Abstract
Attenuated total reflection Fourier transform infrared spectroscopy was used to compare the structure of β-lactoglobulin, basil seed gum, and β-lactoglobulin-basil seed gum mixtures, at different states (powder, solution, and gel). The effects of heating and different ratios of β-lactoglobulin-basil seed gum were also investigated to determine their impact on chemical structure and understand their interaction. The results showed that gelification process proved a pronounced effect upon β-lactoglobulin secondary structure, leading to the formation of intermolecular hydrogen-bonding β-sheet structure. These results confirmed that this structure may be necessary for the formation of a gel network. Basil seed gum had a distinct peak at around 1603 cm–1 that relates to –COO–1 stretching of carboxylate salts, probably uronic acids, which approved its anionic structure. The Fourier transform infrared spectroscopy findings strongly suggested that these two polymers are thermodynamic incompatible as amide I peak was increased in the β-lactoglobulin-basil seed gum mixed system and carbon–nitrogen (CN) stretching peak was observed at 2125 cm–1. On the basis of these findings, it was possible to modify the ability of β-lactoglobulin-basil seed gum to form a gel and as a consequence to control the gelling and emulsifying properties.
INTRODUCTION
β-lactoglobulin (BLG) is a major constituent of whey proteins, which is extensively used in foods due to its high nutritional value and potential to form gels. In its natural state, it exists as a globular protein folded into compact three-dimensional structures, where the relative hydrophobic amino-acid residues are located in the interior, while most of the polar side chains are exposed to the exterior. Heating BLG solutions above a certain temperature causes partial unfolding, exposition of interiorly located amino acid, and aggregation of partially or completely denatured protein.[Citation1,Citation2] It is now well-established that the heat-induced gelation process occurs in two distinct steps. In the first step, the protein solution is heated above the denaturation temperature, which involves unfolding of some polypeptide segments and exposure of hydrophobic and thiol groups. In the second step, the exposed functional groups in the unfolded polypeptide interact with each other, leading to the aggregation of denatured proteins and to the formation of a gel network.[Citation3]
The thermal aggregation of BLG has been investigated by different techniques including differential scanning calorimetry (DSC),[Citation4,Citation5] circular dichroism (CD),[Citation5,Citation6] infrared spectroscopy,[Citation6,Citation7] UV-vis spectroscopy,[Citation8] nucleic magnetic resonance (NMR) spectroscopy,[Citation9] laser Raman spectroscopy,[Citation10] and Fourier transform infrared spectroscopy (FTIR) spectroscopy.[Citation3] All these studies have concentrated on the conformational changes that occur upon heating the protein above or below the temperature of denaturation. The changes have been found to be due to the association of the monomeric protein and the irreversible aggregation of its unfolded state.[Citation4,Citation5] Different states of BLG in solution and gel upon the cooling regime of the gelation process have been made to understand the relationship between the extent of gel formation and the state of protein structure in the gel.[Citation3]
Basil seed gum (BSG) is a novel hydrocolloid which extracted from seeds of basil herb and can be used as a thickening and gelling agent in the food industry.[Citation11] BSG is a hetero-polysaccharide which contains glucomannan, xylan, and glucan.[Citation12] The chemical composition of BSG has been determined to be comprised of two major fractions; including an acid-stable core glucomannan (43%) with glucose/manose ratio of 10:2, and a linked xylan (24.29%) including acidic side chains in the acid-soluble portion. It also has a minor fragment of glucan (2.31%).[Citation13] Some studies on rheological and structural properties of BSG have been recently published.[Citation11,Citation14–Citation16] It was generally found that BSG has a weak-gel behavior at the concentration range of 0.5–3%. The structural analysis on the macromolecular network performed by different techniques such as confocal laser scanning microscopy (CLSM) and scanning electron microscopy (SEM) have been shown to encompass hydrogel properties, which dissolve in an aqueous environment.[Citation15] Hydrogels are often stimuli-responsive in nature due to their biocompatible properties and use extensively in biomedical researchers. An important use of hydrogels is for the delivery of protein-based drugs. It has also been reported that a mixture of BLG and BSG hydrogels forms a mixed biopolymer system.[Citation16] In recent decades, considerable interest has been given to the study of protein-polysaccharide mixtures in all aspects of food and drug applications.[Citation17–Citation22] It was found that the nature of protein-polysaccharide interactions has a great effect on the protein’s functionality.[Citation23]
In spite of the rheological study of BSG and its interaction with BLG, there is no information on their chemical structure at different states. Therefore, the aim of this work was to study the BLG-BSG interactions at different states (powder, solution, and gel) using the FTIR method. To induce variations in the conformation of BLG, BSG, and BLG-BSG under gelling conditions, the effect of heating on the conformation sensitive amide I band in the infrared spectra has also been investigated.
