Abstract
The physico-chemical properties of solvent-extracted oil from the seeds of noni (Morinda citrifolia L.), spinach (Spinacia oleracea L.), lady’s finger (Abelmoschus esculentus (L.) Moench), bitter gourd (Momordica charantia L.), mustard (Brassica nigra (L.) Koch), and the dried kernel (copra) of coconut (Cocos nucifera L.) were characterized. Among these sources, spinach seed had the lowest oil content (4.5 ± 0.4%) while coconut kernel had the highest oil content (63.1 ± 2.8%). Palmitic, oleic, and linoleic acids were the major fatty acids for spinach, lady’s finger and noni seed oils, while erucic, eleostearic, and lauric acids were the major fatty acids for mustard seed oil, bitter gourd seed oil, and coconut kernel oil, respectively. All of the oils possessed at least three major peaks in their triacylglycerol profiles except for bitter gourd seed oil which had only one major peak (1-stearoyl, 2,3-dieleostearoyl). The last endothermic peaks were –12.4, –6.0, 6.8, 57.7, 2.7, and 24.3ºC for noni, spinach, lady’s finger, bitter gourd and mustard seed oils, and coconut oil, respectively. Initially, the solid fat content of bitter gourd seed oil decreased gradually, but became rapidly after 50 until 60ºC. Coconut oil had its solid fat content reduced rapidly around 14 to 28ºC.
INTRODUCTION
According to the 8th Global Oils and Fats Forum 2013,[Citation1] the world’s commercially most important vegetable oils in 2012 (global production calculated in thousands metric tonnes) were palm oil (53,446), soybean oil (42,758), rapeseed oil (24,444), and sunflower oil (14,831). Global consumption of these oils has increased steadily due to growth of global population, energy crisis (used as biofuels), emergence of new functionality (applications in the oleo-chemical industry as surfactant, detergents, lubricants, flavor esters, and other purposes, such as blended with other oils for new functionality and utilization), and nutritional transition trend from saturated animals fat to unsaturated vegetable oils.[Citation2] This has led to either inadequate supply or increases in price, and, thus highlights the need for finding new plants as complimentary oil sources. In the end, it becomes more important to conduct further research on currently known plant oils that have incomplete properties information or varied characteristics due to different hybrids and cultivars. This is the case with the oils extracted from noni, spinach, lady’s finger, and bitter gourd seeds, and these sources of oils may have future applications both in the food and non-food industries. Noni seeds are a waste product of noni juice industry when the juice is commercialized worldwide.[Citation3,Citation4] Noni is a shrub that grows widely in Polynesia and is used as folk medicine, food, and fabric dye by the locals since thousand years ago.[Citation4] Other countries where noni is also grown include Vietnam,[Citation5] Malaysia,[Citation6] China,[Citation7] Costa Rica,[Citation8] and America.[Citation9,Citation10] The Noni seed contains 7% oil, 44% dietary fiber, 43% carbohydrate, 4% protein, and 2% ash.[Citation9] The major fatty acid of noni seed oil is linoleic acid while oleic acid and palmitic acid are the minor fatty acids.[Citation9]
TABLE 1 Oil content (%) of noni, spinach, lady’s finger, bitter gourd and mustard seed oils, and coconut oil
Spinach seed contains approximately 3% oil.[Citation11] Known properties of spinach seed oil include iodine value (IV; 129.2 g of I2/100 g), saponification value (SV; 190.8 mg of KOH/g) and unsaponifiable matter content (2.9%). The major compounds in the unsaponifiable matter are sterols from the category of α-spinasterol and stigmastenol. On the other hand, the lady’s finger seed contains a higher quantity of oil (17.9%) than the noni and spinach seeds.[Citation12] Rao[Citation12] reported that the lady’s finger seed contains 21.1% protein, 23.4% crude fiber, and 4% ash. The lady’s finger seed oil is highly unsaturated and is linoleic acid-rich (41.7%). Other fatty acids in the oil include 23.8% palmitic acid and 27.1% oleic acid. Anwar et al.[Citation13] reported that alcoholysis of the lady’s finger seed oil with methanol produces methyl esters that have fuel properties comparable with diesel. Bitter gourd seed contains 19.3% oil, 11.8% protein, and 34.8% crude fiber.[Citation14] The major fatty acid for bitter gourd seed oil is eleostearic acid[Citation14,Citation15] while the predominant triacylglycerol (TAG) is dieleosteroylstearin.[Citation15]
Mustard seed is oil-rich and has been reported to contain oil content in the range of 17–40%.[Citation16] The major fatty acid of mustard seed oil is erucic acid (0–62%) while eicosenoic acid, linolenic acid, linoleic acid, and oleic acid are the minor fatty acids.[Citation17] However, erucic acid is not suitable for human consumption as this fatty acid is detrimental to the function of heart[Citation18,Citation19] and liver,[Citation20] besides affecting fertility.[Citation21] Traditionally, coconut oil is obtained from the white edible flesh inside the kernel. The major fatty acids of coconut oil are lauric (51.0%) and myristic acids (23.0%) while stearic, palmitic, capric, and caprylic acids are present in trace amounts.[Citation22] Coconut oil can be formulated into an environment-friendly lubricant[Citation23] or used as feedstock of biodiesel.[Citation24] Coconut oil has also been found to possess antimicrobial properties against Candida species and is potentially for usage as an antimicrobial drug in the treatment of fungal infections.[Citation25] Thus, in this study, the physicohysic-chemical properties of oils that have been solvent-extracted from the seeds of the bitter gourd, spinach, lady’s finger, mustard, noni, and dried coconut kernel were characterized in order to determine their potential applications in food systems, and eventually as new edible oil.
MATERIALS AND METHODS
Sources and Extraction of Oil
Bitter gourd, lady’s finger, and spinach seeds were purchased from the Malaysian Agricultural Research and Development Institute (MARDI; Selangor, Malaysia). Mustard seeds, which are used as a spice by the local people, were bought from a local store in Serdang, Selangor, Malaysia. Noni seeds were extracted from fruits which were collected at the “hard-white” maturity stage[Citation26] from trees growing at the Universiti Farm, Universiti Putra Malaysia. These fruits were then stored five fruits per plastic bag (21 × 12 cm) at ambient temperature for four days until the fruits became soft. The seeds were separated from the fruit pulp manually. In order to remove residual pulp, the seeds were placed in a beaker containing 1 L of 0.5% (v/v) Pectinex Ultra SP-L (Novozymes, Bagsværd, Denmark) solution and incubated in a shaking water bath at 50ºC and 200 rpm for 5 h. The seeds were then cleansed under running tap water and oven-dried for 48 h at 90ºC[Citation27] and stored at room temperature until needed. Fresh coconut kernel was removed from eight mature coconut fruits obtained from a local market and then chopped into smaller pieces. The chopped kernel was then oven-dried at 90ºC for 48 h.[Citation28] The dried kernel was stored under cool, dried condition at room temperature and used for oil extraction within three days. Prior to solvent-extraction, the dried copra pieces were flaked manually into thin pieces and then ground finely using a home blender (Panasonic, Japan).
