum cultivars (Prunus domestica L.). Horticultural Science (Prague) 2007, 34, 91–95.
- Usenik, V.; Kastelec, D.; Veberič, R.; Štampar, F. Quality changes during ripening of plums (Prunus domestica L.). Food Chemistry 2008, 111, 830–836.
- Skene, D.S. The fine structure of apple, pear, and plum fruit surfaces, their changes during ripening, and their response to polishing. Annales of Botany 1963, 27, 581–587.
- Bain, J.M.; McBean, D.M. The development of the cuticular wax layer in prune plums and the changes occurring in it during drying. Australian Journal of Biological Sciences 1969, 22, 101–110.
- Storey, R.; Price, W.E. Microstructure of the skin of d’Agen plums. Scientia Horticulturae 1999, 81, 279–286.
- Belmans, K.J.; Keulemans, J.; Debarsy, T.; Bronchart, R. Influence of sweet cherry epidermal characters on susceptibility to cracking. Proceedings of the XXIII International Horticulture Congress, Firenze, Italy, August 27–September 1, 1990; 637 p.
- Simon, G. Review on rain induced fruit cracking of sweet cherries (Prunus avium L.), its causes and the possibilities of prevention. International Journal of Horticultural Science 2006, 12, 27–35.
- Roy, S.; Conway, W.S.; Watada, A.E.; Sams, C.E.; Erbe, E.F.; Wergin, W.P. Changes in the ultrastructure of the epicuticular wax and postharvest calcium uptake in apples. HortScience 1999, 34, 121–124.
- Spurr, A.R. A low-viscosity epoxy resin embedding medium for electron microscopy. Journal of Ultrastructure Research 1969, 26, 31–43.
- Reynolds, E.S. The use of lead citrate at high pH as an electron-opaque stain for electron microscopy. Journal of Cell Biology 1963, 17, 208–212.
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture 1965, 16, 144–158.
- Polish Farmacopoeia, VI. Polskie Towarzystwo Farmaceutyczne: Warszawa, 2002; 150 p.
- Jeffree, C.E.; Baker, E.A.; Holloway, P.J. Ultrastructure and recrystallization of plant epicuticular waxes. New Phytology 1975, 75, 539–549.
- Riederer, M.; Markstädter, C. Cutical waxes: A Critical Assessment of Current Knowledge. In The Plant Cuticles: An Integrated Functional Approach; Kerstiens, G.; Ed.; BIOS Scientific Publishers: Oxford, 1996; 189–200.
- Kolattukudy, P.E. Natural waxes on fruits. Post Harvest Pomology Newsletter 1984, 2, 3–7.
- Müller, C.; Riederer, M. Plant surface properties in chemical ecology. Journal of Chemical Ecology 2005, 31, 2621–2651.
- Mrozek, R.F.; Burkhardt, T.H. Factors causing prune side cracking. Transactions ASAE 1973, 16, 686–695.
- Milad, R.E.; Shackel, K.A. Water relations of fruit end cracking in French prune (Prunus domestica L. cv. French). Journal of the American Society for Horticultural Science 1992, 117, 824–828.
- Knoche, M.; Peschel, S.; Hinz, M.; Bukovac, M.J. Studies on water transport through the sweet cherry fruit surface: II. Conductance of the cuticle in relation to fruit development. Planta 2001, 213, 927–936.
- Knoche, M.; Beyer, M.; Peschel, S.; Oparlakov, B.; Bukovac, M.J. Changes in strain and deposition of cuticle in developing sweet cherry fruit. Physiologia Plantarum 2004, 120, 667–677.
- Knoche, M.; Peschel, S. Deposition and strain of the cuticle of developing European plum fruit. Journal of the American Society for Horticultural Science 2007, 132, 597–602.
- Gibert, C.; Chadoeuf, J.; Vercambre, G.; Génard, M.; Lescourret, F. Cuticular cracking on nectarine fruit surface: Spatial distribution and development in relation to irrigation and thinning. Journal of the American Society for Horticultural Science 2007, 132, 583–591.
- Kerstiens, G. Signalling across the divide: A wider perspective of cuticular structure-function relationships. Trends in Plant Science 1996, 1, 125–129.
- Khanal, B.P.; Grimm, E.; Knoche, M. Russeting in apple and pear: A plastic periderm replaces a stiff cuticle. AoB Plants 2013, 5, pls 048.
- Konarska, A. The structure of the fruit peel in two varieties of Malus domestica Borkh. (Rosaceae) before and after storage. Protoplasma 2013a, 250, 701–714.
- Becker, T.; Knoche, M. Deposition, strain, and microcracking of the cuticle in developing “Riesling” grape berries. Vitis 2012, 51, 1–6.
- Khanal, B.P.; Grimm, E.; Knoche, M. Fruit growth, cuticle deposition, water uptake, and fruit cracking in jostaberry, gooseberry, and black currant. Scientia Horticulturae 2011, 128, 289–296.
- Nguyen-The, C. Structure of epidermis wall, cuticle, and cuticular microcracks in nectarine fruit. Agronomie 1991, 11, 902–920.
- Konarska, A. The relationship between the morphology and structure and the quality of fruits of two pear cultivars (Pyrus communis L.) during their development and maturation. The Scientific World Journal 2013b, http://dx.doi.org/10.1155/2013/846796.
