Abstract
The purpose of this study was to evaluate phenolic profile and antioxidant activity of ten different meads of 1:1 and 1:2 types, produced with addition of fruit juices, root spices, and herbs. The total phenolic content in meads varied from 15.27 to 70.80 mg/dm3. The meads originated from dark honeys exhibited the highest antioxidant activity. The predominant phenolic compounds in meads were hydroxybenzoic acids, especially gallic and protocatechuic acid, originated mainly from honeys. Whereas, among the hydroxycinnamic acids, the major phenolic was chlorogenic acid, derived mainly from plant additives used in meads production. A principal component analysis was applied in order to differentiate the investigated meads. The 1:2 meads type could be best described by chlorogenic acid. Among the 1:1 meads type, those made from the dark honeys could be best described by gallic, p-coumaric, and protocatechuic acid while those made with addition of blackcurrant and raspberry juice could be described by caffeic and ferulic acid.
INTRODUCTION
Mead is a traditional beverage, which contains between 9 to 18% of ethanol by volume. It is obtained by alcoholic fermentation of mead wort with the possible addition of herbs, root spices, or fruit juices.[Citation1] Process of mead wort fermentation, as well as its maturation, is time-consuming due to high sugar content, low pH, and low mineral content of honey, and varies from a few month to several years, depending on the method of mead wort dilution with water, as well as honey variety, yeast strain, yeast nutrition and control of pH.[Citation2,Citation3] According to the production technology, meads can be classified as dry, sweet, and frothy.[Citation4] On the other hand, meads can be classified depending on the proportion to which honey is diluted with water, thus following types of meads are obtained: 1:0.5, 1:1, 1:2. and 1:3 (honey:water, v/v).[Citation2]
Meads may be also divided according to the nature of the substances added to the mead wort before fermentation. Depending on that, natural (without additives), hop, spiced, herbal, and juice meads are obtained. The natural meads are products of alcoholic fermentation of mead wort acidified with small amount of organic acid, such as citric or tartaric acids. Hop meads are obtained from natural meads seasoned with hop flowers and optionally with addition of aromatic compounds. When during the brewing process (before fermentation), mead wort is seasoned with spices or herbs, it is possible to obtain spiced or herbal meads. Whereas juice meads are obtained by diluting mead wort with the appropriate amount of water and fruit juice. The most widely used are sour cherry, raspberry, gooseberry, dogberry, and rowanberry juice.
Phenolic compounds comprise a distinct class of secondary plant metabolites, present in plant material and its derived products, and are capable of scavenging of reactive form of oxygen by inhibiting the initiation or propagation of oxidizing chain reactions. These compounds can also be collected from plants by bees, becoming thus, an important component of natural honey. The antioxidant activity (AA) and color of honeys are, to a great extent, determined by the presence in honeys of compounds polyphenolic in character, especially when they are complexed with metals.[Citation5] Phenolic compounds can also serve as useful markers for floral origin of some honey types, additionally they can act as an indicators of honey authenticity.[Citation6,Citation7] Among these compounds, the most important are phenolic acids, such as: gallic, ferulic, p-coumaric, ellagic, and abscisic acid (the latter indicated as a marker for heather honey), as well as flavonoids, i.e., quercetin (as a marker for sunflower honey), chrysin, kaempferol (for rosemary honey), and hesperetin (for citrus honey).[Citation8–Citation11]
The investigation of phenolic profile of meads may be a very useful tool for the identification of the type of honeys and additives used for seasoning, as well as the way of mead preparation. Despite the large amount of literature data concerning phenolic profile and AA of various types of plant materials and food products, including natural honey, there is a very little information regarding meads, in particular that concerning phenolic content and AA. This information seems to be of key importance owing to the fact that beverages are thought to be the main dietary sources of polyphenols. Moreover, the polyphenols present in beverages are completely bioaccessible, because they pass directly into intestinal fluids.[Citation12]
The aim of this study was to determine the influence of the type of mead (i.e., the proportion to which honey was diluted with water, as well as the type of additives used during production of mead) on its phenolic profile and AA. The study focuses on ten various types of Polish meads of 1:1 and 1:2 types which were produced with different plant additives, including fruit juices, herbs, and root spices. The obtained results enabled to classify the investigated meads by a principal component analysis (PCA) using analytical parameters of the meads (i.e., phenolic acids content).