The oil from ground samples (for a total of three extractions per sample, with each extraction using approximately 500 g, except for noni seeds, 205.0–248.7 g) were extracted with 2 L of petroleum ether (boiling point 40–60ºC, from Merck, Darmstadt, Germany) in a 5 L Soxhlet apparatus for 8 h.[Citation29] Residual solvent was removed using a rotary evaporator (Model N-1, Eyela, Tokyo Rikakikal Co., Ltd., Japan) at 50ºC. The oils were placed in screw capped bottles, flushed with 99.9% nitrogen gas, capped, and then stored at –18ºC before analysis.
Chemical Analyses of Oil
Determination of SV, IV, and free fatty acids (FFAs) were done according to the official standard method.[Citation29] Samples were thawed in an oven at 60ºC for 90 min and mixed gently before the analyses were performed. All analytical determinations were done in triplicate, and the mean and standard deviation were calculated.
Determination of Color
The color of extracted oil was determined using a Lovibond tintometer (Model E, Salisbury, England).[Citation29] The melted sample was added to the tintometer cell up to three-quarters full. The color of each sample was determined at room temperature and by finding the best possible match with the standard color slides that were provided.
Determination of Fatty Acid Composition
The fatty acid composition of the oil samples was determined using the method described by Cocks and van Rede.[Citation30] Briefly, the oil was converted into fatty acid methyl ester (FAME) by dissolving 50 mg of sample in 0.9 mL of n-hexane and 0.1 mL of 30% sodium methoxide. After vortexing for 1 min and leaving to stand for 5 min, 1 µL of the upper layer was injected into a gas chromatograph (Perkin Elmer Clarus 500, Shelton, USA) which was fitted with a flame-ionization detector (FID) and a polar capillary column of BPX70 (0.32 mm internal diameter, 30 m length and 0.25 µm film thickness; SGE International Pty, Ltd., Victoria, Australia). The pressure of the column was set at 10 psi, while the initial temperature for the oven was set at 90ºC. The temperature was then programmed to increase to 220ºC at three different rates: 15ºC/min for the first 5 min, 2ºC/min for the following 20 min, and 15ºC/min for the remaining 1 min. The temperature of the injector and detector were maintained at 240ºC. The FAME peaks were identified by comparing their retention time with peaks of the standard (C6-C24, Even Carbon Number, Saturated FAMEs Kit, Sigma-Aldrich, St. Louis, USA) that was analyzed under the same conditions. Total percentage of saturated and unsaturated fatty acids was calculated by summing up the relative percentage of saturated and unsaturated fatty acids, respectively. Unsaturated and saturated fatty acids ratio was calculated by taking the total percentage of unsaturated fatty acids over the total percentage of saturated fatty acids.
Determination of TAG Profile
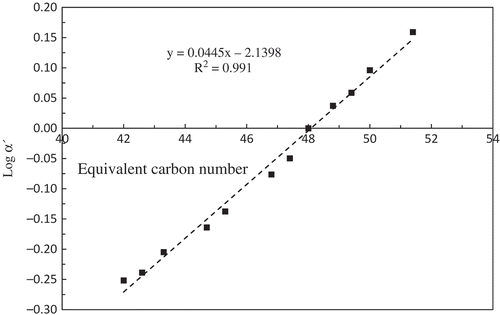
The TAG profile was determined using reversed phase high-performance liquid chromatography (HPLC) according to Ghazali et al.[Citation31] The instrument used was an Alliance Waters 2695 Separations Module HPLC system equipped with a Waters auto-injector, a Waters 2414 refractive index detector, column oven and two Waters 515 HPLC pumps (Waters Corp., Milford, USA). The chromatogram was processed using Water’s Empower Pro Chromatography software version 1.0. The separation column was an RP-18 column (250 × 4 mm) with a particle size of 5 µm (LichroCART 250-4, Merck, Darmstadt, Germany) placed in the column oven at 30ºC. The mobile phase for TAG separation was a mixture of acetone/acetonitrile in the ratio of 63.5:36.5, delivered into the HPLC column at a flow rate of 1 mL/min. An aliquot (10 µL) of oil sample (5% [v/v] in acetone) was injected into the system and the total run time was 45 min. Mean percentage for each peak were calculated by dividing the area of the respective TAG peaks over total area of all TAG peaks (for a sample) and times with 100%. Each sample was run in triplicate and the standard deviation for the respective mean percentage was calculated. In order to identify the TAG peaks, another oil sample, palm oil, which was freshly extracted from oil palm fruit using Soxhlet extraction, was included, as many of its TAG peaks have been identified. Instruments and method that used for running this extra oil sample was same as the other oils sample. The TAG peaks for the palm oil were identified based on the chromatogram of Chen et al.,[Citation32] Lida et al.,[Citation33] and Ghazali et al.[Citation31] The equivalent carbon number (ECN) was assigned for each identified TAG peaks according to the method of Wolff et al.[Citation34] for the palm oil TAG peaks. Graph of log α´ (log of relative retention time of each palm oil TAG peaks) against ECN (for each respective peaks) was plotted and linear equation obtained from this plotted graph was used to identify the ECN of other oil sample. ECN for the reference graph was calculated by the following formula:
Thermal Properties
A differential scanning calorimeter (DSC; PYRISTM Diamond DSC, Perkin Elmer, Shelton, Washington, USA) was used to determine the thermal behavior and solid fat content (SFC) of the oils sample. The instrument was calibrated using indium and was connected to a laboratory Peak Scientific® NG1000A nitrogen generator (Peak Scientific, Billerica, Massachusetts, USA) and a cooling accessory (Intracooler 2P, Perkin Elmer, Shelton, Washington, USA). A data processing software, PYRIS Instrument Managing Software, Version 10.1, was used to analyze the thermal property of oils. Before analysis, the oil samples were placed in an oven at 60ºC for 90 min to ensure complete melting. Approximately 4–9 mg of oil was placed into a volatile aluminum pan (Perkin Elmer, Shelton, Washington, USA) and crimp-sealed with a crimper (Perkin Elmer, Shelton, Washington, USA). The crimped volatile aluminum pan was then placed in the DSC’s sample furnace. An empty-crimped volatile aluminum pan that was used as reference was placed into the DSC’s reference furnace. The DSC furnaces were then covered up with two platinum furnace covers. All the oils sample were subjected to the following temperature programmed: heating from the load temperature (30ºC) to 60ºC and held for 5 min; cooling from 60 to –60ºC with a cooling rate of 10ºC/min and held for 2 min; then, heating from –60 to 60ºC with a heating rate of 10ºC/min and held for another 2 min. Nitrogen gas was purged into the furnaces at 20 mL/min. Peaks that appeared in thermogram can be exothermic or endothermic and their temperature was recorded. Due to the fact that these peaks represent the phase changes process (from solid to liquid or vice versa), temperature of these peaks was collectively known as transition temperature.
The solid fat content percentage (SFC%) for all oil samples were derived from the heating thermogram of the respective oil sample. By using the PYRIS data processing software, the partial peak areas (%) of the melting peaks against temperature were generated. Due to the fact that when the partial area of melting peak was 0%, the SFC% of the oil was 100% and vice versa, the SFC% of the oil sample at different temperature was obtained by reversing the percentage of partial area (from 0–100% to 100–0%).