MATERIALS AND METHODS
Materials
Purified BLG powder containing genetic variant A and B was purchased from Fonterra Company (Lot No. CU27, New Zealand). The composition of BLG powder, provided by the supplier, shows that it contains a mixture of the following: moisture (4.65%), protein (94.16%), fat (0.31%), and mineral (0.1%). The amount of protein as dry basis was 98.8% and its protein’s profile contains 70 g BLG, 14.2 g α-lactalbumin, 2.3 g serum albumin per 100 gram of product. The BSG powder was prepared according to the work described by Razavi et al.[Citation14] Physicochemical characteristics were determined to be the same as our previous works.[Citation12]
Preparation of BSG and BLG Solutions
Solutions of BSG and BLG were prepared by adding appropriate amounts of powder to a portion of deionized water (DW) that contained 0.02% (w/w) sodium azide. These solutions were stirred for 2 h at room temperature and then placed on a roller shaker overnight to complete the hydration process. All samples were refrigerated (4°C) before the experiments. The formation of BLG gels was done by dissolving BLG powder in DW containing 10 mM of CaCl2. Protein-polysaccharide mixtures were prepared by maintaining a constant BSG concentration (1%, w/w) and varying the BLG concentration (10, 5, 2, and 1%). In order to study FTIR at gel state, solution was heated at 80°C for 10 min and then cooled to room temperature.
FTIR
All spectra were collected using a Digilab FTS-3000 Fourier transformed infrared spectrometer and a mounted crystalline Zinc Selenide, attenuated total internal reflection (ATR) sampling attachment (Pike Technologies inc., MIRacle™ Single Reflection ATR) with a typical spectral response of 520 to 15,000 cm–1. The infrared light is focused onto the photodiode of a liquid nitrogen-cooled, narrow band mercury-cadmium-telluride (MCT) detector with a normal spectral response of 650 to 7000 cm–1. There is nominally one reflection with a spot size of approximately 100 microns. The spectrometer and attachments purged with dry compressed air to reduce the possibility of atmospheric water or CO2 contamination of the spectra and samples.
Twenty microliters of the sample were transferred onto a ZnSe crystal cleaned with water and ethanol and dried under a flow of dry nitrogen-gas before analysis. The spectra presented are an average of accumulation of 64 scans. All spectra were recorded at room temperature, approximately 23 ± 0.5°C with a spectral resolution of 2 cm–1. The spectra were converted into absorbance units by taking the negative of the log ratio of a sample spectrum to that of an air spectrum. The data was then transferred to data processing programs (ExcelTM, Microsoft Corp., USA and SigmaplotTM v. 10, Systat Software Inc, USA), where numerical consideration and final graphs were prepared. All the experiments were carried out in three replicates.