MATERIALS AND METHODS
Chemicals
Acetonitrile, ethyl acetate, methanol, sodium carbonate, sodium chloride, and potassium persulphate were purchased from POCh (Gliwice, Poland). Acetic and hydrochloric acid were obtained from Chempur (Piekary Śląskie, Poland). The Folin-Ciocalteau reagent, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2´-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid diammonium salt; ABTS) were purchased from Sigma-Aldrich Chemie (Steinheim, Germany). All the standards of phenolic acids were from Sigma-Aldrich Chemie (Steinheim, Germany) or Fluka Chemie AG (Buchs, Switzerland).
Mead Samples
The experimental material were ten various types of commercial Polish meads, including both 1:1 and 1:2 mead types () which were purchased from APIS Apiculture Cooperative (Lublin, Poland). The products were stored in the dark at 4ºC until further analysis.
TABLE 1 The additives used for production of the meads of 1:1 (A1–A5) and 1:2 (B1–B5) types
Determination of Total Phenolic Content (TPC) in Meads
The TPC was determined using Folin-Ciocalteu reagent following the reported procedure.[Citation13] In summary, 0.5 cm3 of appropriate diluted mead was mixed with 2.5 cm3 of 0.2M Folin-Ciocalteu reagent. After 5 min of sample incubation, 2 cm3 of 7.5% Na2CO3 solution (m/v) was added to the sample, and the obtained mixture was diluted with distilled water to volume of 5 cm3. The resulted mixture was incubated for 2 h, and then its absorbance was measured in a UV/Vis V-530 spectrophotometer (Jasco, Japan) at λ = 760 nm against a blank sample. The measurements were performed in triplicate. The TPC was expressed as equivalents of gallic acid (GAE) in mg per 1 dm3 of mead, based on a calibration curve. The latter was plotted for standard solutions of GAE used in concentration range of 0–200 mg per 1 dm3 according to the above described procedure.
Determination of AA Using a DPPH Assay
The AA of mead was assayed using a DPPH radical method following the reported procedure.[Citation14] Shortly, 0.1 cm3 of mead sample was mixed with 3.9 cm3 of methanolic solution of DPPH (25 mg per dm3) and left for 15 min. The control tests were made with water instead of the mead. Then an absorbance of the mixture was measured in a UV/Vis V-530 spectrophotometer (Jasco, Japan) at λ = 515 nm against methanol. The measurements were performed in triplicate. The AA (AADPPH) of meads was expressed as percentage of DPPH inhibition according to the formula:
Determination of AA Using an ABTS Assay
The AA of meads was assayed using an ABTS radical method following the reported procedure.[Citation15] In summary, 0.1 cm3 of the mead sample was mixed with 6 cm3 of ABTS cation radical solution and after 30 min an absorbance of the obtained mixture was measured in a UV/Vis V-530 spectrophotometer (Jasco, Japan) at wavelength of 734 nm against phosphate buffer. The control tests were made with water instead of the mead. The measurements were performed in triplicate. The AA (AAABTS) of the meads was expressed as percentage of ABTS cation radical inhibition according to the formula:
Extraction and Chromatographic Analysis of Phenolic Compounds
Phenolic compounds were extracted from the investigating meads with using ethyl acetate, as described by Socha.[Citation16] In summary, 25 cm3 of mead sample was concentrated under reduced pressure at temp. 35°C, in a rotary evaporator in order to remove alcohol without destroying phenolic compounds. The dealcoholized solution was diluted to primary volume by distilled water. The resulting sample was adjusted to pH = 2 with HCl solution and then saturated with NaCl. The resulting solution was extracted three times with ethyl acetate, using 25 cm3 of the solvent. Fraction of ethyl acetate were collected and evaporated to dryness in a vacuum evaporator. The dry residue after evaporation was dissolved in 5 cm3 of methanol. For each sample the extraction procedure was conducted in two replications.