Statistical Analysis
All measurements with replication were statistically analyzed by using Minitab 14 software. Test of one-way ANOVA (Analysis of variance) was carried out and the average values were compared for significance difference using Turkey’s test. Statistical significance differences were considered at the level of p < 0.05.
RESULTS AND DISCUSSION
Oil Content
shows the oil contents of noni, spinach, lady’s finger, bitter gourd and mustard seeds, and coconut copra and it can be seen that the oil content (15.1 ± 1.8%) of the noni seeds used in this study was slightly higher than value (12.5%) reported by West et al.[Citation9] This slight difference can be due to factors such as cultivation region, temperature, or climate. On the other hand, Bai et al.[Citation37] recovered 20.13% of oil from noni seeds using a supercritical CO2 extraction method. Apart from varietal differences, the greater extraction yield may be because supercritical fluid extraction method is more effective than solvent-extraction method as supercritical fluid possess diffusion coefficient that is similar to gases and solubility that is comparable to liquid.[Citation38]
Among the oil samples, spinach seed oil has the lowest oil content (4.5 ± 0.4%) and is similar to the value reported by Itoh et al.,[Citation11] making it least suitable as a source of industrial oil unless it has a special property such as having antimicrobial activity. On the other hand, the lady’s finger seed, or more commonly known as okra seed, has an oil content 3.6 times more than spinach seeds, making it a potential source of industrial oil. This value is similar to the oil contents reported previously, i.e., 16.3[Citation39] and 17.9%.[Citation12] The oil content of bitter gourd seed (20.2 ± 0.8%) is considered as high since it is comparable to the commercially important oil-bearing cotton seed (18–20%).[Citation40] This value is similar to the oil content (19.3 ± 1.8%) reported by Nyam et al.[Citation14] but different from the value (41–45%) reported by Chang et al.[Citation15] Higher oil content value reported by Chang et al.[Citation15] is probably due to the de-shelling of seeds process before they extracted the oil, thereby increasing the oil content significantly on a weight basis. Mustard seed has the second highest oil content value among oils sample used in this study (36.7 ± 0.7%). This value is within those reported by Fadhil and Abdulahad[Citation41] (33%) and Tiwari et al.[Citation42] (38%). The oil content for coconut copra is 63.1 ± 2.8%. This value is slightly less than the range reported in the literature (65–68%) for dry process extraction method for copra.[Citation40,Citation43] The slight difference could be due to a variety of factors and the most possible factor for this case is the different extraction methods that were used.
Chemical Analysis
The chemical properties of noni, spinach, lady’s finger, bitter gourd and mustard seeds, and coconut copra are shown in . FFA content is one of the parameters that used to judge the quality of oil where values obtained for all oils sample were below 2%, and hence, better than the set parameter (2.3%) for good quality of crude oil.[Citation44] Oil with high FFA (30–50%) is usually obtained from bruised, crushed, or fermented sources. Color is one of the food properties that can give out it impact in a variety of ways. Natural colorants that usually exist in food are compounds such as carotenoids, anthocynanins, and flavonoids.[Citation45] These colored compounds can act as an indicator of the quality of crude oil because oxidation, polymerization, or other chemical reactions can cause color changes in the original oil.[Citation45] In this study, results obtained for R/Y (red color over yellow color ratio) is below 0.3 for all oils sample indicating that all oils sample appear visually more yellow than red.
TABLE 2 Properties of noni, spinach, lady’s finger, bitter gourd and mustard seed oils, and coconut oil
Results obtained for IV for all the oils sample are also shown in . IV indicates the degree of unsaturation of the respective oils and noni seed oil is significantly higher (p < 0.05) than those of the other oil samples, due to its very high content of linoleic acid (72%; ). Noni and spinach seed oils have an IV that are similar to that of safflower (136–148 g of I2/100 g) and sunflower seed oils (118–141 g of I2/100 g) which are both mainly used as edible oil and can also being utilized as industrial lubricant basestock.[Citation46,Citation47] The IV of lady’s finger seed oil is comparatively lower than noni seed oil due to its higher content of saturated fatty acids () and its IV is slightly lower that the value reported by Anwar et al.[Citation13] probably due to varietal differences. Bitter gourd seed oil has a lower IV when compared with linseed oil (IV, 127.8–202.8 g of I2/100 g) that has a similar percentage of tri-unsaturated fatty acids (56% of linolenic acid).[Citation48,Citation49] This is probably because of the presence of eleostearic acid which possesses conjugated double bonds, known to result in lower IV compared to oils with non-conjugated double bonds.[Citation50] The IV obtained is similar to the value reported by Nyam et al.[Citation14]
TABLE 3 Fatty acids relative percentage of noni, spinach, lady’s finger, bitter gourd and mustard seed oils, and coconut oil
The IV for mustard seed oil is within the IV range of commercial edible oils such as sesame oil (104–120 I2/100 g) and cotton seed oil (99–119 I2/100 g).[Citation46,Citation51] However, unlike sesame oil and cotton seed oil that have their degree of unsaturation contributed mainly by oleic and linoleic acids,[Citation46,Citation52] a large part of the degree of unsaturation of mustard seed oil is contributed by erucic acid (). Compared with other oil samples, the IV of coconut oil is the lowest, and statistically different (p < 0.05) from the other oil samples, largely due to an abundance of medium-chain saturated fatty acids (). The IV is within the value range (8.8–10.4 g of I2/100 g) reported by Gopalakrishnan et al.[Citation53]
One-way ANOVA analysis shows that the SV of noni seed oil is not significantly different (p > 0.05) from those for lady’s finger and bitter gourd seed oils but was significantly different from the SV of the other oils used in this study. As SV is inversely related to the mean molecular weight of the oil, the data obtained indicates that noni, lady’s finger, and bitter gourd seed oils have relatively similar molecular weights. Such property can be deduced from their fatty acid profiles () as they all contain fatty acids of similar chain lengths. The SV of these three seed oils are within the reported SV range of commercial edible oils such as sunflower (188–194 mg of KOH/g) and soybean (189–195 mg of KOH/g) seed oils.[Citation46,Citation54] The unsaponifiable matter in the three oils have been reported to be 1.2, 0.37, and 1.12–1.71% for noni,[Citation9] lady’s finger,[Citation55] and bitter gourd seed[Citation56] oils, respectively. The SV of spinach seed oil is found to be lower than the SV of the three oils despite the presence of a higher content of palmitic acid. Itoh et al.[Citation11] reported that spinach seed oil has a high percentage of unsaponifiable matter (2.9%) and oil without unsaponifiable materials has a SV of 190.8 mg of KOH/g. The difference is probably due varietal differences and possible high unsaponifiable content in the spinach seed oil under study. The SV of mustard seed oil is the lowest because of its high content of erucic acid () and is within the value reported for Brassica family (159–179 mg of KOH/g).[Citation57] As expected, the SV of coconut oil is statistically the highest (p < 0.05) when compared with other oil samples due mainly to the presence of medium chain fatty acids such as lauric and myrtistic acids (). The SV of coconut oil is within the value range reported in the literature, 248–265 mg of KOH/g.[Citation52]