RESULTS AND DISCUSSION
BLG Structure at Different States
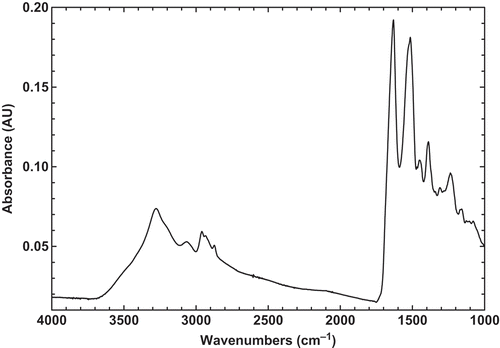
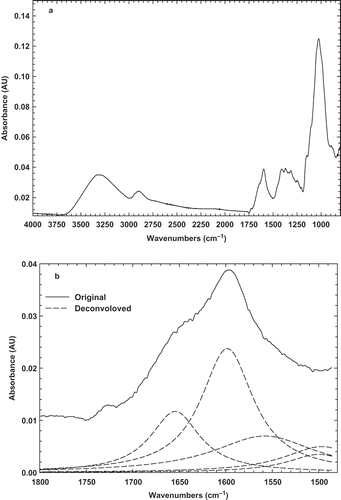
In this study, the FTIR technique was used to investigate the chemical structure of BSG, BLG, and their mixture at different states. The FTIR technique is a versatile and powerful technique for determining the different percent of secondary structure of globular protein in aqueous solution, in complex systems, such as a biological system or in functional states, such as a gel or a film.[Citation3] In addition, this method provides a sensitive diagnostic tool for monitoring changes in the conformation of biopolymers. The original infrared spectrum of BLG powder in the region of 4000–1000 cm–1 was recorded at room temperature (23°C; ). The major peaks which are characteristic of proteins were observed with a maximum at around 1634 and 1518 cm–1, which are indicative of the amide I band (1750–1600 cm–1) and amide II band (1550–1510 cm–1), respectively. Generally, amide I band has a composite profile, consisting of several spectral components related to different secondary structures.[Citation24] The amide I region, which is mainly due to the C=O stretching vibration and to a small extent to C-N stretching and N-H bending vibrations of the peptide bonds, is sensitive to the secondary structure of proteins.[Citation25] The peak observed around 1634 cm–1 is typical of that observed for proteins known to be predominantly β-sheet in structure and is in agreement with previous X-ray diffraction and FTIR spectroscopy studies data.[Citation3]
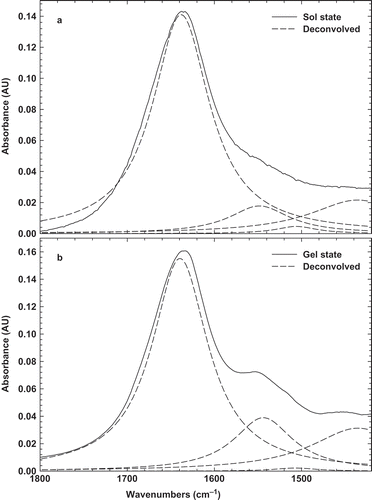
A comparison of the chemical structure of BLG in solution and gel states was carried out by FTIR over the same spectral region (4000–1000 cm–1) and a maximum peak was observed for BLG in both states at around 1622 cm–1 ( and ). This peak is also characteristic of the amide I’ and has been found in pressure, solvent, and thermal denaturation of proteins.[Citation26,Citation27] The peak is believed to be the result of the disruption of hydrogen bonds within the native protein secondary structure and the formation of new hydrogen bonds, associated with the aggregation process upon cooling, i.e., formation of intermolecular hydrogen bonds.[Citation28] Besides, the peak at around 1622 cm–1 presents a conversion from α-helix and intramolecular β-native toward β-aggregated structures. As already observed for other proteins,[Citation29,Citation30] the development of such a band is characteristic of the formation of a protein gel or aggregates. These spectra are very similar to those obtained for BLG powder, suggesting a partial denaturation of protein, which is in agreement with previous work that only reversible modifications of the environment of tryptophan residues occur, indicating a moderate swelling of the protein tertiary structure.[Citation5]
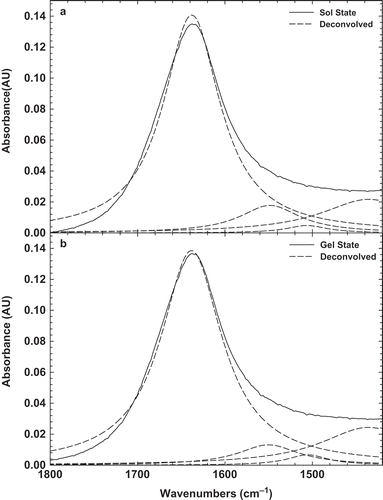
FIGURE 2 Original FTIR and deconvolved spectra of 10% BLG solution (a) before heating sol; and (b) after heating gel at room temperature.
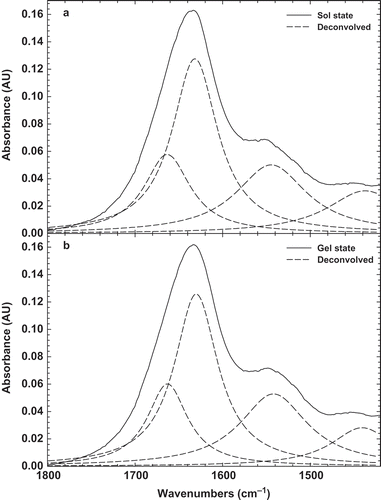
FIGURE 3 (a) Original FTIR spectrum; and (b) deconvolved spectrum of BSG powder at room temperature.