The qualitative and quantitative analysis of phenolic compounds in the meads was based on the reported procedure.[Citation16] Hydroxybenzoic acids, including gallic and protocatechuic acid were identified at λ = 280 nm, whereas hydroxycinnamic acids, including caffeic, chlorogenic, p-coumaric, and ferulic acid were identified at λ = 340 nm. The chromatographic analysis was carried out on a reversed-phase ODS column (Thermo scientific, USA: 250 × 4.6, particle size 5 µm) at a temperature of 30ºC in a gradient mode with two phases: phase A: aqueous solution of acetic acid (2.5 cm3 per 100 cm3), and B: acetonitrile. The eluent flow rate was of 1 cm3 per 1 min. The chromatographic analysis was conducted as follows: the first 10 min—linear gradient through increasing phase B contribution from 3 to 8%, followed by an increase in phase B contribution to 15, 20, 30, and 40% at 20, 30, 40, and 50 min, respectively. Finally, the column was eluted isocratically for 10 min with acetonirtile before the next injection. The qualitative analysis of phenolic acids was carried out by comparing their retention times with those of standards obtained from Sigma-Aldrich (Steinheim, Germany) and Fluka Chemie AG (Buchs, Switzerland). The calibration curves of the analyzed phenolic acids were made in triplicate for each individual standard and were plotted separately for each standard at four different concentrations (0.02–0.2 mg cm–3). The analyses of particular phenolic acids in mead samples were carried out in duplicate.
Statistical Analysis
In order to determine the statistically significant differences between the means, the obtained data were treated by a one-way analysis of variance, and the least significant differences (LSD) using Fischer test at a significance level α = 0.05 was calculated. The Pearson’s linear correlation coefficients between selected parameters were also calculated. Additionally, a PCA was applied in order to differentiate the meads under study in terms of phenolic acids. Calculations were performed with statistical software package Statistica 9.0 (StatSoft Inc. Tulsa, USA).
RESULTS AND DISCUSSION
TPC
presents the values of TPC in analyzed meads. On the basis of obtained results it was stated that mead types of 1:1 (honey/water) were characterized by a significantly higher content of phenolic compounds as compared to 1:2 types, which was associated with a higher amount of honey used for the production of 1:1 mead types. The values of TPC in 1:1 mead types varied in the range from 30.54 mg/dm3 in “Dominikański” to 70.8 mg/dm3 in “Kasztelański” meads. The highest content of phenolic compounds in “Kasztelański” mead, among all the samples may be due to the fact, that the main components used for production of this mead were rich in antioxidants dark-colored buckwheat honey and spices (i.e., roots and herbs). As it was demonstrated in the previous study concerning Polish honeys,[Citation14] buckwheat honey was characterized by the highest value of TPC among all the investigated monofloral honeys. Among the meads of 1:1 type, a high content of phenolic compounds (i.e., 62.8 mg/dm3) also exhibited “Maliniak” mead (), what was probably associated with the presence of raspberry juice, known as a rich source of phenolic compounds, in particular anthocyanin pigments. With respect to the 1:2 mead types investigated (), the highest content of TPC was observed in “Apis” mead (i.e., 21.51 mg/dm3), which was obtained from mead wort enriched with rowanberry juice, whereas “Piastowski” mead, obtained from mead wort enriched only with root spices and herbs was the poorest in this respect (i.e., 15.27 mg/dm3).
FIGURE 1 Total phenolic content in the meads expressed as gallic acid equivalents. Means with different letters are significantly different (p < 0.05).