Fatty Acid Composition
The fatty acid composition () shows that mustard seed oil is the most unsaturated (98.2%) of the oil samples, followed by noni and spinach seed oils. The high degree of unsaturation of mustard seed oil is largely due to the presence of erucic, oleic, linoleic, and linolenic acids where MUFA content made up 61.2% of the total. The MUFA content is close to those of other oils with high MUFA content such as olive, hazelnut, and almond oils[Citation58–Citation60] and Moringa oleifera, Myrcianthes pungens, and Rollinia sylvatica seed oil.[Citation61,Citation62] High MUFA in mustard seed oil is largely due to erucic acid, which has been reported to cause nutritional deficiency and cardiac lesion in tested animals and, thus is prohibited from being used as an edible vegetable oil in the U.S., Canada, and Europe regions.[Citation63] The fatty acid profile of mustard seed oil is in accordance with the result reported by Velasco et al.[Citation64] Mustard seed oil together with noni seed oil (89% unsaturated fatty acid) would probably be the more susceptible to oxidative rancidity than other oils as mustard seed oil is high in linoleic and linolenic acids (total 37.0%) while noni seed oil is rich in linoleic acid (72.1%). In fact, the fatty acid composition of mustard seed oil is quite similar to that of rapeseed [Brassica napus, and Brassica rapa (campestris)] oil.[Citation17] However, through the effort of selective breeding, low erucic acid B. napus, and B. rapa have been successfully grown since 1968 and 1974, respectively. Subsequent research resulted in the release of low erucic acid and low glucosinolate cultivars of both species which are used to produce canola oil.[Citation65]
Both spinach and bitter gourd seed oils contain a non-typical fatty acid, namely eleostearic acid, in their fatty acids profile (). The presence of eleostearic acid makes these two oils suitable for food usage as conjugated double bonds have been found to be cytotoxic to human tumor cells.[Citation66] When fatty acid with conjugated double bonds was tested on human normal cells, no detrimental effects were exhibited and the cytotoxic effect is believed to act selectively on fast-growing human tumor cells.[Citation67] Furthermore, the use of bitter gourd seed in food is certainly not a modern discovery because traditional Indian culinary had long employed bitter gourd fruit together with its seeds in a dish which is more specially known as “Karela sabzi.”[Citation68] Tung oil which contains 90% eleostearic acid is used industrially in paints and coatings due to its “fast-drying” property.[Citation15] With its higher oil content compared to spinach seed, bitter gourd seed may be a potential source of oil that have useful applications in the food and non-food industries. Bitter gourd oil is especially interesting as it is the only seed oil under study with a saturated fatty acid to unsaturated fatty acid ratio of 43:57, while the other oils are largely more unsaturated.
Lady’s finger seed oil is rich in palmitic, oleic, and linoleic acids (). Therefore, this oil has the potential to be formulated into food products that can provide energy and essential fatty acids such as fat powder with high proportion of polyunsaturated fatty acids.[Citation12,Citation69,Citation70] The three most abundant fatty acids in the oil account for 97.8% of total fatty acid which is in agreement with the range (94.8–99%) reported in the literature.[Citation12,Citation39,Citation71] On the other hand, coconut oil is rich in medium chain fatty acids, namely lauric and myristic acids (), and is more suitable as cooking/frying oil when compared to the other seed oils because it contains a low level (0.7%) of polyunsaturated fatty acids making it less susceptible to oxidative deterioration. Besides, coconut oil also contains a high amount of short and medium chain fatty acids (84.5%) that makes it suitable as feedstock in the production of biodiesel,[Citation72] lubricant,[Citation23] flavor esters,[Citation73] and α-amylase.[Citation74]
TAG Profile
The TAG profiles of the extracted oils are shown in –. Included is the TAG profile palm mesocarp oil () from which a plot of log α´ versus ECN was generated (). The equation derived from the linear regression plot was used to determine the ECN of the TAG peaks for the seed oils used in the study (). Due to linoleic acid being the dominant fatty acid in noni seed oil, the most abundant TAG in the oil was found to be trilinolein (LLL) (31.6 ± 0.03%), followed by PLL (21.3 ± 0.07%), and OLL (20.1 ± 0.05%), where P, L and O are palmitic, linoleic and oleic acids. For spinach seed oil, the major TAG were ones that contain eleostearic acid such as LEE (where E is eleostearic acid) and linoleic acid (e.g., PLL and POL/PLO) which were both present in nearly equal proportions. To date, there is as yet no report on the TAG profile and composition of noni seed and spinach seed oils.
TABLE 4 Triacylglycerol composition of noni, spinach, lady’s finger, bitter gourd and mustard seed oils, coconut oil, and palm olein
FIGURE 1 HPLC chromatograms for (A) noni seed oil; (B) spinach seed oil; (C) lady’s finger seed oil; (D) bitter gourd seed oil; (E) mustard seed oil; (F) coconut oil; (G) palm olein.
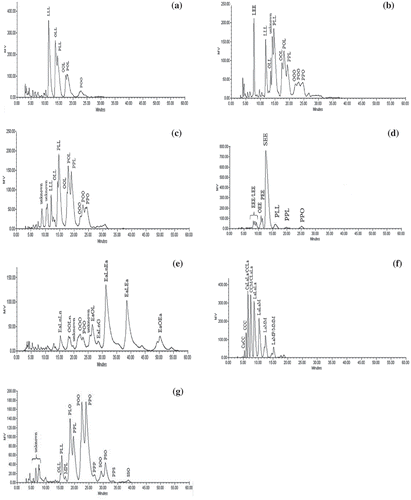
As lady’s finger seed oil is rich in palmitic and linoleic acids, its TAG profile is dominated by PLL, PLO/POL, and PPL in an almost equal proportion. Since different subspecies of lady’s finger seed produce oil with varying fatty acids composition and TAG profile,[Citation71] the TAG composition obtained is thus, slightly different from the values reported by Fiad.[Citation71] Being abundant in eleostearic and stearic acids, bitter gourd seed oil has SEE (where S is stearic acid) as its major TAG. LEE, which is the dominant TAG of tung oil[Citation15] and spinach seed oil, is <6% in bitter gourd seed oil. Other minor TAG in bitter gourd seed oil with similar quantities is EEE, PEE, and OEE. On the other hand, the TAG profile of mustard seed oil is dominated by EaLnEa (31.9 ± 2.03%) and EaLEa (28.3 ± 1.86%), where Ea and Ln represent erucic and linolenic acid, respectively. EaOEa is present in a lower amount, since O (oleic acid) appears to be used to form other TAG such as OOO and POO. For coconut oil, the major TAG are all composed of short and medium chain fatty acids such as LaLaLa, CLaLa/CCM, LaLaM, and CaLaLa/CCLa, where La, C, Ca, and M stand for lauric, caprylic, capric, and myristic acids, respectively. A total of 74.1% of coconut oil is composed by these short- to medium-chain TAGs. The TAG composition of coconut oil is approximately similar as the values reported in the literature.[Citation35]
Thermal Behavior
The heating and cooling thermograms of the oil samples are shown in –3f and –, respectively. Due to the presence of a greater variety of dominant TAG in noni and lady’s finger seed oils, the thermograms of these two oils exhibited more endothermic and exothermic peaks when compared with other oil samples. All endothermic peaks of noni seed oil are found at temperature below 0ºC (D1 = –56.4ºC, D2 = –38.8ºC, D3 = –29.5ºC, D4 = –20.4ºC, D5 = –12.4 ºC; ). The endset temperature (ET, temperature where the last peak goes back to the baseline, and indicates complete melting) of noni seed oil is 4.6ºC. Compared with noni seed oil, lady’s finger seed oil which has more saturated fatty acids (), has its last endothermic peak above 0ºC (B1 = –32.0ºC, B2 = –19.6ºC, B3 = –3.2ºC, B4 = –0.3ºC, and B5 = 6.8ºC) and also a higher ET of 13.3ºC (). The cooling thermogram of noni seed oil contains two broad and blunt exothermic peaks that are approximately equal in size (D6 = –14.7ºC and D7 = –42.8ºC; ). Lady’s finger seed oil has two unequal size exothermic peaks (B6 = –5.7ºC and B7 = –34.3ºC) in its cooling thermogram, with B6 larger and broader than B7 peak ().