Since, the spectral region (1600–1700 cm−1) is attributed to different structure, a more detailed comparison of the BLG in solution and gel states was obtained from deconvolved spectra[Citation31] ( and ). Under physiological conditions, BLG exists as a dimer and its secondary structure consist of nine β-strands (~50%), single α-helix (10~15%), turns (~20%), and random arrangements (~15%).[Citation32] The band at 1634 cm–1 corresponds to β-sheets, while that at 1665 cm–1 has been assigned to the consisting turn β-sheets. The other bands at 1550 and 1440 cm–1 corresponds to turns or unordered structure. The strongest band at 1634 cm–1 is highly characteristic of amide groups involved in the extended β-sheet structure.[Citation9] Since, the strongest peak was seen at 1634 cm–1, it can be found the native protein in solution is mainly constituted by β-sheet. The similar results were also seen in the previous works.[Citation33,Citation34] The shift or disappearance of the band at 1634 cm–1 under denaturing conditions indicates that the presence BLG as a dimer may be another factor impeding the crosslinking.[Citation34] It is clear that BLG in the gel state displays a marked reduction of α-helical, β-sheet, and unordered structures at the expense of intermolecular hydrogen bonded β-sheet structure. The formation of intermolecular β-sheet in gel of BLG is not particular to this globular protein and has been already observed in the gels of some proteins like bovine serum albumin[Citation30] and glycinin.[Citation29]
BSG Structure at Different States
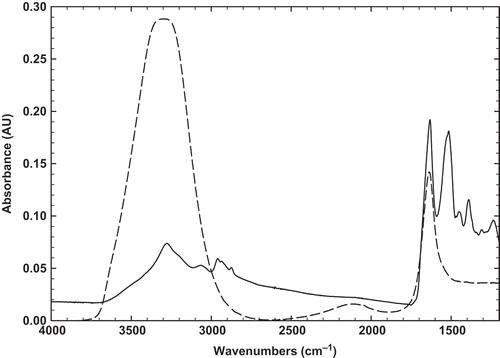
The original FTIR spectrum of BSG powder was also recorded at the same region in order to be comparable with BLG spectrum (). The spectrum exhibits all typical bands and peaks characteristic of polysaccharides. The 2800–3000 cm–1 wave number range is associated with the stretching modes of the C-H bonds of methyl groups (–CH3), the broad band at 3400 cm–1 results from the presence of -OH groups, and three major peaks reveals at around 1603, 1413, and 1023 cm–1. As it is found, the peak 1603 cm–1 may be assigned as –COO– anti-symmetric and symmetric stretching bands of carboxylate salts, probably uronic acids. The second peak (1413 cm–1) may be related to C-N stretching and primary amide. The third peak at 1023 cm–1, which is seen at 900–1200 cm–1 range, represents various vibrations of C-O-C glycosidic and C-O-H bonds.[Citation35] Similar spectra were obtained for other carbohydrates such as guar gum.[Citation36] It should be pointed that 1020–1050 cm–1 range was also attributed to S=O and C-O starching, which may be attributed to BSG emulsifying properties.[Citation35] Therefore, it may be concluded that BSG is a hydrocolloid which have a little amount of peptides in its compound and possess emulsifying characteristics. The similar findings were also shown in water extractable crude polysaccharides from cherry, raspberry, and ginseng berry fruits.[Citation37] In addition, these bands had an important effect on the gel forming and emulsifying abilities that can interact with water to have hydrogen bonds and make a proper gel and emulsion.