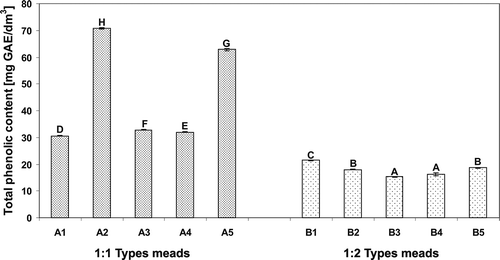
Comparing the content of total phenolics in the meads under study with that concerning natural honeys, it was found that majority of studied meads are poorer sources of phenolic compounds than natural honeys, even despite using various floral additives during meads production. On the other hand, it was ascertained in our study that in the case of some 1:1 mead types, for which the values of TPC exceeded 50 mg per dm3 (i.e., A2 and A5; ), the level of TPC was similar to that found by Bertoncelj[Citation17] and Beretta[Citation18] in acacia honeys. The latter authors found that the level of TPC in honeys strictly depends on both the type of honey and its color, and varied in the range from 5.25 to 78.96 mg per 100 g of honey. Consistently, the highest level of TPC was observed by above mentioned authors in dark buckwheat honey, whereas the significantly lower values of this parameter were observed in light monofloral honeys. Similarly in our study, the highest level of TPC was observed in A2 mead () obtained from buckwheat honey. Additionally, Majewska and Myszka[Citation19] when investigated Polish meads reported that only in the case of mead of 1:0.5 types their level of TPC was similar to that observed in natural honeys.
AA
The values of AA of meads under study, expressed as percentage of DPPH and ABTS radicals scavenging, are shown in and . The results showed that all the studied samples exhibited AA in the both assays, but its values fluctuated considerably. The 1:1 mead types generally demonstrated a significantly higher AA in comparison to 1:2 mead types (both in the reaction with ABTS and DPPH free radicals), which was associated with a higher proportion of honey used in 1:1 mead types production. Antioxidant properties of natural honey are mainly associated to the presence of bioactive substances in its composition, such as: enzymes (i.e., glucose oxidase, catalase), tocopherols, phenolic acids, flavonoids, amino acids, L-ascorbic acid, Maillard reaction products, carotenoid-like substances and possibly other minor components.[Citation13–Citation15]
FIGURE 2 Antioxidant activity of the meads measured using DPPH assay. Means with different letters are significantly different (p < 0.05).
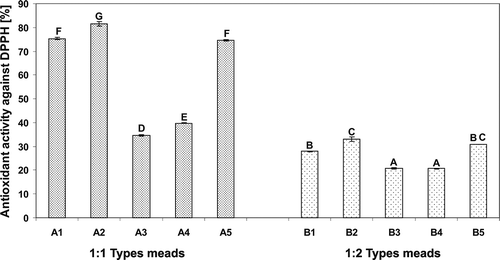
FIGURE 3 Antioxidant activity of the meads measured using ABTS assay. Means with different letters are significantly different (p < 0.05).
