Abstract
“Mad honey” is a complex mixture of numerous chemicals produced by honeybees from Rhododendron flowers. Consumption of mad honey leads to diarrhea, perspiration, dizziness, changes in consciousness, syncope, diplopia, as well as blurred vision, hypotension, and bradycardia due to the presence of grayanotoxins (GTXs). Therefore, it is important to detect the level grayanotoxins in mad honey. Besides its toxicity, mad honey also has antioxidant activity. This study was designed to determine the level of grayanotoxin-III toxin and antioxidant activity of ten different mad honey samples collected from the Black Sea region of Turkey. Liquid chromatography-tandem mass spectrometry was used for the quantitation of grayanotoxin-III. Antioxidant activity was evaluated using total phenolic contents, total ferric reducing antioxidant power, scavenging of 2,2-diphenyl-1-picrylhydrazyl and 3-(2-pyridyl)-5, 6-diphenyl-1,2,4-triazine-4’,4’’-disulfonic acid radicals. Quantities of grayanotoxin-III levels ranged from 68.754 to 0.701 µg grayanotoxin-III/g honey. Mad honey MH7 from Artvin/Hopa had the highest grayanotoxin-III level. Although there were varying levels of grayanotoxin-III, mad honey samples were outstanding in terms of antioxidant activity. MH3 had the highest antioxidant potential. Although toxicity effect comprises, a metered dose of mad honey might also be explored as a potential source in clinical trials due to high bioactivity levels.
INTRODUCTION
The composition of honey is highly dependent on the flowers visited by bees and the weather conditions of the area from which the honey is harvested. The flowers of Rhododendron species are widely spread over countries such as Spain, Portugal, Japan, Brazil, the United States, Nepal, Great Britain, and especially Turkey. The Rhododendron family contains more than 750 plant species, most of which, although not all, contain toxins (e.g., grayanotoxins [GTXs]). Five species are found in Turkey, including R. ponticum (purple flower), R. luteum (yellow flower), R. ungernii (white flower), R. smirnovii (pink flower), and R. caucasicum (Caucasian rhododendron) and their 12 taxa, and these can grow from sea level to an altitude of approximately 3200 m.[Citation1]
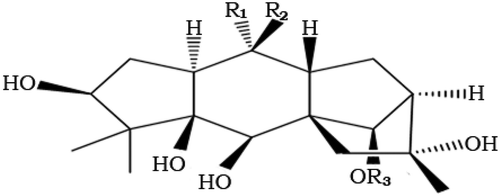
The honey produced from the nectar of plants from the Rhododendron family is known as “mad honey,” or “bitter honey” in Turkey. Consumption of this honey causes a wide spectrum of symptoms such as diarrhea, perspiration, dizziness, changes in consciousness, syncope, diplopia, as well as blurred vision, hypotension, and bradycardia, in a dose-dependent manner.[Citation1,Citation2] Food poisoning is a common problem for consumers of mad honey due to the presence of GTXs, which have been identified as highly toxic in clinical case.[Citation3] These nitrogen free toxins are polyhydroxylated cyclic diterpenes, and also known as andromedotoxin, acetylandromedol, and rhodotoxin[Citation4] (). While GTX-I and GTX-II are found in smaller amounts, the GTX-III isoform is the principal toxin in mad honey. More than 25 GTX isoforms have been isolated from the Rhododendron family.[Citation5] In Turkey, only R. ponticum, and R. luteum produce GTXs.[Citation4,Citation6,Citation7]
Moreover, each type of honey has various bioactivity properties and antioxidant effects. Rhododendron honey is consumed as an alternative medicine against various diseases in the northeastern (Black Sea) region of Turkey, despite its toxic effects. This honey contains thousands of different phenolic compounds which possess antioxidant properties that defend cells against attacks by free radicals.[Citation8,Citation9] Normally, the levels of oxidants and antioxidants are well-balanced in a healthy cell. However, this balance can change due to oxidative stress caused by unstable molecules (free radicals), which are the primary source of harmful biopolymers including nucleic acids, proteins, carbohydrates, and polyunsaturated fatty acids.[Citation10] Antioxidants can effectively protect the cells against such damages. They are, therefore, vitally important for the homeostasis of cells and tissues.[Citation11,Citation12]
In recent studies, Rhododendron plants and their products have been analyzed using gas chromatographic applications with mass spectrometry (GC-MS).[Citation4,Citation6,Citation13] However, the liquid chromatography-tandem mass spectrometry (LC-MS/MS) method is becoming more popular for several toxicological analyses due to its advantages of easy sample preparation without derivatization, sample conservation without contamination, faster analysis, and lower detection limits. Tasdemir et al.[Citation13] listed the disadvantages of GC-MS for GTX analysis as GTXs being unstable on heating and having low vapor pressure, thus requiring derivatization before GC analysis.