FIGURE 3 DSC melting curves of for (A) noni seed oil; (B) spinach seed oil; (C) lady’s finger seed oil; (D) bitter gourd seed oil; (E) mustard seed oil; (F) coconut oil.
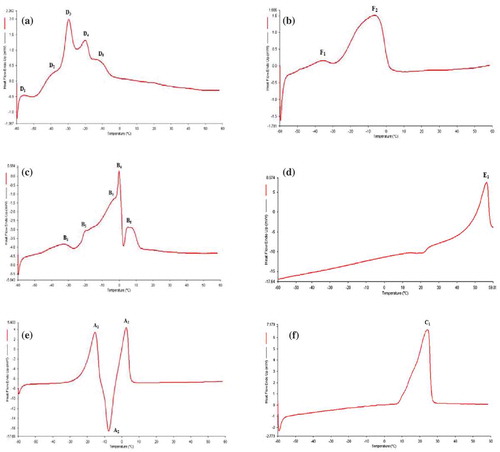
FIGURE 4 DSC cooling curves of for (A) noni seed oil; (B) spinach seed oil; (C) lady’s finger seed oil; (D) bitter gourd seed oil; (E) mustard seed oil; (E) coconut oil.
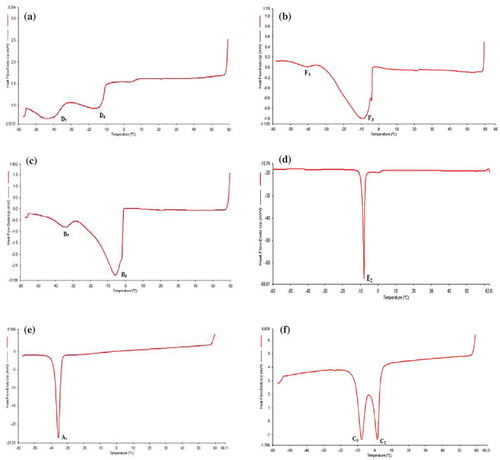
The heating thermogram of spinach seed oil () showed two endothermic peaks (F1 = –34.4ºC and F2 = –6.0ºC) with peak F1 as a small shoulder peak while peak F2 as a large and broad peak and an ET at 6.3ºC. In the cooling thermogram of spinach seed oil (), two exothermic peaks (F3 = –9.7ºC and F4 = –40.4ºC) can be observed, with peak F4 as the broad and large peak. On the other hand, bitter gourd seed oil has only one tall endothermic peak (E1 = 57.7ºC) in its heating thermogram and an ET at 59.7 ºC (). Such a high transition temperature may be largely due to the high content of stearic acid (41.2%). The cooling thermogram of the oil () shows a single, sharp exothermic peak (E2 = –8.7ºC).
The heating thermogram of mustard seed oil () shows two endothermic peaks (A1 = –15.4ºC and A3 = 2.7ºC) and one exothermic peak (A2 = –7.6ºC). The ET of mustard seed oil is at 7.9ºC. Existence of peak A2 is most probably due to recrystallization of the melted fat crystal. Although further investigation is needed to confirm the true cause of peak A2 existence, it can be deduced as an exothermic peak caused by recrystallization through crystallization principle.[Citation75] Cooling thermogram of mustard seed oil () shows a single, narrow and sharp exothermic peak (A4 = –35.45ºC).
Coconut oil has only one large and broad endothermic peak (C1 = 24.3ºC) in its heating thermogram () with ET at 28.1ºC, because it contains mostly of medium-chain saturated fatty acids. In the cooling thermogram (), two not-completely-resolve peaks (C2 = 1.8ºC and C3 = –7.6ºC) were observed and this indicates that coconut oil contains TAGs that crystallized at very similar temperature.
SFC%
The SFC% of the oil samples were derived from their heating thermograms and are shown in . The SFC of noni () and lady’s finger () seed oils share two common characteristics: A gradual decrease in SFC% with an increase in temperature and the formation of a half-solid (50% solid) state at temperatures below 0ºC. The latter feature is shared by spinach seed oil () but it melts at a higher rate. This indicates that these three oils will be useful in any applications that require high liquidity as only extensive cooling can change these oils into total or half-solid state. Potential applications of such oils are as salad oil or whipped-soft margarine that does not solidify when stored at refrigerator temperature.
FIGURE 5 Solid fat content (%) for (A) noni seed oil; (B) spinach seed oil; (C) lady’s finger seed oil; (D) bitter gourd seed oil; (E) mustard seed oil; (F) coconut oil.
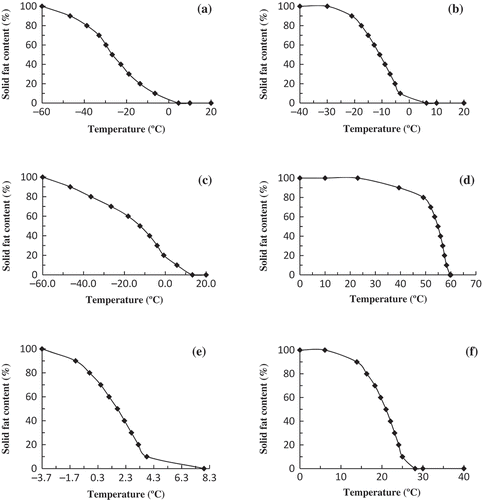
The SFC% of bitter gourd seed oil is shown in . The half-solid state of the oil is 54.9ºC. Subsequent increase of 4.8ºC from this half-solid state liquefies the oil completely at 59.7ºC. This narrow melting temperature range from half-solid to liquid state is valuable in any applications that require immediate phase change from solid to liquid. The SCF% of mustard seed oil (shown in ) decreased rapidly between –1.3 to 3.8ºC. Its onset of melting temperature occurred at –3.7ºC. At ambient tropical temperature, this mustard seed oil would remain mainly in the liquid state. In contrast, coconut oil can be regarded as hard oil since the SFC% is 100% at 0ºC. The SFC% decreases gradually from 0 to 14ºC, then decreased more rapidly after 14ºC () to melt completely at 28.1ºC. This indicates that food substances that need sharp melting behavior may employ coconut oil as cocoa butter substitute and ice-cream fat which are supposed to melt rapidly within body temperature.[Citation76,Citation77]
CONCLUSION
The study shows that, apart from spinach seed, the samples used have an oil content >15% and the physico-chemical characterization of the oils provides valuable information including their potential applications and utilization in food and nonfood industries. All the extracted oil samples have FFA (%) value that is <2% and thus, intensive neutralization will not be needed if being processed for food usage. The noni seed oil has the highest red color index while lady’s finger and mustard seed oils have the same highest yellow color index. The IV and SV of all extracted oil samples are in accordance with the values reported in the literature. It is interesting to note that the fatty acid compositions of the extracted oils, except bitter gourd seed oil, show high proportions of fatty acids with antibacterial activity.[Citation78–Citation81] Fatty acids with antibacterial activities include linoleic acid in noni, spinach, and lady’s finger seed oils, linolenic acid in mustard seed oil, and lauric acid in coconut copra oil. Although bitter gourd seed oil does not contain any fatty acid that has antibacterial activity, it contains a high level of eleostearic acid, a conjugated trienoic acid that may have the ability to inhibit microbial growth. The SFC% profile of noni, lady’s finger, and spinach seed oils show potential application in food usage as salad oil or whipped-soft margarine. Bitter gourd seed oil may have an application that is similar as coconut oil used to its narrow phase-changing temperature range.