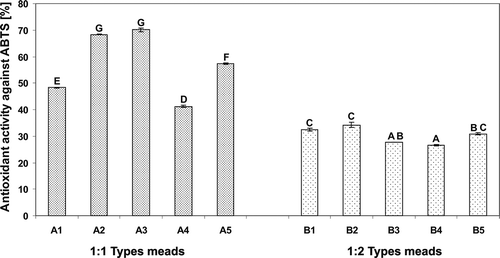
The AA of 1:1 mead types, varied from 34.63% in “Koronny” mead to 81.63% in “Kasztelański” mead, and from 41.25% in “Kurpiowski” mead to 70.11% in “Koronny” mead, in the test with DPPH () and ABTS radicals (), respectively. “Kasztelański” mead, produced from the dark-brown colored buckwheat honey exhibited the highest AA measured using DPPH assay, while in the case of the ABTS assay the highest AA was observed for “Koronny” mead, derived from the dark autumn honeys. The colored compounds in mead that are responsible for its antioxidant properties usually originating from both the honeys and additives (e.g., root spices, herbs, fruit juices) used for mead production. With respect to honeys, among the colored substances the most important are some flavonoids (in particular anthocyanin pigments), carotenoid-like substances, Maillard reaction and fructose caramelization products, as well as products reaction of polyphenols occurring during honey storage.[Citation17] As reported by many researchers, a strong correlation was found between AA and the color of honeys. Namely, Beretta,[Citation18] and Frankel[Citation20] found that honeys with dark color exhibited higher TPC and consistently higher AA. Above results are also in agreement with the data reported by Bertoncelj[Citation17] who proved that light colored Slovenian honey (e.g. acacia honey) exhibited a lower AA in the DPPH test compared to the darker ones. With respect to the 1:2 mead types in our study, their AA measured in the reaction with DPPH radical varied from 20.66% in “Podczaszy” mead to 33.10% in “Bernardyński” mead (), while in the reaction with ABTS cation radical AA ranged from 26.56% in “Podczaszy” mead to 34.25% in “Bernardyński” mead (). The highest AA of the latter mead, among 1:2 types meads may be due to the presence of chokeberry juice, which is a much richer source of phenolic compounds (in particular anthocyanin pigments), as compared to other fruit juices, used as additives in the production of investigated meads ().
In this study, a significant (α = 0.05) linear correlation (r = 0.70) was observed between the AA determined using ABTS and DPPH assay. Comparing our results with those reported for natural honeys we found that correlation between these two assays was a slightly weaker in comparison to that reported for Lihtuanian honeys[Citation15] (r = 0.74), and for Polish honeys[Citation14] (r = 0.81), however, stronger (r = 0.62) in comparison to that reported for Southern Italy honeys of different botanical origin.[Citation21] The differences in reactivity of analyzed meads between ABTS and DPPH assay may be due to differences in kinetic between these two assays, as well as differences in concentration of the substrates.[Citation14] In contrary to a DPPH method, an ABTS assay is applicable both for lipophilic and hydrophilic antioxidants.[Citation15]
In our study there were high and significant (α = 0.05) linear correlations between the TPC and AA determined in the reaction with both DPPH (r = 0.86) and ABTS radicals (r = 0.81). High and significant correlation coefficients between TPC and AA measured using ABTS and DPPH assay (r = 0.90 and r = 0.79, respectively) were also observed by Majewska and Myszka[Citation19] in Polish meads produced with various ratio of honey to water (v/v). Similar results were obtained in most studies but concerning honeys. The significantly higher correlation between the TPC and AA determined using DPPH assay was reported for Slovenian honeys[Citation17] (r = 0.93). These results indicate that phenolic compounds are the main components responsible for antioxidant behavior of meads under study but obviously other factors are involved, including non-phenolic compounds originating from natural honeys and additives used for mead production.
The Profile of Phenolic Acids
The amounts of individual phenolic acids in meads evaluated chromatographically are specified in . The following two groups of phenolic acids were identified in the studied meads: gallic, protocatechuic, and vanillic acid as hydroxybenzoic acids, and chlorogenic, caffeic, p-coumaric, and ferulic acid as hydroxycynnamic acids. It was found that additives used in mead production have a significant effect on the profile of phenolic acids. In the case of 1:2 mead types () the most important factor that influenced the level of phenolic acids seems to be the type of fruit juice added to the mead wort. Namely, the highest level of total phenolic acids (sum of individual acids) in the 1:2 mead types was evaluated in “Apis” mead produced with the addition of rowanberry juice, while the lowest sum was observed in “Podczaszy” mead made with sour cherry juice. In the case of 1:1 mead types, the highest value of total phenolic acids was found in “Kasztelański” mead which may result from the use of dark colored buckwheat honey and spices from roots and herbs in its production.
