Abstract
In vitro dissolution is a major indicator of potential in vivo calcium absorption. It can be used to assess the bioavailability of Ca from different sources. The aim of this study was to analyze the in vitro dissolution of calcium carbonate from the eggshell samples collected before and after the incubation period. The samples of chicken eggshell were characterized by good dissolution, better than that of precipitated CaCO3. The dissolution of the eggshell before incubation was found to be faster than that after incubation. Good dissolution (after 30 min both types of eggshells were dissolved in over 75%) of the chicken eggshell and the presence of other valuable microelements (boron, strontium) make this biomaterial an excellent source for dietary supplements production.
Keywords:
INTRODUCTION
The total content of calcium in the human body is about 1200 g, which corresponds to 1.40–1.66% of the total body weight. Of this almost 99% exists as the component of bones, bound in the form of apatite, while the rest is present in the ionized form in extracellular and intracellular fluids.[Citation1] The physiological role of calcium and its compounds includes a number of well-known functions. Calcium is not only the major component of bones and teeth, it also participates in the regulation of hormone secretion and activation, muscle contraction, neuronal conduction via ion channels, regulation of inflammatory processes, maintaining the permeability of cell membranes, and many others.[Citation2] The average daily requirement for calcium, established by the National Academy of Sciences, Food and Nutrition Board USA varies from 0.8 to 1.3 g/day, depending on the age and sex life stage group.[Citation3]
Long-term calcium deficiency can lead to serious health problems. In children it can cause rickets, a disease which is characterized by soft and deformed bones and growth retardation. In adults, calcium deficiency can lead to osteomalacia or softening of the bones.[Citation4] Low levels of calcium in blood can cause muscle spasms and cramps.[Citation2] Many studies indicate that low calcium intake also contributes to high blood pressure and osteoporosis.[Citation5,Citation6]
Recent studies have shown that the problem of calcium deficiency occurs worldwide and affects people in all age and sex groups.[Citation7] Even when the optimal dose of calcium is consumed, the results of supplementation can be unsatisfactory.[Citation8] This may be caused by low bioavailability of some calcium preparations due to their poor solubility in the stomach acid and, as a result, a low absorption rate of Ca ions in the gastrointestinal tract. The efficiency of calcium bioabsorption depends on a number of factors, including age, sex, health status, type of supplemented calcium salt, the presence of other substances in the intestines such as lactose, basic amino acids, organic acids, indigestible oligosaccharides, and fatty acids. The proper calcium absorption process also requires the presence of bile acids. Calcium absorption restricts the presence of phytates, oxalates, insoluble dietary fiber fractions, the high content of phosphorus and an increased pH value of the digestive juices.[Citation9–Citation11]
The crucial role of calcium in maintaining health and an often inadequate intake of this element from food make calcium supplementation very common. There are many calcium sources available for supplementation, however there are several of them which are the most popular.[Citation12,Citation13] Calcium carbonate is the most widely used calcium salt with 40% of calcium content and generally good dissolution in acid solutions.[Citation14] Pure calcium carbonate exists in three polymorphic forms, of which calcite is the most stable form that forms transparent crystals known as Iceland spar.[Citation15] Apart from mineral sources, CaCO3 can be obtained either synthetically from calcium hydroxide or chalk (calcium carbonicum praecipitatum, precipitated calcium carbonate), or else it is derived from fossilized or fresh animal shells. Those sources differ from each other not only in composition, but also in dissolution rates, which was proven by Brennan et al.[Citation16]
Natural sources of calcium usually contain many other valuable microelements and, therefore, seem to be a better choice than the precipitated calcium carbonate. Chicken eggshell (CE) is a traditional, yet still relatively poorly recognized source of calcium. It was proven that besides calcium carbonate, which is the main component of an eggshell (95%), this natural biomaterial is composed of other valuable elements such as strontium and boron which play the key role in the prevention of osteoporosis.[Citation17] The high bioavailability of calcium from unfertilized CE was also proven in the piglet study.[Citation18] However, there are no studies and, hence no information about the dissolution of calcium carbonate from the CE.