FUNDING
The authors would like to acknowledge the Ministry of Education, Malaysia, for the research grant (ERGS/1/2012/STG04/UPM/01/9 Vot. 5527111) awarded to H. M. Ghazali.
Additional information
Funding
REFERENCES
- Basiron, Y. Market Challenges and Global Opportunities for Palm Oil, 8th Global Oils & Fats Forum 2013, Orlando, Florida, Oct 3, 2013.
- Popkin, B.M.; Gordon-Larsen, P. The Nutrition Transition: Worldwide Obesity Dynamics and Their Determinants. International Journal of Obesity 2004, 28, S2–S9.
- Potterat, O.; Hamburger, M. Morinda citrifolia (Noni) Fruit-Phytochemistry, Pharmacology, Safety. Planta Medica 2007, 73, 191–199.
- Dixon, A.R.; McMillen, H.; Etkin, N.L. Ferment This: The Transformation of Noni: A Traditional Polynesian Medicine (Morinda citrifolia, Rubiaceae). Economic Botany 1999, 53, 51–68.
- Bui, A.K.T.; Bacic, A.; Pettolino, F. Polysaccharide composition of the fruit juice of Morinda citrifolia (Noni). Phytochemistry 2006, 67, 1271–1275.
- Zin, Z.M.; Hamid, A.A.; Osman, A.; Saari, N. Antioxidative Activities of Chromatographic Fractions Obtained from Root, Fruit, and Leaf of Mengkudu. Food Chemistry 2006, 94, 169–178.
- Liu, C.H.; Xue, Y.R.; Ye, Y.H.; Yuan, F.F.; Liu, J.Y.; Shuang, J.L. Extraction and Characterization of Antioxidant Compositions from Fermented Fruit Juice of Morinda citrifolia (Noni). Agricultural Sciences in China 2007, 6, 1494–1501.
- Dussossoy, E.; Brat, P.; Bony, E.; Boudard, F.; Poucheret, P.; Mertz, C.; Michel, A. Characterization, Anti-Oxidative and Anti-Inflammatory Effects of Costa Rican Noni Juice (Morinda citrifolia L.). Journal of Ethnopharmacology 2011, 133, 108–115.
- West, B.J.; Jarakae Jensen, C.; Westendorf, J. A New Vegetable Oil from Noni (Morinda citrifolia) Seeds. International Journal of Food Science & Technology 2008, 43, 1988–1992.
- Yang, J.; Paulino, R.; Janke-Stedronsky, S.; Abawi, F. Free-Radical-Scavenging Activity and Total Phenols of Noni Juice and Powder in Processing and Storage. Food Chemistry 2007, 102, 302–308.
- Itoh, T.; Tamura, T.; Matsumoto, T. Sterols, Methylsterols, and Triterpene Alcohols in Three Theaceae and Some Other Vegetable Oils. Lipids 1974, 9, 173–184.
- Rao, P.U. Chemical Composition and Biological Evaluation of Okra (Hibiscus esculentus) Seeds and Their Kernels. Plant Foods for Human Nutrition 1985, 35, 389–396.
- Anwar, F.; Rashid, U.; Ashraf, M.; Nadeem, M. Okra (Hibiscus esculentus) Seed Oil for Biodiesel Production. Applied Energy 2010, 87, 779–785.
- Nyam, K.L.; Tan, C.P.; Lai, O.M.; Long, K.; Che Man, Y.B. Physicochemical Properties and Bioactive Compounds of Selected Seed Oils. LWT–Food Science and Technology 2009, 42, 1396–1403.
- Chang, M.K.; Conkerton, E.J.; Chapital, D.C.; Wan, P.J.; Vadhwa, O.P.; Spiers, J.M. Chinese Melon (Momordica charantia L.) Seed: Composition and Potential Use. Journal of the American Oil Chemists’ Society 1996, 73, 263–265.
- Tsunoda, S.; Hinata, K.; Gomez-Campo, C. Eco-Physiology of Wild and Cultivated Forms in Brassica and Allied Genera. In: Brassica Crops Wild Allies; Japanese Science Society Press: Tokyo, 1980; 109–120.
- Appelqvist, L.Å. Composition of Seeds of Cruciferous Oil Crops. Journal of the American Oil Chemists’ Society 1971, 48, 851–859.
- Clandinin, M.T. The Role of Dietary Long Chain Fatty Acids in Mitochondrial Structure and Function. Effects on Rat Cardiac Mitochondrial Respiration. Journal of Nutrition 1987, 108, 273.
- Christophersen, B.O.; Bremer, J. Erucic Acid—An Inhibitor of Fatty Acid Oxidation in The Heart. Biochimica et Biophysica Acta (BBA)—Lipids and Lipid Metabolism 1972, 280, 506–514.
- Flatmark, T.; Christiansen, E.N.; Kryvi, H. Evidence for a Negative Modulating Effect of Erucic Acid on the Peroxisomal β-Oxidation Enzyme System and Biogenesis in Rat Liver. Biochimica et Biophysica Acta (BBA)—Lipids and Lipid Metabolism 1983, 753, 460–466.
- Abdellatif, A.M.M.; Vles, R.O. Pathological Effects of Dietary Rapeseed Oil in Rats. Annals of Nutrition and Metabolism 1970, 12, 285–295.
- Bezard, J.; Bugaut, M.; Clement, G. Triglyceride Composition of Coconut Oil. Journal of the American Oil Chemists’ Society 1971, 48, 134–139.
- Jayadas, N.H.; Prabhakaran Nair, K. Tribological Evaluation of Coconut Oil as an Environment-Friendly Lubricant. Tribology International 2007, 40, 350–354.