TABLE 2 Phenolic acids content in Polish meads types expressed in mg per 1 dm3
The major phenolic compounds, identified in studied meads were hydroxybenzoic acids, especially gallic and protocatechuic acid (). GAE content was higher in 1:1 mead types (0.73–7.56 mg/dm3) than in 1:2 mead types (0.55–1.15 mg/dm3), what was in accordance with the proportion of honey used in mead production, and indicating that bee honeys used in its production were the main source of GAE. These results are in accordance with the previous data concerning Polish honey.[Citation14] The latter authors reported that GAE was the main phenolic acid found in the studied honeys (including monofloral and multiflower honeys), and its highest content was observed in buckwheat honey. Similarly, “Kasztelański” mead which proved to be the richest in GAE content () was made from buckwheat honey. In our study, the average content of GAE in all meads studied (i.e., 1.84 mg/dm3) was very similar to that evaluated[Citation6] in Czech meads (i.e., 1.73 mg/dm3). According to the latter authors, the highest content of GAE was observed in mead enriched with propolis and almond extract, as well as those with addition of propolis and herbal extract.
The second predominant phenolic acid in the studied meads was protocatechuic acid and its content ranged from 0.30 to 2.01 mg/dm3. The highest amount of this acid was found in “Koronny” mead of 1:1 type (), produced from dark colored autumn honeys, and enriched with herbal spices, whereas the lowest was observed in “Piastowski” mead of 1:2 type produced on the basis of light colored honey without any addition of fruit juice. The meads of 1:1 types were characterized by a significantly higher content of this acid, what may suggest that the honeys were the main source of this phenolic. As in the case of GAE, the values of protocatechuic acid content (with the average of 1.14 mg/dm3) were similar to that found in Czech meads (i.e., 0.87 mg/dm3) by Kahoun.[Citation6] According to the latter authors, a high content of this acid was found in meads produced with the addition of propolis and herbal spices, as well as in those with addition of some fruit juices, especially raspberry and sour cherry juice.
The following hydroxybenzoic acid identified in the studied meads, present in the smallest amount, was vanillic acid (). The highest content of this acid was observed in “Kurpiowski” mead made with the additives, such as blackcurrant juice and root spices. The average content of this acid was 0.30 mg per dm3 of mead and was a significantly lower than that observed in Czech meads[Citation6] (i.e., 0.66 mg/dm3). However, the latter authors also reported the lowest concentration of this acid among all the hydroxybenozic acids. Similarly, as in the case of protocatechuic acid, the latter authors observed the highest amount of vanillic acid in meads produced with raspberry and sour cherry juices, as well as in those with the addition of propolis and herbs.
The amounts of hydroxycinnamic acids identified in studied meads were significantly lower than those of hydroxybenzoic acids (). These results are not in accordance with the data reported for Czech meads,[Citation6] where the predominant phenolic acids in meads were hydroxycinnamic acids (especially chlorogenic acid). Moreover, the contents of individual hydroxycinnamic acids in Czech meads were significantly higher as compared to Polish meads. According to these authors the highest amounts of hydrocinnamic acids were found in meads enriched with propolis (being a bee product with strong antioxidant properties), or sour cherry juice, as well as in meads enriched with extracts from a special kind of herbs (i.e., aromatic, mountain herbs, and drug plants).
The major hydrocinnamic acid identified in Polish meads was chlorogenic acid (). The highest content of this compound was found in “Bernardyński” mead of 1:2 type, produced on the basis of chokeberry juice and root spices, whereas the smallest was observed in “Podczaszy” mead of 1:2 type, enriched with root spices and sour cherry juice. The level of chlorogenic acid in 1:2 mead types was significantly higher and varied from 0.08 to 1.38 mg/dm3 as compared to 1:1 mead types (i.e., 0.22–0.57 mg/dm3; ), in spite of the smaller proportion of honey used in their production. It can be assumed that the main source of this phenolic acid in 1:2 mead types were plant additives used during mead production. Chlorogenic acid was also a predominant hydrocinnamic acid determined in Czech meads.[Citation6] The highest content of chlorogenic acid was found by the latter authors in mead produced with the addition of propolis and almond extract, followed by meads with propolis and chosen herbs, and by mead with the addition of sour cherry juice.