The purpose of this study was to determine the levels of GTXs and antioxidant activity of in mad honey samples collected from different locations in the Black Sea region of Turkey. In this general perspective study, LC-MS/MS was used to determine the level of GTX-III directly from mad honey because of some known analytical advantages. In addition, antioxidant properties of samples were detected through total phenolic contents (TPCs)—ferric reducing antioxidant power (FRAP) assay, 2,2-diphenyl-1-picrylhydrazyl (DPPH), and 3-(2-pyridyl)-5, 6-diphenyl-1,2,4-triazine-4’,4’’-disulfonic acid (ABTS) radical scavenging activities.
MATERIALS AND METHODS
Reagents
GTX-III standard was supplied as grayanotoxin III Hemi (ethyl acetate) from Sigma-Aldrich (St. Louis, MO, USA). Hypergrade methanol for LC-MS, hydrochloric acid (HCl), glacial acetic acid and Folin-Ciocalteau reagent were obtained from LiChrosolv® (Merck KGaA, Darmstadt, Germany). High quality ultra-pure water was supplied by Human Zeneer Navi Power I Integrate (Human Corporation, Korea). Gallic acid, sodium carbonate (Na2CO3), sodium acetate trihydrate (NaCH3COO.3H2O), iron (III) chloride hegzahydrate (FeCl3.6H2O), 2,4,6-Tris(2-pyridyl)-s-triazine (TPTZ), DPPH (2,2-Diphenyl-1-(2,4,6-trinitrophenyl) hydrazyl), 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), and potassium persulfate (K2S2O8) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Trolox (6-hydroxy-2, 5, 7, 8-tetramethylchroman-2carboxylic acid) was obtained from Fluka Chemie GmbH (Buchs, Switzerland). LC syringe filters (RC-membrane, 0.2 µm) were obtained from Sartorius Minisart RC 15, Sartorius (Darmstadt, Germany).
Honey Sample
For study sensitivity, only high percentage Rhododendron pollen of ten mad honey samples were collected and used. All samples were tagged as monofloral mad honey by melissopalynological analysis. The melissopalynological characteristics were performed following the method described by Louveaux et al.[Citation14] Acetolyzed pollen grains were mounted on glycerin jelly and sealed with paraffin.[Citation15] For determining the frequency classes of pollen type, 500 pollen grains were counted and classified as dominant pollen (more than 45%). MH1, MH2, MH3, MH4, MH5, MH6, MH7, MH8, MH9, and MH10 codes used for description of mad honey samples. Each code represents the origin of honey. MH1 was from Bartın, MH2 Trabzon-Şalpazarı, MH3 Giresun-Görele, MH4 Kastamonu, MH5 Artvin, MH6 Rize, MH7 Artvin-Hopa, MH8 Trabzon-Vakfıkebir, MH9 Rize, MH10 Zonguldak.
Preparation of Honey
Approximately 5 g of honey sample was extracted with 30 mL methanol in a flask attached to a condenser at 60ºC in 6 h. Extract was subsequently filtered to remove particles, and the final volume was determined, with 5 mL methanolic extract being set aside for antioxidant activity analyses. The remaining extract was evaporated until dry using a rotary evaporator (IKA, Werke, USA) at 40ºC. The residues were dissolved in 10 mL distilled water and transferred to a C18 solid phase extraction (SPE) cartridge, which was initially conditioned with 5 mL methanol followed by 5 mL water. The cartridge was washed with 5 mL water to remove unbound materials. GTX-III was eluted from C18 SPE using 5 mL methanol. Finally, the organic solvents were evaporated in a rotary evaporator under reduced pressure at 40°C. The residue was weighed and dissolved in methanol for LC/MS-MS analysis.
Preparation of GTX-III Standard
First, 1 mg grayanotoxin-III hemi (ethyl acetate) was dissolved with 1 mL hypergrade methanol. In order to establish the calibration graphs and method validation, different dilutions ranging from 0.03125 to 2.5 μg/mL of standard were used.