In fact, there are two available types of CE that can be used as a material for the production of dietary supplements.[Citation19] One of them is derived from the unfertilized eggs (mostly from the baking industry), the other originates from the fertilized eggs, after the process of incubation (from the broiler farms).[Citation20] So far neither of them has had any wider application and they are a troublesome waste.[Citation21] Recently, we have proven that during the embryonic development of a chicken inside the egg elution and subsequent absorption of some elements from the shell occur.[Citation17] We have also confirmed that the mechanism of calcium liberation from the insoluble calcium carbonate is based on the formation of calcium bicarbonate Ca(HCO3)2,[Citation22] according to the reaction:
The liberated calcium is then used by the growing chicken embryo for its skeletal system development. Undoubtedly, there are statistically significant differences between those two types of CE in their composition and structure.[Citation17] The question we are trying to answer now is whether or not those differences affect the dissolution of CaCO3, and as a direct consequence, the bioavailability of calcium from those sources.
The aim of this study was to analyze the in vitro dissolution of calcium carbonate from CE collected before and after the incubation as compared with other well-known sources of CaCO3. As it was proven,[Citation23] the in vitro dissolution is a major indicator for the potential absorption of calcium in vivo, and therefore, it can be used to assess the bioavailability of Ca from different sources.[Citation24]
MATERIALS AND METHODS
Determination of Ca Concentration in the Eggshells
Materials preparation
Sections (9 cm2) from the mid-region of 12 eggshells from a commercial strain of broiler breeder, Gallus gallus domesticus, were collected on the 1st and 21st day of the incubation process at a farm localized in Mazovian Province, Poland. The samples were washed with water, then the membranes were removed and finally the eggshells were dried in air and powdered, this method of the CE preparation was previously successfully applied.[Citation25] Next 500 mg of each sample was weighted and placed in a digestion polytetrafluoroethylene (PTFE) vessel, 6 mL of 65% nitric acid (Suprapur; Merck, Darmstad, Germany) was added and the samples were placed in the microwave sample preparation system MULTIWAVE made by Anton Paar (Perkin Elmer, Waltham, MA). The sample digestion was performed in accordance with the program given in .
TABLE 1 Digestion program of eggshell samples
After the digestion, the samples were quantitatively transferred into 10 mL flasks (class A, Brand®) and filled up to the mark with deionized water (Milli Q, Billerica, MA). Because of the expected high concentrations of calcium all the previously prepared samples were diluted 1:100 with deionized water (Milli Q, Billerica, MA) to enable the analysis of Ca concentration.
ICP-OES Ca concentration analysis
An inductively coupled plasma-optical emission spectrometer (Optima 3100XL made by Perkin Elmer) was used for the measurements of Ca in the eggshells. The setup parameters of the spectrometer are presented in . The calibration of the spectrometer was performed using aqueous calibration standards. Standard solutions were prepared from 1000 mg⁄L stock solution (Merck) by dilution in 5% (v/v) HNO3. Seven standard solutions of different concentrations in the range of 0–500 mg⁄L were prepared. The quality control of the method was performed using the standard reference materials NIST 1486 (bone meal), NCS ZC73012 (cabbage). The measured values were within 95–105% of the certified values.
TABLE 2 Operating parameters of ICP OES
This method of the determination of the Ca concentration in eggshells was successfully used and described in our earlier article.[Citation17]
Dissolution Testing
Materials preparation
The dissolution testing was performed for: six samples of each type of the CE (from the first and last day of incubation) for which the calcium concentration was analyzed; two commercially available dietary supplements containing calcium carbonate derived from the oyster shell, differing in the composition of excipients; one over-the-counter (OTC) drug containing the calcium carbonate produced by chemical precipitation in the form of capsules and an Iceland spar which is the chemically pure calcium carbonate in the polymorphic form of calcite. All the samples were crushed using an agate mortar and a pestle. The homogeneity and the degree of powder fineness was determined using the sieving procedure, as described in European Pharmacopoeia X (EP),[Citation26] for very fine powders. This method ensures the size of the particles between 90 and 125 micrometers in diameter.