The second predominant hydroxycinnamic acid identified in all mead samples was p-coumaric acid. Its amount varied from 0.03 to 1.20 mg/dm3, and its highest level was observed in “Dominikański” mead of 1:1 type, produced with the addition of multifruit juice. As stated in previous studies,[Citation14] p-coumaric acid was the major compound among the hydrocinnamic acids identified in Polish honeys, as well as in Australian and New Zealand honeys[Citation11,Citation22] what may indicate that the amount and type of honey used during mead production are the main factor that influence the level of this phenolic in meads, in contrary to chlorogenic acid which originated mainly from plant additives.
The following hydroxycinnamic acid determined in all Polish meads was caffeic acid. Its level ranged from 0.03 to 0.87 mg/dm3. A relatively high amount of caffeic acid in meads “Kurpiowski” and “Maliniak”, enriched with blackcurrant and raspberry juice was determined, in comparison to those made from rich in antioxidants dark honeys. It may suggest that the main source of this phenolic were additives used for production of the investigated meads, in particular fruit juices. Caffeic acid is, in fact, well-known as main phenolic compound in many plant species, including various fruit juices (e.g., blackcurrant juice in the case of A4 and raspberry juice in the case of A5 mead that were the richest in caffeic acid). These data are in accordance with the results[Citation6] concerning phenolic profile of Czech meads. The latter authors reported that the highest content of caffeic acid was determined in meads produced with the addition of fruit juices, especially sour cherry juice, as well as blackcurrant juice.
Ferulic acid was identified in all meads samples (), however, its average content (0.24 mg/dm3) was at significantly lower degree in comparison to the other determined hydroxycinnamic acids. Similarly to caffeic acid content, the highest content of ferulic acid was found in 1:1 mead types, such as “Kurpiowski” and “Maliniak”, which were enriched with fruit juices (i.e., blackcurrant and raspberry juice; ). Ferulic acid was also determined in Czech meads,[Citation6] however, its average level (0.54 mg/dm3) was also significantly lower than those of the other hydroxycinnamic acids identified in the studied samples. As reported by latter authors, the highest content of this phenolic was found in meads produced with the addition of propolis and chosen types of herbs.
PCA
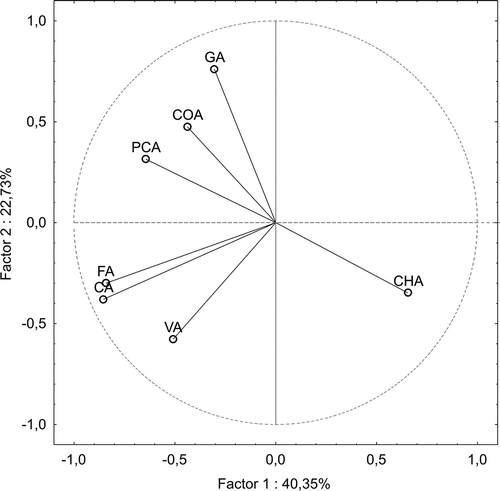
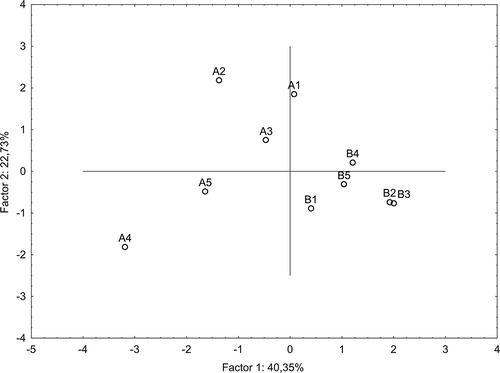
The analytical data of the meads examined were processed statistically using a PCA in order to show grouping of the meads. The PCA explained 63.08% of total variation of the chemical variables (including phenolic acids content) in the first two dimension, with 40.35 and 22.73% explained by the first and second factor, respectively. With respect to the hydroxycinnamic acids, the variables were “split” into three groups (): first which loaded positively on factor I (i.e., chlorogenic acid content), and was unrelated to the second group located in the lower left quadrant (i.e., caffeic and ferulic acid content, which highly correlated with each other), and to the third group located in the upper left quadrant (i.e., p-coumaric acid content). With respect to the hydroxybenzoic acids, the variables were “split” into those in the upper left quadrant (i.e., gallic and protocatechuic acid contents, and that in the lower left quadrant (i.e., vanillic acid content). The perpendicular orientations reflected that these two last groups of variables