LC-MS/MS Analysis
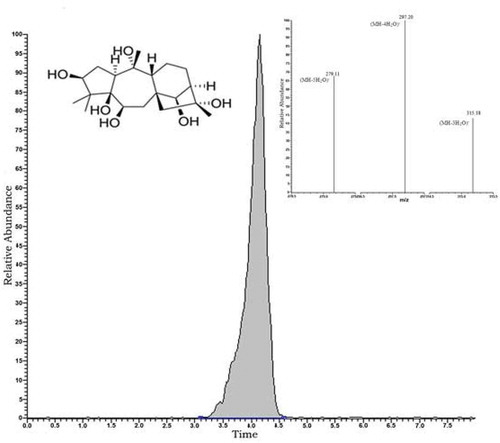
A Thermo-Scientific LC coupled with a TSQ Quantum Access Max triple-stage quadruple-mass spectrometer (San Jose, CA, USA) was used for all analyses. The analytical column was a Phenomenex C-18 (15 cm × 3 mm × 5 µm; Torrance, California, USA). GTX-III was eluted under isocratic conditions using a mobile phase consisting 50:50 water/methanol solution containing 1% acetic acid at flow rate of 0.3 mL/min in 8 min. MS data were acquired by running electrospray ionization (ESI) in negative ion mode using selected reaction monitoring (SRM) after describing the real molecular weight of GTX-III by full scan in the range of 200–500 m/z. The instrument was tuned on MS/MS mode by optimizing the response of m/z 369 as the negative ion form of m/z 370 using 1 µg/mL GTX-III into the mobile phase at 10 µL/min flow rate with a flash syringe (). The fragmentation pattern study was applied for m/z 279, m/z 297, m/z 315 mass ions as product masses of m/z 369 (). For high precision and accuracy, the method was validated, and limit of detection and quantification, repeatability, and recovery were calculated ().
TABLE 1 Summary of Validation Study Results of GTX-III
Recovery Analysis
To calculate the recovery, Rhododendron-nectar-free flower honey was prepared as a methanolic extract. This extract was divided into four parts; 1 mL of GTX-III standard as 0.25–0.5–1 mg/L was spiked to each of three extracts. The remaining fourth extract was considered as unspiked. The spiked samples were also processed using the same mad honey sample preparation procedure before running on LC/MS-MS. The percent recovery for the MS analysis is shown in .
Antioxidant Activity
TPC assay
TPCs were analyzed using Folin-Ciocalteu’s phenol reagent method, taking gallic acid as the standard.[Citation16] Briefly, 20 µL samples (1 mg/mL), 400 µL of 0.2 N Folin-Ciocalteu reagent and 680 µL of distilled water were mixed, and the mixture was vortexed. Following 3-min incubation, 400 µL of Na2CO3 (10%) solution was added and vortexed. Absorbances of the mixtures were measured at 760 nm after 2 h. The concentration of total phenolic compounds was calculated as g of gallic acid equivalents per kg of dry weight, using a standard curve for gallic acid in a concentration range between 0.015 and 0.5 mg/mL (R2 = 0.997).
FRAP (reducing ability) assay
The ability of the mad honey methanolic extracts samples to reduce ferric tripyridyltriazine (Fe3+-TPTZ) complex was measured according to the methods previously described by Benzie and Strain,[Citation17] using a slight modification. The test involves the reduction of ferric tripyridyltriazine (Fe3+-TPTZ) complex to a blue colored Fe(II)-TPTZ by antioxidant agents of samples. Working FRAP reagent was prepared by mixing 25 mL of 300 mM acetate buffer, pH 3.6, with 2.5 mL of 10 mM TPTZ solution in 40 mM HCl and 2.5 mL of 20 mM FeCl3.6H2O solution. Next, 3 mL of freshly prepared FRAP reagent and 100 µL of the samples were mixed to incubate for 4 min at 37°C, and the absorbance was noted at 595 nm against reagent blank containing distilled water. Trolox was used a positive control to construct a reference curve (62.5–1000 µM), FRAP values were expressed as µM Trolox equivalent of g honey.