In order to obtain more details on BSG structure, the deconvolved peak was presented in . It can be seen there are two peaks at 1656 and 1602 cm–1, which are attributed to amid I band and C=O stretching, respectively.[Citation35] Other bands were seen at 1490 and 1560 cm–1, which are indicating of amid II, C-N stretching and N-H bending.[Citation38] The original and deconvolved FTIR spectra of 3% BSG solution before (sol state) and after heating (gel state) were recorded at room temperature, as shown in and . It can be seen that there is only one distinct peak at around 1603 cm–1, indicating –COO– antisymmetric and symmetric stretching bands of carboxylate salts. It should be noted that there is not any statistical difference (p < 0.5) between sol and gel state of BSG that can be inferred chemical structure was not changed by heating the solution. Natural gums were known to contain a fraction of uronic derivatives, which would impart a weakly anionic character to the gum macromolecule.[Citation39] It was also worth pointing out that a peak near 1602 cm–1 may rise from in-plane deformations of water molecules hydrogen-bonded to the polysaccharide molecule.[Citation40] In addition, the bands 1656, 1490 cm–1 were disappeared and only band at 1602 cm–1 was enhanced, which indicates that C=O stretching in the amid I region is the main structure of BSG in sol and gel states. Therefore, it can be concluded that due to the presence of carboylate salts, BSG is an anionic gum, which can show proper emulsifying properties. It was also shown that this band (1602 cm–1) is related to the ring vibration and tyrosine (phenyl groups).[Citation41]
Interaction of BLG-BSG at Structure Level in Sol and Gel States
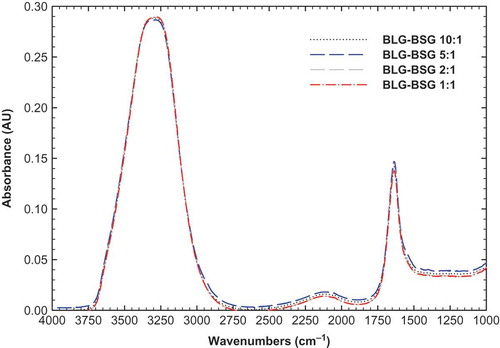
shows the FTIR spectra of different ratios of BLG-BSG mixtures (10:1, 5:1, 2:1, and 1:1) in the region of 4000–1000 cm–1. It can be seen that all of the mixtures had one distinct peak at the maximum amount of 1638 cm–1, which reduced slightly by increasing the BSG amount in the ratio. This spectrum peak could be attributed to the non-esterified carboxyl groups.[Citation42] The shift in this band from 1603 to 1638 cm–1 in the spectrum of BSG-BLG is indicating a change in the environment of this group, particularly water absorption, through BSG interaction with BLG. The maximum peak for BLG-BSG mixture in the ratio of 10:1 to 1:1 was changed from 1638 to 1635 cm–1. In addition, another peak was observed in the new region around 2125 cm–1, which is indicative of CN stretching caused by interaction between BSG with amide groups of BLG.[Citation43] It should be noted that there is a very distinct peak at around 3300 cm–1, indicating the O-H stretching of water molecules.
When blended polymers are thermodynamically compatible and intermolecular interactions prevail, the resultant FTIR spectra of the blends differ from those of the constituent polymers. Conversely, blends of two incompatible (phase-separated) polymers yield FTIR spectra in which the spectra of the two polymers are superimposed. The FTIR result strongly suggests that these two polymers are thermodynamic incompatible as amide I peak was increased in the BLG-BSG mixture (). In fact, the phase separation of network attributed to a distinct peak at 1638 cm–1 (amide I). It supports the idea that intermolecular hydrogen bonds interact between junction zones and thus, stabilize the gel network.
The FTIR spectra of BLG-BSG mixed system at ratio 10:1, before and after heating at 80°C for 10 min, are shown in . It can be found that heating caused to denature the mixture and aggregation was occurred. Similar behavior was also observed for BLG, as shown in . In such kind of biopolymer mixture, which contains a gel-forming biopolymer (BLG), phase separation and gelation become competing processes and the intervention of gelation introduces the possibility of non-equilibrium influences on the formed microscopic structure. Therefore, a number of different structures were formed, depending on the degree of miscibility of the polymers in solution, on the relative time scales of phase separation and gelation, and on the thermal regime, which the mixture was subjected. Since, these biopolymers have shown sufficient spectral difference, it is possible to determine the phase diagram of BSG-BLG by FTIR, which was performed for aqueous amylopectin/gelatin mixture above gel temperature of each constituent.[Citation44]
CONCLUSION
All FTIR spectra in sol and gel states have shown hydroxyl band peaks in the 3368–3384 cm–1 range as well as 1044 cm–1 C-O stretching, amide I (1640 cm–1). The subtle changes in shape, shift and/or intensity of FTIR peaks indicate intermolecular alteration. All protein samples contain the amide II (1548–1550 cm–1) peak due to stretching of N-H and vibration of C-N.[Citation45] Conformational and structural changes are the dominant initial steps driving the aggregation and gelling process. It was obvious from the results presented that gelification process has a pronounced effect upon BLG and BSG secondary structure, leading to the formation of intermolecular hydrogen-bonding β-sheet structure as evidenced by the appearance of the band at around 1622 and 1602 cm–1, respectively. From the results herein, it can be concluded that the cooling regime of the thermal gelation process does not induce drastic changes in the structure of BLG, although it has critical changes on BSG. The FTIR result may strongly suggests that BLG-BSG mixed system is a typical thermodynamic incompatible as amide I peak was increased in the BLG-BSG mixed system comparing with the BLG, BSG in solution and gel states. It supports the idea that intermolecular hydrogen-bonding interactions between junction zones stabilized the gel network. The results also clearly demonstrate the potential of attenuated total reflection Fourier transform infrared spectroscopy (ATR-FTIR) spectroscopy in determining secondary structure of a protein in its functional state, i.e., gel, and to relate its functional properties to specific molecular conformations.