- Kumar, D.; Kumar, G.; Singh, C.P. Fast, Easy Ethanolysis of Coconut Oil for Biodiesel Production Assisted by Ultrasonication. Ultrasonics Sonochemistry 2010, 17, 555–559.
- Ogbolu, D.O.; Oni, A.A.; Daini, O.A.; Oloko, A.P. In vitro Antimicrobial Properties of Coconut Oil on Candida Species in Ibadan, Nigeria. Journal of Medicinal Food 2007, 10, 384–387.
- Nelson, S. Noni Cultivation and Production in Hawaii, Proceedings of the 2002 Hawaii Noni. University of Hawaii at Manoa, College of Tropical Agriculture and Human Resources, 2003.
- McWatters, K.H.; Chinnan, M.S.; Hung, Y.C.; Branch, A.L. Effect of Predecortication Drying Temperature on Cowpea Paste Characteristics and Functionality in Preparation of Akara. Cereal Chemistry 1988, 65, 23–27.
- Guarte, R.C.; Mühlbauer, W.; Kellert, M. Drying Characteristics of Copra and Quality of Copra and Coconut Oil. Postharvest Biology and Technology 1996, 9, 361–372.
- AOAC. Official Methods of Analysis, 14th ed; Association of Official Analytical Chemists: Washington, DC, 1984; 187–188.
- Cocks, L.V.; van Rede, C. Laboratory Handbook for Oil and Fat Analysts. Academic Press Inc.: New York, NY, 1966.
- Ghazali, H.M.; Hamidah, S.; Man, Y.C. Enzymatic Transesterification of Palm Olein with Nonspecific and 1, 3-Specific Lipases. Journal of the American Oil Chemists’ Society 1995, 72, 633–639.
- Chen, C.W.; Chong, C.L.; Ghazali, H.M.; Lai, O.M. Interpretation of Triacylglycerol Profiles of Palm Oil, Palm Kernel Oil, and Their Binary Blends. Food Chemistry 2007, 100, 178–191.
- Lida, H.N.; Sundram, K.; Siew, W.L.; Aminah, A.; Mamot, S. TAG Composition and Solid Fat Content of Palm Oil, Sunflower Oil, and Palm Kernel Olein Blends before and after Chemical Interesterification. Journal of the American Oil Chemists’ Society 2002, 79, 1137–1144.
- Wolff, J.P.; Mordret, F.X.; Dieffenbacher, A. Determination of Triglycerides in Vegetable Oils in Terms of Their Partition Numbers by High Performance Liquid Chromatography. Pure and Applied Chemistry 1991, 63, 1173–1182.
- Adhikari, P.; Shin, J.A.; Lee, J.H.; Hu, J.N.; Zhu, X.M.; Akoh, C.C.; Lee, K.T. Production of Trans-Free Margarine Stock by Enzymatic Interesterification of Rice Bran Oil, Palm Stearin, and Coconut Oil. Journal of the Science of Food and Agriculture 2010, 90, 703–711.
- Laureles, L.R.; Rodriguez, F.M.; Reaño, C.E.; Santos, G.A.; Laurena, A.C.; Mendoza, E.M.T. Variability in Fatty Acid and Triacylglycerol Composition of the Oil of Coconut (Cocos nucifera L.) Hybrids and Their Parentals. Journal of Agricultural and Food Chemistry 2002, 50, 1581–1586.
- Bai, X.P.; Zhao, X.L.; Guo, Z.Y.; Liu, X.Q.; Xu, F.L. Optimization of Supercritical CO2 Extraction of Noni (Morinda citrifolia L.) Seed Oil Using Response Surface Methodology. Advanced Materials Research 2012, 361, 743–747.
- Brunner, G. Gas Extraction. An Introduction to Fundamentals of Supercritical Fluids and the Application to Separation Processes; Springer: New York, NY, 1994.
- András, C.D.; Simándi, B.; Örsi, F.; Lambrou, C.; Missopolinou, Tatala, D.; Panayiotou, C.; Doleschall, F. Supercritical Carbon Dioxide Extraction of Okra (Hibiscus esculentus L.) Seeds. Journal of the Science of Food and Agriculture 2005, 85, 1415–1419.
- Langstraat, A. Characteristics and Composition of Vegetable Oil-Bearing Materials. Journal of the American Oil Chemists’ Society 1976, 53, 241–247.
- Fadhil, A.B.; Abdulahad, W.S. Transesterification of Mustard (Brassica nigra) Seed Oil with Ethanol: Purification of the Crude Ethyl Ester with Activated Carbon Produced from De-Oiled Cake. Energy Conversion and Management 2014, 77, 495–503.
- Tiwari, P.N.; Gambhir, P.N.; Rajan, T.S. Rapid and Nondestructive Determination of Seed Oil by Pulsed Nuclear Magnetic Resonance Technique. Journal of the American Oil Chemists’ Society 1974, 51, 104–109.
- Nik Norulaini, N.A.; Setianto, W.B.; Zaidul, I.S.M.; Nawi, A.H.; Azizi, C.Y.M.; Omar, A.K. Effects of Supercritical Carbon Dioxide Extraction Parameters on Virgin Coconut Oil Yield and Medium-Chain Triglyceride Content. Food Chemistry 2009, 116, 193–197.
- Babatunde, G.M.; Pond, W.G. Nutritive Value of Nigerian Rubber Seed (Hevea brasiliensis) Meal and Oil. III. Performance Characteristics, Relative Organ Weights, Hematocrit, and Plasma Metabolites of Growing Female Rats Fed Corn Diets Containing Rubber Seed Meal, Soya Bean Meal, or Casein. Animal Feed Science and Technology 1988, 20, 125–133.
- Maskan, M. Change in Colour and Rheological Behaviour of Sunflower Seed Oil During Frying and after Adsorbent Treatment of Used Oil. European Food Research and Technology 2003, 218, 20–25.
- Codex Alimentarius Commission. Codex Alimentarius: Fats, Oils and Related Products, 2nd ed., Codex Standard for Named Vegetable Oils. Codex Stan 210-1999; Secretariat of the Joint FAO/WHO Food Standards Programme, FAO: Rome, Lazio, 2001; 11–25.
- Padavich, R.A.; Honary, L. A Market Research and Analysis Report on Vegetable-Based Industrial Lubricants. Training 1995, 2014, 4–28.
- Zhang, Z.S.; Wang, L.J.; Li, D.; Jiao, S.S.; Chen, X.D.; Mao, Z.H. Ultrasound-Assisted Extraction of Oil from Flaxseed. Separation and Purification Technology 2008, 62, 192–198.
- Painter, E.P. Some Relationships between Fat Acid Composition and the Iodine Number of Linseed Oil. Oil Soap 1944, 21, 343–346.
- Forbes, W.C.; Neville, H.A. Wijs Iodine Numbers for Conjugated Double Bonds. Industrial and Engineering Chemistry, Analytical Edition 1940, 12, 72–74.
- Azcan, N.; Danisman, A. Alkali Catalyzed Transesterification of Cottonseed Oil by Microwave Irradiation. Fuel 2007, 86, 2639–2644.
- Gunstone, F.D.; Harwood, J.L.; Padley, F.B. The Lipid Handbook, 2nd Ed; Chapman & Hall: London, 1994.