ABTS radical scavenging assay
This radical scavenging method was improved by Re et al.[Citation18] The stock solutions included 7 mM ABTS solution and 2.4 mM potassium persulfate solution. These stock solutions were mixed in equal amounts and incubated to react for 12 h at room temperature in darkness. The solution was diluted by mixing 1 mL ABTS solution with methanol to obtain an absorbance of 0.706 ± 0.001 units at 734 nm using the spectrophotometer. Subsequently, 20 µL honey extract and 2 mL diluting ABTS solution volume were mixed. The resulting values were expressed as 50% scavenging concentrations (SC50).
DPPH radical scavenging assay
Radical scavenging activity of methanolic extracts against DPPH radical was spectrophometrically determined at 517 nm.[Citation19] Briefly, various concentrations of 0.75 mL of extracts of mad honey were mixed with 0.75 mL of 0.1 mM of DPPH in methanol. Radical scavenging activity was measured using Trolox as standard, and the values were expressed as SC50 (mg sample per mL), the concentration of samples causing 50% scavenging of DPPH radicals.
RESULTS AND DISCUSSION
There are some studies on the physicochemical and biochemical properties of mad honeys,[Citation20–Citation22,Citation23] but this article increases the efficiency and actuality of the previous studies. Until today, there is not any extensive research about the GTX-III determination taken from directly mad honey samples and also there is not any study comparing between honey toxicity and its bioactivity properties. When investigated with the general perspective research of mad honey, results obtained from different areas could be seen. For instance, the 72 volatile compounds were identified from mad honey samples in a study.[Citation21] The other findings of Silici and Karaman,[Citation24] and Sascha et al.[Citation25] demonstrated that mad honeys have a strong source about phenolics. In addition, Silici et al.[Citation26] compared between Rhododendron and multi-flower honeys about elemental analysis. The authors stated that Rhododendron honeys exhibited higher concentrations of Cu, Co, Cr, Ni, Se, Zn, Ca, and Mg than the multi-flower honeys have.
GTX-III Concentrations
Ten Rhododendron honey samples, so-called “mad honey,” were analyzed using LC-MS/MS. The molecular weight of GTX-III is 370 g/mol, appearing at m/z 369 in negative ion mode. GTX-III concentrations ranged from 0.701 to 68.754 µg GTX-III/g honey among the mad honey samples from different locations in the Black Sea region of Turkey (). Literature has shown a huge lack of knowledge about in vitro GTX-III value from directly honey samples. For this reason, the comparison and explication of the quantitative GTX-III data obtained could not be made with others. Among the analyzed sample in the present study, the MH7 from the Artvin/Hopa region was critically important, as it has previously been reported that mad honey from this region causes GTX poisoning by blocking sodium channels in the cell membrane.[Citation1] As MH7 had the highest level of GTX-III with the amount of 68.754 µg GTX-III/g honey, followed by MH10, MH1, MH4, MH6, MH3, MH9, MH2, MH8, and MH5, in that order (). However, the crucial point for the evaluation of these findings was the question of “What is the threshold concentration for GTX-III to be toxic?” Yılmaz et al.[Citation2] reported the amount of mad honey causes poisoning ranges between 5 and 30 g. However, the lethal dose of mad honey was not stated explicitly. We cannot, therefore, conclude that mad honey samples other than MH7 were below the toxicity threshold.
TABLE 2. GTX-III and Antioxidant Activity (TPC, FRAP, ABTS, DPPH) Results from Honeys*
Furthermore, the lethal dose of mad honey may also be dependent on the human metabolic system and resistance developing to mad honey following gradual consumption. Therefore, mad honey poisoning symptoms may vary from person to person, as extensively studied by Gunduz et al.[Citation1] Of 70 cases of mad honey-related poisoning, 52% were sinus bradycardia, 18.5% non-specific bradycardia, 11.4% nodal rhythm, 11.4% atrioventricular (AV) block, 1.4% Wolff-Parkinson-White syndrome (WPW), 1.4% second degree heart block, and 1.4% asystole.[Citation1]
Antioxidant Activity
Many studies have also demonstrated that honeys can be regarded as a member of alternative medicine family. A Sumerian tablet (2100–2000 BC) clearly shows that the people of Sumer used honey both as an ointment and as food in ancient times.[Citation27] Especially mad honey has been used in traditional Chinese, Indian, European, and American folk medicines due to its rich bioactivity value. And also, it has been consumed by Anatolian people for the treatment of gastric pains, bowel disorders, and hypertension.[Citation28,Citation29] Despite mad honey’s toxicity, domestic beekeepers produce mad honey on a routine basis for apitherapy, using honeybee products for various therapeutic purposes and applications. However, the biological activities of mad honey, and particularly its antioxidant capacity, have not been extensively studied. We, therefore, investigated the antioxidant activity of 10 mad honey samples using up-to-date assays.