NOMENCLATURE
| ATR-FTIR: | = | Attenuated total reflection Fourier transform infrared spectroscopy |
| BLG: | = | Beta-lactoglobulin |
| BSG: | = | Basil seed gum |
| BLG-BSG: | = | Beta-lactoglobulin—basil seed gum mixture |
| CD: | = | Circular dichroism |
| CLSM: | = | Confocal laser scanning microscopy |
| DSC: | = | Differential scanning calorimetry |
| DW: | = | Deionized water |
| MCT: | = | Mercury-cadmium-telluride |
| NMR: | = | Nucleic magnetic resonance |
| SEM: | = | Size exclusion microscopy |
ACKNOWLEDGMENT
This study was conducted at the Laser Imaging and Vibrational Spectroscopy facility located in the Department of Chemistry, North Carolina State University, USA. The authors are gratefully acknowledged Dr. Simon Lappi for valuable information and his assistance in working with FTIR.
REFERENCES
- Akkermans, C.; Venema, P.; Van der Goot, A.J.; Gruppen, H.; Bakx, E.J.; Boom, R.M.; Van der Linden, E. Peptides are Building Blocks of Heat-Induced Fibrillar Protein Aggregates of β-Lactoglobulin Formed at pH 2. Journal of Biomacromolceules 2008, 9(5), 1474–1479.
- Creusot, N.; Gruppen, H. Enzyme-Induced Aggregation and Gelation of Proteins. Journal of Biotechnology Advances 2007, 25(6), 597–601.
- Allain, A.; Paquin, P.; Subirade, M. Relationships Between Conformation of β-Lactoglobulin in Solution and Gel States as Revealed by Attenuated Total Reflection Fourier Transform Infrared Spectroscopy. International Journal of Biological Macromolecules 1999, 26(5), 337–344.
- Boye, J.I.; Alli, I. Thermal Denaturation of Mixtures of α-lactalbumin and β-Lactoglobulin: A Differential Scanning Calorimetric Study. Food Research International 2000, 33(8), 673–682.
- Wada, R.; Fujita, Y.; Kitabatake, N. Effects of Heating at Neutral and Acid pH on the Structure of β-Lactoglobulin A Revealed by Differential Scanning Calorimetry and Circular Dichroism Spectroscopy. Biochimica et Biophysica Acta (BBA)-General Subjects 2006, 1760(6), 841–847.
- Dong, A.; Matsuura, J.; Allison, S.D.; Chrisman, E.; Manning,M.C.; Carpenter, J.F. Infrared and Circular Dichroism Spectroscopic Characterization of Structural Differences Between β-Lactoglobulin A and B. Biochimstry 1996, 35, 1450–1457.
- Aberkane, L.; Jasniewski, J.; Gaiani, C.; Scher, J.; Sanchez, C. Thermodynamic Characterization of Acacia Gum-β-Lactoglobulin Complex Coacervation. Langmuir 2010, 26(15), 12523–12533.
- Boye, J.I.; Ismail, A.A.; Alli, I. Effects of Physicochemical Factors on the Secondary Structure of β-Lactoglobulin B. Journal of Dairy Research 1996, 63, 97–109.
- Goetz, J.; Koehler, P. Study of the Thermal Denaturation of Selected Proteins of Whey and Egg by Low Resolution NMR. LWT–Food Science and Technology 2005, 38(5), 501–512.
- Ikeda, S.; Li-Chan, E.C.Y. Raman Spectroscopy of Heat-Induced Fine-Stranded and Particulate β-Lactoglobulin Gels. Journal of Food Hydrocolloids 2004, 18(3), 489–498.