Dissolution procedure
The dissolution procedure was adapted from the publication of Brennan et al.[Citation16] The dissolution rate of the samples was measured by the method of EP[Citation27] (Apparatus 2-rotating paddle) using an Erweka DT 70 six-spindle dissolution tester. The samples were placed in vessels containing 900 mL of the dissolution medium: 0.1 M HCl, pH = 1.00 (prepared from 2 N TitriPUR Hydrochloric acid, MERCK and deionized water) at 37°C and stirred constantly by rotating paddles at 75 rpm for 90 min. Aliquots (5 mL) were withdrawn from each vessel after 10, 30, 60, and 90 min. All samples were filtered through 0.45 μm cellulose acetate membranes and refrigerated at 4°C until analyzed for calcium concentrations. Dissolution was calculated by dividing the measured calcium content of a particular sample at a given time by the stated calcium content of the sample.
WD-XRF Ca concentrations analysis
In order to obtain a rapid and accurate method for performing the analysis of calcium in a wide range of concentrations a wavelength dispersive X-ray fluorescence spectrometry (WD-XRF) method was chosen.[Citation28] This technique was described in detail in the EP. The WD-XRF method has an advantage over other techniques as it is a fast and reproducible method, mainly because it does not require sample preparation; although its detection limits are higher. Calcium determination by WD-XRF was performed using an Advant’XP WD-XRF spectrometer (THERMO ARL, En Vallaire, Switzerland), with 60 mA electric current, 40kV voltage, 2.4 kW power and Rh tube, fitted with a flow detector, operating at He atmosphere. The samples were measured using plastic X-ray cells 25 mm deep and an X-ray film chemically resistant (Mylar, 6 μm thick, from PANalytical), free of calcium and capable of giving good transmission for long wavelength elements.
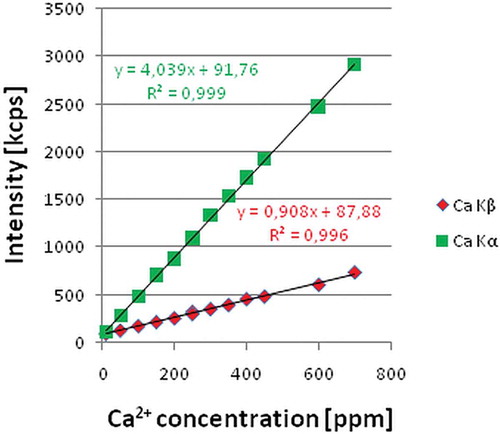
Elemental analysis was carried out on the Ca Kα and Kβ lines and counting time was set to 10 s. Following the EP indications[Citation29] the calibration curve method was employed, for which 12 standard solutions were prepared at the following concentrations: 10; 50; 100; 150; 200; 250; 300; 350; 400; 450; 600; and 700 mg/L. Calcium standard solution 1000 mg/L Ca stock solution (Merck) and dissolution test medium (0.1 M HCl) were used to prepare the standard solutions at the chosen concentrations. The resulting regression curves are presented in . Due to the higher value of regression coefficient, the Kα line was chosen for further analysis.
Statistical Analysis
The results were expressed as mean values with the corresponding standard deviation (SD). Differences between samples were estimated by analysis of variance (ANOVA) followed by Tukey’s “Honest Significant Difference” test. Normality and homoscedasticity of the data were assessed for all parameters, using the Shapiro-Wilk Test and Levene’s Test, respectively. The statistical significance level was set to 0.05. The results were statistically analyzed in Statistica (version 6.0).