TPC of natural products is a significant marker of their antioxidant potential. We used the modified Folin-Ciocalteu assay, also known as the gallic acid equivalence (GAE) method to determine TPC values of the mad honey samples, which ranged between 0.134 and 0.606 mg GAE/g honey (). Lachman et al.[Citation30] investigated 40 honey samples of different origins (multi-floral, lime, rape, raspberry, mixture, and honeydew honeys) and reported TPCs ranging between 0.216 and 0.900 mg GAE/g. In another study, by Kucuk et al.,[Citation23] catechin standard was used to determine the total polyphenols of mad honey and other honey samples. They reported results ranging from 0.132 to 0.239 mg catechin/100 g honey. Since different standards were used to determine TPCs, the results of our study cannot be compared directly.
TPC values may not be sufficient for definitive conclusions regarded the antioxidant potential of biological samples. We, therefore, used some additional assays, such as FRAP, ABTS, and DPPH radical scavenging activity,[Citation31] to determine the antioxidant activity of our mad honey samples. The FRAP results from our samples were between 0.619 and 1.616 µmol Trolox/g honey (), in accordance with earlier studies. Gorjanovic et al.[Citation32] reported that the FRAP values of their honey samples ranged from 0.040 to 4.980 µmol Trolox/g. Ruiz-Navajas et al.[Citation33] reported FRAP results between 1.91 and 6.07 µmol Trolox/g (converted values from µg). ABTS and DPPH radical scavenging activity are popular methods for screening the free radical-scavenging ability of the compounds in biological samples, represented as SC50, 50% scavenging of radicals.[Citation34,Citation35] The lower SC50 value, the higher the antioxidant activity. The scavenging activities of ABTS and DPPH in our mad honey samples were in the range of 0.302–0.918 and 0.030–0.097 g/mL, respectively. The radical scavenging of the honey extracts was lower than the Trolox in both methods (). Based on our findings, MH3 had the highest antioxidant potential with greater FRAP, ABTS, DPPH values, as well as the highest TPC.
CONCLUSION
Much research effort has been centered on examining the toxicity of Rhododendron honey, or mad honey, but this is the first study to determine GTX, particularly GTX-III, concentrations in mad honey using the latest LC-MS/MS technology. We used the LC-MS/MS method for the identification and quantification of the GTX-III isoform in ten mad honey samples from different locations in the Black Sea Region of Turkey. In addition to determining GTX-III concentrations, the antioxidant capacity of these mad honey samples was evaluated using the TPC, FRAP, ABTS (SC50), and DPPH (SC50) methods. We concluded that the GTX-III concentrations and antioxidant potentials of the mad honey samples were not correlated with another. The MH7 sample from Artvin/Hopa had the highest GTX-III value, while the MH3 sample from Giresun/Görele had the highest antioxidant capacity. Since the LC-MS/MS technology provides rapid, repeatable, and highly precise detection of GTX-III concentrations, our study could help guide further animal and clinical studies, such as determining lethal doses and effects in experimental animals, as well as quantification of GTX-III in case of clinical poisoning.
NOMENCLATURE
| ABTS: | = | 3-(2-pyridyl)-5, 6-diphenyl-1,2,4-triazine-4’,4’’-disulfonic acid |
| DPPH: | = | 2,2 diphenyl 1-picrylhydrazyl |
| FRAP: | = | Ferric reducing antioxidant power |
| GAE: | = | Gallic acid equivalent |
| GC-MS: | = | Gas chromatography-mass spectrometry |
| GTX: | = | Grayanotoxin |
| LC/MS-MS: | = | Liquid chromatography-tandem mass spectrometry |
| ESI: | = | Electrospray ionization |
| SRM: | = | Selected reaction monitoring |
| MH: | = | Mad honey |
| SC50: | = | cavenging concentration of 50% |
| SPE: | = | Solid phase extraction |
| TPC: | = | Total phenolic content |
| TPTZ: | = | 2,4,6 Tris(2-pyridyl)-s-triazine |
ACKNOWLEDGMENT
The authors would like to thank R.T.E. University for assistance with the LC-MS/MS device.