- Hosseiniparvar, S.H.; Matia-Merino, L.; Goh, K.K.T.; Razavi, S.M.A.; Mortazavi, S.A. Steady Shear Flow Behavior of Gum Extracted from Basil Seed (Ocimum basilicum L.): Effect of Concentration and Temperature. Journal of Food Engineering 2010, 101(3), 236–243.
- Rafe, A.; Razavi, S.M.A. Dynamic Viscoelastic Study on the Gelation of Basil Seed Gum. International Journal of Food Science and Technology 2013, 48(3), 556–563.
- Mirhosseini, H.; Tabatabaee Amid, B. A Review Study on Chemical Composition and Molecular Structure of Newly Plant Gum Exudates and Seed Gums. Food Research International 2012, 46(1), 387–397.
- Razavi, S.M.A.; Mortazavi, S.A.; Matia-Merino, L.; Hosseini-Parvar, S.H.; Motamedzadegan, A.; Khanipour, E. Optimization Study of Gum Extraction from Basil Seeds (Ocimum basilicum L.). International Journal of Food Science and Technology 2009, 44(9), 1755–1762.
- Rafe, A.; Razavi, S.M.A.; Farhoosh, R. Rheology and Microstructure of Basil Seed Gum and β-Lactoglobulin Mixed Gels. Food Hydrocolloids 2013, 30(1), 134–142.
- Rafe, A.; Razavi, S.M.A.; Khan, S. Rheological and Structural Properties of β-Lactoglobulin and Basil Seed Gum Mixture: Effect of Heating Rate. Food Research International 2012, 49(1), 32–38.
- Dickinson, E.; McClements, D. J. Protein-Polysaccharides Interactions. In Advances in Food Colloids; Dickinson, E.; Ed.; Blackie Academic & Professional: London, 1995; 81–101.
- Tolstoguzov, V.B. Some Physico-Chemical Aspects of Protein Processing in Foods. Multicomponent Gels. Food Hydrocolloids 1995, 9(4), 317–332.
- Dickinson, E.; James, J.D. Influence of High-Pressure Treatment on β-Lactoglobulin-Pectin Associations in Emulsions and Gels. Food Hydrocolloid 2000, 14(4), 365–376.
- Gil, E.S.; Frankowski, D.J.; Bowman, M.K.; Gozen, A.O.; Hudson, S.M.; Spontak, R.J. Mixed Protein Blends Composed of Gelatin and Bombyx Mori Silk Fibroin: Effects of Solvent-Induced Crystallization and Composition. Biomacromolecules 2006, 7(3), 728–735.
- Gil, E.S.; Frankowski, D.J.; Hudson, S.M.; Spontak, R.J. Silk Fibroin Membranes from Solvent-Crystallized Silk Fibroin/Gelatin Blends: Effects of Blend and Solvent Composition. Materials Science and Engineering 2007, 27(3), 426–431.
- Aberkane, L.; Jasniewski, J.; Gaiani, C.; Hussain, R.; Scher, J.; Sanchez, C. Structuration Mechanism of β-lactoglobulin-Acacia Gum Assemblies in Presence of Quercetin. Food Hydrocolloids 2012, 29(1), 9–20.
- De Kruif, C.G.; Tuinier, R. Polysaccharide Protein Interactions. Food Hydrocolloids 2001, 15(4–6), 555–563.
- Vetri, V.; Militello, V. Thermal Induced Conformational Changes Involved in the Aggregation Pathways of β-Lactoglobulin. Biophysical Chemistry 2005, 113(1), 83–91.
- Pelton, J.T.; McLean, L.R. Spectroscopic Methods for Analysis of Protein Secondary Structure. Analytical Biochemistry 2000, 277(2), 167–176.
- Ngarize, S.; Herman, H.; Adams, A.; Nazlin, H. Comparison of Changes in the Secondary Structure of Unheated, Heated, and High-Pressure-Treated β-Lactoglobulin and Ovalbumin Proteins Using Fourier Transform Raman Spectroscopy and Self-Deconvolution. Journal of Agriculture and Food Chemistry 2004, 52(21), 6470–6477.
- Long, G.; Ji, Y.; Pan, H.; Sun, Z.; Li, Y.; Qin, G. Characterization of Thermal Denaturation Structure and Morphology of Soy Glycinin by FTIR and SEM. International Journal of Food Properties 2015, 18(4), 763–774.