- Archibald, R.D.; Melton, L.D. The anatomy of the fleshy pericarp of maturing Moorpark apricots, Prunus armeniaca. New Zealand Journal of Botany 1987, 25, 181–184.
- King, G.A.; Henderson, K.G.; Lill, R.E. Growth and anatomical and ultrastructural studies of nectarine fruit wall development. Botanical Gazette 1987, 148, 443–455.
- Schneider, A.; Dargent, R. Localisation et comportement du mycélium de Taphrina deformans dans le mésophylle et sous la cuticule des feuilles de pêcher (Prunus persica). Canadian Journal of Botany 1977, 55, 2485–2495.
- Holloway, P.J. Structure and Histochemistry of Plant Epicuticular Membranes—An Overview. In The Plant Cuticle; Cutler, D.F.; Alvin, K.L.; Price, C.E.; Eds.; Academic Press: London, 1982; 1–32.
- Jeffree, C.H. The Fine Structure of the Plant Cuticle. In Biology of the Plant Cuticle; Riederer, M.; Müller, C.; Eds.; Blackwell Publishing: Oxford, 2006; 11–125.
- Heredia, A. Biophysical and biochemical characteristics of cutin, a plant barrier biopolymer. Biochimica et Biophysica Acta 2003, 1620, 1–7.
- Kolattukudy, P.E. Biosynthetic Pathways of Cutin and Waxes, Their Sensitivity to Environmental Stresses. In Plant Cuticles, An Integrated Functional Approach; Kersteins, G.; Ed.; BIOS Scientific Publishers: Oxford, 1996; 83–108.
- Riederer, M. Introduction: Biology of the Plant Cuticle. In Biology of the Plant Cuticle; Riederer, M.; Müller, C.; Eds.; Blackwell Publishing: Oxford, 2006; 1–10.
- Da-Peng, Z.; Min, L.; Yi, W. Ultrastructural changes in the mesocarp cells of grape berry during its development. Acta Botanica Sinica 1997, 39, 389–396.
- Bae, R.N.; Kim, K.W.; Kim, T.C.; Lee, S.K. Anatomical observations of anthocyanin rich cells in apple skins. HortScience 2006, 41, 733–736.
- Łata, B.; Trampczyńska, A.; Paczesna, J. Cultivar variation in apple peel and whole fruit phenolic composition. Scientia Horticulturae 2009, 121, 176–181.
- Treutter, D.; Wang, D.; Farag, M.A.; Baires, G.D.A.; Rühmann, S.; Neumüller, M. Diversity of phenolic profiles in the fruit skin of Prunus domestica plums and related species. Journal of Agricultural and Food Chemistry 2012, 60, 12011–12019.
- Cinquanta, L.; Di Matteo, M.; Esti, M. Physical pre-treatment of plums (Prunus domestica). Part 2. Effect on the quality characteristics of different prune cultivars. Food Chemistry 2002, 79, 233–238.
- Kim, D.O.; Jeong, S.W.; Lee, C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chemistry 2003, 81, 321–326.
- Miletić, N.; Popović, B.; Mitrović, O.; Kandić, M. Phenolic content and antioxidant capacity of fruits of plum cv. “Stanley” (Prunus domestica L.) as influenced by maturity stage and on-tree ripening. Australian Journal of Crop Science 2012, 6, 681–687.
- Kader, A.A.; Mitchell, E.G. Postharvest Physiology. In Peaches, Plums, and Nectarines: Growing and Handling for Fresh Market; LaRue, J.H.; Johnson, S.R.; Eds.; The Regents of the University of California: Oakland, CA, 1989; 158–164.
- Kayano, S.; Kikuzaki, H.; Fukutsaka, N.; Mitani, T.; Nakatani, N. Antioxidant activity of prune (Prunus domestica L.) constituents and a new synergist. Journal of Agricultural and Food Chemistry 2002, 50, 3708–3712.
- Leong, L.P.; Shui, G. An investigation of antioxidant capacity of fruits in Singapore markets. Food Chemistry 2002, 76, 69–75.
- Öztürk, A.; Demirsoy, L.; Demirsoy, H.; Asan, A.; Gül, O. Phenolic compounds and chemical characteristics of pears (Pyrus communis L.). International Journal of Food Properties 2014, 18(3), 536–546.
- Leccese, A.; Bartolini, S.; Viti, R. Genotype, harvest season, and cold storage influence on fruit quality and antioxidant properties of apricot. International Journal of Food Properties 2012, 15(4), 864–879.
- Jakobek, L.; Seruga, M. Influence of anthocyanins, flavonols, and phenolic acids on the antiradical activity of berries and small fruits. International Journal of Food Properties 2012, 15(1), 122–133.
- De Gara, L.; De Pinto, M.C.; Tommasi, F. The antioxidant systems vis-à-vis reactive species during plant-pathogen interaction. Plant Physiology and Biochemistry 2003, 41, 863–870.
- Hodges, D.M.; Lester, G.E.; Munro, K.D.; Toivonen, P.M.A. Oxidative stress: Importance for postharvest quality. HortScience 2004, 39, 924–929.
- Tomás‐Barberán, F.A.; Espin, J.C. Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. Journal of the Science of Food and Agriculture 2001, 81, 853–876.