FUNDING
The study was supported by TAGEM AR-GE/15, and author Huseyin Sahin was supported by a grant from the research fund of Tubitak Bideb for his Ph.D. research.
Additional information
Funding
REFERENCES
- Gunduz, A.; Tatlı, O.; Turedi, S. Mad Honey Poisoning from the Past to the Present. Turkish Journal of Emergency Medicine 2008, 8, 46–49.
- Yılmaz, O.; Eser, M.; Şahiner, A.; Altıntop, L.; Yeşildağ, O. Hypotension, Bradycardia, and Syncope Caused by Honey Poisoning. Resuscitation 2006, 68, 405–408.
- Popescu, R.; Kopp, B. The Genus Rhododendron: An Ethnopharmacological and Toxicological Review. Journal of Ethnopharmacology 2013, 147, 42–62.
- Wong, J.; Youde, E.; Dickinson, B.; Hale, M. School of Agricultural and Forest Science; University of Wales Press: Bangor, UK, 2002; 1–73.
- Qiang, Y.; Zhou, B.; Gao, K. Chemical Constituents of Plants from the Genus Rhododendron. Chemistry & Biodiversity 2011, 8, 792–814.
- Hough, R.L.; Crews, C.; White, D.; Driffeld, M.; Campbell, C.D.; Maltin, C. Degradation of Yew, Ragwort, and Rhododendron Toxins During Composting. Science of the Total Environment 2010, 408, 4128–4137.
- Çeter, T.; Güney, K. Rhododendron and mad honey. Bee Science 2011, 11(4), 124–129.
- Tezcan, F.; Kolayli, S.; Sahin, H.; Ulusoy, E.; Erim, F.B. Evaluation of Organic Acid, Carbohydrate Composition, and Antioxidant Properties of Some Authentic Turkish Honeys. Journal of Food and Nutrition Research 2011, 50, 33–40.
- Alzahrani, H.A.; Boukraa, L.; Bellik, Y.; Abdellah, F.; Bakhotmah, B.A.; Kolayli, S.; Sahin, H. Evaluation of the Antioxidant Activity of Three Varieties of Honey from Different Botanical and Geographical Origins. Global Journal of Health Science 2012, 4(6), 192–196.
- Badarinath, A.V.; Mallikarjuna RAo, K.; Madhu Sudhana Chetty, C.; Ramkanth, S.; Rajan, T.V.S.; Gnanaprakash, K.A. Review on In-Vitro Antioxidant Methods: Comparisons Correlations, and Considerations., International Journal of PharmTech Research 2010, 2(2), 1276–1285.
- Sant’ana, L.D.; Ferreira, A.B.B.; Lorenzon, M.C.A.; Berbara, R.L.L; Castro, R.N. Correlation of Total Phenolic and Flavonoid Contents of Brazilian Honeys with Colour and Antioxidant Capacity. International Journal of Food Properties 2014, 17(1), 65–76.
- Sagdic, O.; Silici, S.; Ekici, L. Evaluation of the Phenolic Content, Antiradical, Antioxidant, and Antimicrobial Activity of Different Floral Sources of Honey. International Journal of Food Properties 2013, 16(3), 658–666.
- Tasdemir, D.; Demirci, B.; Demirci, F.; Dönmez, A.A.; Baser, K.H.C.; Rüedi, P. Analysis of the Volatile Components of Five Turkish Rhododendron Species by Headspace Solid-Phase Microexraction and GC-MS (HS-SPME-GC-MS). Zeitschrift fur Naturforschung C 2003, 58(11–12), 797–803.
- Louveaux, J.; Maurizio, A.; Vorwohl, G. Methods of Melissopalynology. Bee World 1978, 59, 139–157.
- Erdtman, G. The Acetolysis Method, a Revised Description. Svensk Botanisk Tidskrift 1960, 54, 561–564.
- Singleton, V.L.; Rossi, J.L. Colorimetry of Total Phenolics with Phosphomolybdic Phosphotungstic Acid Reagents. American Journal of Enology and Viticulture 1965, 16, 144–158.
- Benzie, I.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power:” The FRAP Assay. Analytical Biochemistry 1996, 239(1), 70–76.
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radical Biology and Medicine 1999, 26, 1231–1237.