- Hu, X.; Kaplan, D.; Cebe, P. Determining Beta-Sheet Crystallinity in Fibrous Proteins by Thermal Analysis and Infrared Spectroscopy. Macromolceules 2006, 39(18), 6161–6170.
- Renkema, J.M.S.; Knabben, J.H.M.; Vliet, T.V. Gel Formation by β-Conglycinin and Glycinin and Their Mixtures. Food Hydrocolloids 2001, 15(4–6), 407–414.
- Militello, V.; Casarino, C.; Emanuele, A.; Giostra, A.; Pullara, F.; Leone, M. Aggregation Kinetics of Bovine Serum Albumin Studied by FTIR Spectroscopy and Light Scattering. Journal of Biophysical Chemistry 2004, 107(2), 175–187.
- Sakai, K.; Sakurai, K.; Sakai, M.; Hoshino, M.; Goto, Y. Conformation and Stability of Thiol-Modified Bovine β-Lactoglobulin. Protein Science 2000, 9, 1719–1729.
- Boye, J.I.; Ismail, A.A.; Alli, I. Effects of Physicochemical Factors on the Secondary Structure of β-Lactoglobulin. Journal of Dairy Research 1996, 63(1), 97–109.
- Lefevre, T.; Subirade, M. Structural and Interaction Properties of β-Lactoglobulin as Studied by FTIR Spectroscopy. International Journal of Food Science and Technology 1999, 34(5–6), 419–428.
- Eissa, A.; Puhl, C.; Saad, K. Enzymatic Crosslinking of β-Lactoglobulin: Conformational Properties using FTIR Spectroscopy. Biomacromolecules 2006, 7(6), 1707–1713.
- Nikonenko, N.A.; Buslov, D.K.; Sushko, R.G.Z.; Bankov, B. Analysis of the Structure of Carbohydrates with Use of the Regularized Deconvolution Method of Vibrational Spectra. BAÜ Fen Bil. Enst. Dergisi 2002, 4, 2.
- Ma, X.; Pawlik, M. Intrinsic Viscosities and Huggins Constants of Guar Gum in Alkali Metal Chloride Solutions. Carbohydrate Polymers 2007, 70(1), 15–24.
- Kelly Ross, K.; Siow, Y.; Brown, D.; Isaak, C.; Fukumoto, L.; Godfrey, D. Characterization of Water Extractable Crude Polysaccharides from Cherry, Raspberry, and Ginseng Berry Fruits: Chemical Composition and Bioactivity. International Journal of Food Properties 2015, 18(3), 670–689.
- Kong, J.; Yu, S. Fourier Transform Infrared Spectroscopic Analysis of Protein Secondary Structures. Acta Biochimica et Biophysica Sinica 2007, 39(8), 549–559.
- Wang, Q.; Ellis, P.R.; Ross-Murphy, S.B. Dissolution Kinetics of Guar Gum Powders-II. Effects of Concentration and Molecular Weight. Carbohydrate Polymers 2003, 53(1), 75–83.
- Synytsya, A.; Copikova, J.; Matejka, P.; Machovic, V. Fourier Transform Raman and Infrared Spectroscopy of Pectins. Carbohydrate Polymers 2003, 54(1), 97–106.
- Gullekson, C.; Lucas, L.; Hewitt, K.; Kreplak, L. Surface-Sensitive Raman Spectroscopy of Collagen I Fibrils. Biophysical Journal 2011, 100(7), 1837−1845.
- Gnanasambandam, R.; Proctor, A. Preparation of Soy Hull Pectin. Food Chemistry 1999, 65, 461–467.
- Jung, C. Insight into Protein Structure and Protein-Ligand Recognition by Fourier Transform Infrared Spectroscopy. Journal of Molceular Recognition 2000, 13(6), 325–351.
- Durrani, C.M.; Pryrtupa, D.A.; Donald, A.M.; Clark, A.H. Phase Diagram of Mixtures of Polymers in Aqueous Solution Using Fourier Transform Infrared Spectroscopy. Macromolecules 1993, 26, 981–987.
- Gounga, M.E.; Xu, S.; Wang, Z. Whey Protein Isolate-Based Edible Films as Affected by Protein Concentration, Glycerol Ratio, and Pullulan Addition in Film Formation. Journal of Food Engineering 2007, 83(4), 521–530.