RESULTS AND DISCUSSION
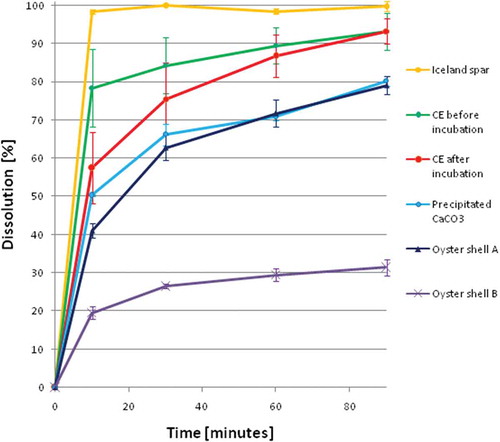
The dissolution of the studied samples (mean ± SD) is shown in and . Using the ANOVA analysis we proved that there are statistically significant differences between the dissolution of the samples of each type. The samples of Iceland spar were characterized by the best (100%) and almost instant dissolution, whereas the dissolution of precipitated CaCO3 was much slower. An effect responsible for this was reported previously,[Citation14] and is now confirmed in our study. Carbon dioxide is one of the products of CaCO3 dissolution in acid media. This gas can be adsorbed on the particles of undissolved calcium carbonate, isolating this salt from the acid medium and, in consequence, lowering the dissolution of CaCO3. Moreover, as it was stated by Brennan et al.,[Citation14] some materials may be characterized by poor dissolution due to the ability of the CaCO3 particle to hold onto a CO2 gas bubble generated in the acid media, which slows down further dissolution. Apparently, in the case of pure calcite crystals the dissolution is more effective than in the case of precipitated calcium carbonate.
TABLE 3 In vitro dissolution of calcium carbonate preparations at 10, 30, 60, and 90 minutes
The preparations from the oyster shell (A and B) were generally characterized by poor dissolution. We also observed statistically significant differences of the dissolution at each of the control time points between those two dietary supplements. Poor dissolution of those samples does not necessarily mean that the CaCO3 from oyster shell is not a highly bioavailable calcium source. As it was stated by Brennan et al., the type and quantity of the filler in tablet (pharmaceutical excipients) can be responsible for the differences in dissolution between the samples prepared from the same material.[Citation14] In our study, those two types of preparations were used as a reference group rather than a representative group of oyster shell material. The aim of the study was not to analyze the dissolution of CaCO3 from oyster shell and since there is no literature data providing information about the dissolution of CaCO3 from different types of oyster shell, we were forced to use the commercially available products.
The dissolution of calcium carbonate from the CE is “more” statistically significant than the dissolution of this salt from other samples, except the pure calcite. Better dissolution of CE than that of precipitated CaCO3 can be explained by the form in which calcium carbonate is present in those two materials. In contrast to precipitated CaCO3 in which calcium carbonate exists mostly in the form of vaterite, CE is composed mostly (95%) of calcite, which, in our experimental conditions was characterized by the best dissolution (Iceland spar samples). Better dissolution of CE in comparison with the oyster shell based samples might be a result of the addition of excipients (mostly starch) during the formulation of those commercially available preparations as well as other polymorphic forms of calcium carbonate in the oyster shell (aragonite).
Significantly higher values of SDs for CE than for other samples can be explained as a result of inhomogeneity between the samples of the same type of CE. In contrast to other tested materials each sample of CE came from a separate eggshell and had a slightly different calcium concentration, which was determined using ICP-OES. Statistical analysis confirmed that there are significant differences between the dissolution of the samples of CE before and after incubation. Those differences concern the dissolution after 10 and 30 min from the experiment start, however, the total dissolution after 90 min was almost the same. CE samples collected from the eggs that did not undergo the process of incubation were characterized by faster dissolubility. During the incubation the content of calcium decreased[Citation12] from 40to 35%. Furthermore, the form in which this element is bound changed from CaCO3 to Ca(HCO3)2.[Citation18] Those changes may be responsible for the differences in the dissolution of calcium carbonate from CE. The lower calcium content and higher porosity of the CE after the incubation can explain its slower dissolution, which is probably inhibited by the formation of CO2, similarly as in the case of amorphous calcium carbonate.