- Molyneux, P. The Use of the Stable Free Radical Diphenylpicrylhyrazyl (DPPH) for Estimating Antioxidant Activity. Songklanakarin Journal of Science and Technology 2004, 26, 211–219.
- Silici, S.; Sarioglu, K.; Dogan, M.; Karaman, K. HPLC-DAD Analysis to Identify the Phenolic Profile of Rhododendron Honeys Collected from Different Regions in Turkey. International Journal of Food Properties 2014, 17(5), 1126–1135.
- Silici, S.; Sagdic, O.; Ekici, L. Total Phenolic Content, Antiradical, Antioxidant, and Antimicrobial Activities of Rhododendron Honeys. Food Chemistry 2010, 121(1), 238–243.
- Buratti, S.; Benedetti, S.; Cosio, M.S. Evaluation of the Antioxidant Power of Honey, Propolis and Royal Jelly by Amperometric Flow Injection Analysis. Talanta 2007, 71(3), 1387–1392.
- Kucuk, M.; Kolayli, S.; Karaoglu, S.; Ulusoy, E.; Baltaci, C.; Candan, F. Biological Activities and Chemical Composition of Three Honeys Of Different Types from Anatolia. Food Chemistry 2007, 100, 526–534.
- Silici, S.; Karaman, K. Chemometric Approaches for the Characterization of Turkish Rhododendron and Honeydew Honeys Depending on Amino Acid Composition. Journal of Liquid Chromatography and Related Technologies 2014, 37(16), 864–877.
- Rohn, S.; Rawel, H.M.; Kroll, J. Inhibitory Effects of Plant Phenols on the Activity of Selected Enzymes. Journal of Agricultural and Food Chemistry 2002, 50, 3566–3571.
- Silici, S.; Uluözlü, Ö.D.; Tüzen, M.; Soylak, M. Assessment of Trace Element Levels in Rhododendron Honeys of Black Sea Region, Turkey. Journal of Hazardous Materials 2008, 156, 612–618.
- Mandal, M.D.; Mandal, S. Honey: Its Medicinal Property and Antibacterial Activity. Asian Pacific Journal of Tropical Medicine 2011, 1(2), 154–160.
- Demir, H.; Denizbasi, A.; Onur, O. Mad Honey Intoxication: A case Series of 21 Patients. ISRN Toxicology 2011, 2011, 1–3.
- Sibel, S.; Yonar, M.E; Sahin, H.; Atayoğlu, A.T.; Ozkok, D. Analysis of Grayanatoxin in Rhododendron Honey and Effect on Antioxidant Parameters in Rats. Journal of Ethnopharmacology 2014, 156, 155–161.
- Lachman, J.; Orsak, M.; Hejtmankova, A.; Kovarova, E. Evaluation of Antioxidant Activity and Total Phenolics of Selected Czech Honeys. LWT-Food Science and Technology 2010, 43, 52–58.
- Cao, G.; Prior, R.L. Comparison of Different Analytical Methods for Assessing Total Antioxidant Capacity of Human Serum. Clinical Chemistry 1998, 44(6), 1309–1315.
- Gorjanovic, S.Z.; Alvarez-Suarez, J.M.; Novakovic, M.M.; Pastor, F.T.; Pezo, L.; Battino, M.; Suznjevic, D.Z.J. Comparative Analysis of Antioxidant Activity of Honey of Different Floral Sources Using Recently Developed Polarographic and Various Spectrophotometric Assays. Journal of Food Composition and Analysis 2013, 30(1), 13–18.
- Ruiz-Navajas, Y.; Viuda-Martos, M.; Fernandez-Lopez, J.; Zaldivar-Cruz, J.H.; Kuri, V.; Perez-Alvarez, J.A. Antioxidant Activity of Artisanal Honey from Tabasco, Mexico. International Journal of Food Properties 2011, 14(2), 459–470.
- Thabti, I.; Elfalleh, W.; Tlili, N.; Ziadi, M.; Campos, M.G.; Ferchichi, A. Phenols, Flavonoids, and Antioxidant and Antibacterial Activity of Leaves and Stem Bark of Morus Species. International Journal of Food Properties 2014, 17(4), 842–854.
- Gokbulut, I.; Bilenler, T.; Karabulut, I. Determination of Chemical Composition, Total Phenolic, Antimicrobial, and Antioxidant Activities of Echinophora Tenuifolia Essential Oil. International Journal of Food Properties 2013, 16(7), 1442–1451.