- Gopalakrishnan, N.; Narayanan, C.S.; Mathew, A.G.; Arumughan, C. Lipid Composition of Coconut Cake Oil. Journal of the American Oil Chemists’ Society 1987, 64, 539–541.
- Itoh, T.; Tamura, T.; Matsumoto, T. Sterol Composition of 19 Vegetable Oils. Journal of the American Oil Chemists’ Society 1973, 50, 122–125.
- Jamieson, G.S.; Baughman, W.F. The Composition of Okra Seed Oil. Journal of the Franklin Institute 1920, 189, 395–396.
- Ali, M.A.; Sayeed, M.A.; Reza, M.S.; Yeasmin, Mst.S.; Khan, A.M. Characteristics of Seed Oils and Nutritional Compositions of Seeds from Different Varieties of Momordica charantia Linn. Cultivated in Bangladesh. Journal of Food Science 2008, 26, 275–283.
- Mikolajczak, K.L.; Miwa, T.K.; Earle, F.R.; Wolff, I.A.; Jones, Q. Search for New Industrial Oils. V. Oils of Cruciferae. Journal of the American Oil Chemists’ Society 1961, 38, 678–681.
- Fasina, O.O.; Craig-Schmidt, M.; Colley, Z.; Hallman, H. Predicting Melting Characteristics of Vegetable Oils from Fatty Acid Composition. LWT–Food Science and Technology 2008, 41, 1501–1505.
- Lee, D.S.; Noh, B.S.; Bae, S.Y.; Kim, K. Characterization of Fatty Acids Composition in Vegetable Oils by Gas Chromatography and Chemometrics. Analytica Chimica Acta 1998, 358, 163–175.
- Ramos-Escudero, F.; Morales, M.T.; Asuero, A.G. Characterization of Bioactive Compounds from Monovarietal Virgin Olive Oils: Relationship Between Phenolic Compounds-Antioxidant Capacities. International Journal of Food Properties 2014, 18, 348–358.
- Abdulkarim, S.M.; Long, K.; Lai, O.M.; Muhammad, S.K.S.; Ghazali, H.M. Frying Quality and Stability of High-Oleic Moringa oleifera Seed Oil in Comparison with Other Vegetable Oils. Food Chemistry 2007, 105, 1382–1389.
- Andrade, J.M.D.M.; Marin, R.; Apel, M.A.; Raseira, M.D.C.B.; Henriques, A.T. Comparison of the Fatty Acid Profiles of Edible Native Fruit Seeds from Southern Brazil. International Journal of Food Properties 2012, 15, 815–822.
- FDA. Detention without Physical Examination of Expressed Mustard Oil. FDA, 2011, Import Alert 26–04. http://www.accessdata.fda.gov/cms_ia/importalert_89.html (accessed Mar 2, 2014).
- Velasco, L.; Goffman, F.D.; Becker, H.C. Variability for the Fatty Acid Composition of the Seed Oil in a Germplasm Collection of the Genus Brassica. Genetic Resources and Crop Evolution 1998, 45, 371–382.
- White, W. Production of rapeseed in Canada. In: The Story of Rapeseed in Western Canada; Saskatchewan Wheat Pool: Regina, Saskatchewan, 1974.
- Igarashi, M.; Miyazawa, T. Newly Recognized Cytotoxic Effect of Conjugated Trienoic Fatty Acids on Cultured Human Tumor Cells. Cancer Letter 2000, 148, 173–179.
- Cornelius, A.S.; Yerram, N.R.; Kratz, D.A.; Spector, A.A. Cytotoxic Effect of Cis-Parinaric Acid in Cultured Malignant Cells. Cancer Research 1991, 51, 6025–6030.
- Veg Recipes of India. Karela Sabzi Recipe, How to make Karela Sabzi Recipe. www.vegrecipesofindia.com/karela-sabzi-recipe-punjabi-karela-sabzi (accessed Jun 7, 2014).
- Adelakun, O.E.; Ade-Omowaye, B.I.O.; Adeyemi, I.A.; Van de Venter, M. Mineral Composition and the Functional Attributes of Nigerian Okra Seed (Abelmoschus esculentus Moench) Flour. Food Research International 2012, 47, 348–352.
- Shivakumar, K.M.; Chetana, R.; Reddy, S.Y. Preparation and Properties of Encapsulated Fat Powders Containing Speciality Fat and ω/Pufa-Rich Oils. International Journal of Food Properties 2012, 15, 412–425.
- Fiad, S. Component Triacylglycerols of Six Seed Oils of Malvaceae. Journal of the American Oil Chemists’ Society 1991, 68, 23–25.
- Tupufia, S.C.; Jeon, Y.J.; Marquis, C.; Adesina, A.A.; Rogers, P.L. Enzymatic Conversion of Coconut Oil for Biodiesel Production. Fuel Processing Technology 2013, 106, 721–726.
- Sun, J.; Yu, B.; Curran, P.; Liu, S.Q. Lipase-Catalysed Transesterification of Coconut Oil with Fusel Alcohols in a Solvent-Free System. Food Chemistry 2012, 134, 89–94.
- Ramachandran, S.; Patel, A.K.; Nampoothiri, K.M.; Francis, F.; Nagy, V.; Szakacs, G.; Pandey, A. Coconut Oil Cake––A Potential Raw Material for the Production of α-Amylase. Bioresource Technology 2004, 93, 169–174.
- Marangoni, A.G. Fat Crystal Networks. In Crystallography; Fennema, O.R.; Hui, Y.H.; Karel, M.; Walstra, P.J.; Whitaker, R.; Eds.; CRC Press: New York, NY, 2005; 15–30.
- Ali, A.; Selamat, J.; Che Man, Y.B.; Suria, A.M. Effect of Storage Temperature on Texture, Polymorphic Structure, Bloom Formation, and Sensory Attributes of Filled Dark Chocolate. Food Chemistry 2001, 72, 491–497.
- Young, F.V.K. Palm Kernel and Coconut Oils: Analytical Characteristics, Process Technology, and Uses. Journal of the American Oil Chemists’ Society 1983, 60, 374–379.
- Yang, D.; Pornpattananangkul, D.; Nakatsuji, T.; Chan, M.; Carson, D.; Huang, C.M.; Zhang, L. The Antimicrobial Activity of Liposomal Lauric Acids Against Propionibacterium Acnes. Biomaterials 2009, 30, 6035–6040.
- Sado-Kamdem, S.L.; Vannini, L.; Guerzoni, M.E. Effect of α-Linolenic, Capric, and Lauric Acid on the Fatty Acid Biosynthesis in Staphylococcus aureus. International Journal of Food Microbiology 2009, 129, 288–294.
- McGaw, L.J.; Jäger, A.K.; Van Staden, J. Isolation of Antibacterial Fatty Acids from Schotia Brachypetala. Fitoterapia 2002, 73, 431–433.
- Dilika, F.; Bremner, P.D.; Meyer, J.J.M. Antibacterial Activity of Linoleic and Oleic Acids Isolated from Helichrysum Pedunculatum: A Plant Used During Circumcision Rites. Fitoterapia 2000, 71, 450–452.