CONCLUSIONS
For the first time the WD-XRF method was successfully applied for the assessment of the dissolution efficiency of different materials containing calcium carbonate. Despite significant differences in the dissolution kinetics between the samples this analytical method enabled a quick and accurate qualitative analysis of calcium. There are statistically significant differences in the dissolution of CaCO3 from different materials in the 0.1 M HCl, under the conditions specified in the EP. The samples of CE were characterized by good dissolution, better than that of precipitated calcium carbonate. Our results coincide with the animal study[Citation16] in which CE digestibility was better than that of precipitated CaCO3. We also proved that the dissolution of the CE before incubation is faster than that after incubation. These differences in the kinetics of dissolution can be a result of differences in the structure and composition of CE being the direct consequence of the incubation. Good dissolution of the CE and the presence of other valuable microelements (boron, strontium) make this biomaterial an excellent source of calcium for dietary supplements production.
ACKNOWLEDGMENTS
The authors are grateful to Professor E. Sieradzki (Medical University of Warsaw) for scientific support. The authors would also like to thank Ms. A. Łuczak (Medical University of Warsaw) for her kind assistance and help.
REFERENCES
- Desobry‐Banon, S.; Vetier, N.; Hardy, J. Health Benefits of Yogurt Consumption. International Journal of Food Properties 1999, 2(1), 1–12.
- Wosje, K.S.; Specker, B.L. Role of Calcium in Bone Health During Childhood. Nutrition Reviews 2000, 58(9), 253–268.
- Dietary Reference Intakes (DRIs): Estimated Average Requirements, Food and Nutrition Board, Institute of Medicine, National Academies. Retrieved from http://www.iom.edu/Activities/Nutrition/SummaryDRIs/~/media/Files/Activity%20Files/Nutrition/DRIs/5_Summary%20Table%20Tables%201-4.pdf
- Chevalley, T.; Rizzoli, R.; Nydegger, V.; Slosman, D.; Rapin, C.H.; Michel, J.P.; Vasey, H.; Bonjour, J.P. Effects of Calcium Supplements on Femoral Bone Mineral Density and Vertebral Fracture Rate in Vitamin D Repleted Elderly Patients. Osteoporosis International 1994, 4, 245–252.
- Griffith, L.E.; Guyatt, G.H.; Cook, R.J.; Bucher, H.C.; Cook, D.J. The Influence of Dietary and Nondietary Calcium Supplementation on Blood Pressure: An Updated Metaanalysis of Randomized Controlled Trials. American Journal of Hypertension 1999, 12(1), 84–92.
- Sidhu, J.S.; Kabir, Y.; Huffman, F.G. Functional Foods from Cereal Grains. International Journal of Food Properties 2007, 10(2), 231–244.
- Prentice, A. Diet, Nutrition, and the Prevention of Osteoporosis. Public Health Nutrition 2004, 7(1A), 227–243.
- Pan, D.; Lu, H.; Zeng, X. A Newly Isolated Ca Binding Peptide from Whey Protein. International Journal of Food Properties 2013, 16(5), 1127–1134.
- Bronner, F. Gastrointestinal Absorption of Calcium. In Calcium in Human Biology; Nordin, B.E.C., Ed.; Springer-Verlag: London, 1988; 93–123.
- Gueguen, L.; Pointillart, A. The Bioavailability of Dietary Calcium. Journal of the American College of Nutrition 2000, 19, 119S–136S.
- Fairweather-Tait, S.J.; Teucher, B. Iron and Calcium Bioavailability of Fortified Foods and Dietary Supplements. Nutrition Reviews 2002, 60(11), 360–367.
- Hanzlik, R.P.; Fowler, S.C.; Fisher, D.H. Relative Bioavailability of Calcium from Calcium Formate, Calcium Citrate, and Calcium Carbonate. Journal of Pharmacology and Experimental Therapeutics 2005, 313(3), 1217–1222.
- Levenson, D.I.; Bockman, R.S. A Review of Calcium Preparations. Nutrition Reviews 1994, 52, 221–232.
- Oikonomou, N.A.; Krokida, M.K. Water Absorption Index and Water Solubility Index Prediction for Extruded Food Products. International Journal of Food Properties 2012, 15(1), 157–168.
- Stipp, S.L.S.; Gutmannsbauer, W.; Lehmann, T. The Dynamic Nature of Calcite Surfaces in Air. American Mineralogist 1996, 81(1–2), 1–8.
- Brennan, M.J.; Duncan, W.E.; Wartofsky, L.; Butler, V.M.; Wray, H.L. In Vitro Dissolution of Calcium Carbonate Preparations. Calcified Tissue International 1991, 49, 308–312.
- Szeleszczuk, L.; Kuras, M.; Pisklak, D.; Wawer, I. Analysis of the Changes in Elemental Composition of the Chicken Eggshell During the Incubation Period. Journal of Elementology (In press). DOI:10.5601/jelem.2014.19.2.673
- Schaafsma, A.; Beelen, G.M. Eggshell Powder, A Comparable or Better Source of Calcium Than Purified Calcium Carbonate: Piglet Studies. Journal of the Science of Food and Agriculture 1996, 79, 1596–1600.
- Aboonajmi, M.; Najafabadi, T.A. Prediction of Poultry Egg Freshness Using Vis-Nir Spectroscopy with Maximum Likelihood Method. International Journal of Food Properties 2014, 17(10), 2166–2176.
- Aboonajmia, M.; Setarehdanb, S.K.; Akramc, A.; Nishizud, T.; Kondoe, N. Prediction of Poultry Egg Freshness Using Ultrasound. International Journal of Food Properties 2014, 17(9), 1889–1899.
- Garnjanagoonchorn, W.; Changpuak, A. Preparation and Partial Characterization of Eggshell Calcium Chloride. International Journal of Food Properties 2007, 10(3), 497–503.
- Pisklak, D.M.; Szeleszczuk, L.; Wawer, I. 1H and 13C Magic-Angle Spinning Nuclear Magnetic Resonance Studies of the Chicken Eggshell. Journal of Agricultural and Food Chemistry 2012, 60, 12254–12259.
- Pantako, T.O.; Amiot, J. Correlation Between In Vitro Solubility and Absorption in Rate of Calcium, Magnesium, and Phosphorus from Milk Protein Diets. Sciences des Aliments 1994, 14(2), 139–158.
- Roig, M.J.; Alegria, A.; Barbera, R.; Farre, R.; Lagarda, M.J. Calcium Bioavailability in Human Milk, Cow Milk, and Infant Formulas—Comparison Between Dialysis and Solubility Methods. Food Chemistry 1999, 65, 353–357.
- Oke, I.A.; Olarinoye, N.O.; Adewusi, S.R.A. Adsorption Kinetics for Arsenic Removal from Aqueous Solutions by Untreated Powdered Eggshell. Adsorption 2008, 14, 73–83.
- European Directorate for the Quality of Medicines and HealthCare. European Pharmacopoeia 8.0; Council of Europe: Strassbourg, France, 2014; 270–271.
- European Directorate for the Quality of Medicines and HealthCare. European Pharmacopoeia 8.0; Council of Europe: Strassbourg, France, 2014; 257.
- Ismail, M.; Abdul Hadi, N.; Haroun, R.Z.; Musa, S.N.A.; Imam, M.U. Energy Dispersive X-Ray Microanalysis of Elemental Distribution in Raw and Germinated Brown Rice Varieties. International Journal of Food Properties 2014, 17(7), 1449–1459.
- European Directorate for the Quality of Medicines and HealthCare. European Pharmacopoeia 8.0; Council of Europe: Strassbourg, France, 2014; 58.