are strongly unrelated to one another. Grouping of the meads was performed visually using the PCA plot (), where the investigated samples are presented by function of the first two principal components. The 1:2 types meads (except of mead B4) could be best described by parameter such as chlorogenic acid content. With respect to the 1:1 types meads, two groups could be observed. The first group, including “Kasztelański” and “Koronny” mead (A2 and A3; ) could be best described by parameters such as: p-coumaric, gallic, and protocatechuic acid content. Thus, these compounds seem to be characteristic for the 1:1 types meads mentioned above which were made from the dark honeys being a rich source of hydroxybenzoic acids (especially, gallic and protocatechuic acid). The second group, including “Kurpiowski” and “Maliniak” (A4 and A5) mead could be best described by parameters such as caffeic and ferulic acid content. The mentioned above phenolic acids seem to be characteristic for the 1:1 types meads made from light honeys but enriched with some fruit juices (i.e., blackcurrant and raspberry juice, as in the case of latter meads).
CONCLUSIONS
Polish meads differed from each other in phenolic compounds profile, as well as in AA, which was mainly related to the way of mead production, i.e., proportion to which mead wort is diluted with water and the type of additives used. The 1:1 mead types were characterized by a significantly higher sum of individual phenolic acids, as well as AA than 1:2 mead types, what is related with the larger proportion of honey (being a rich source of antioxidant) used in their production. “Kasztelański” and “Koronny” mead of 1:1 type made from the dark honeys proved to be a rich in phenolic compounds and also was characterized by the highest AA measured using DPPH assay for the former, and ABTS assay for the latter. The predominant group of phenolic compounds identified in Polish meads were hydroxybenzoic acids, especially gallic and protocatechuic acid, whereas chlorogenic acid was predominant among the hydroxycinnamic acids. The phenolic profile and AA of Polish meads also depended on the type of additive used in their production (i.e., fruit juices, herbs, and root spices). With respect to the results of PCA, the 1:2 types meads could be best described by chlorogenic acid, indicating that this phenolic originated mainly from the additives used during meads production. In contrary, the 1:1 types meads may be divided into two groups: First including meads obtained from the dark honeys, which could be described by gallic, p-coumaric, and protocatechuic acid, and the second group including meads made from the light honeys with addition of some fruit juices (i.e., blackcurrant and raspberry juice) which could be described by caffeic and ferulic acid. High and statistically significant correlations between total polyphenols content and AA of analyzed meads were observed, indicating that phenolic compounds may play an important role in shaping AA of meads.
REFERENCES
- Polish Standard. Honey wines. Polish Committe for Standarization, Warsaw, 1999. PN A-79123-1999.
- Sroka, P.; Tuszyński, T. Changes in organic acid contents during mead wort fermentation. Food Chemistry 2007, 104, 1250–1257.
- Navrátil, M.; Sturdík, E.; Gemeiner, P. Batch and continuous mead production with pectate, immobilised, ethanol-tolerant yeast. Biotechnology Letters 2001, 23, 977–982.
- Gomes, T.; Barradas, C.; Dias, T.; Verdial, J.; Morais, J.S.; Ramalhosa, E.; Estevinho, L.M. Optimization of mead production using response surface methodology. Food and Chemical Toxicology 2013, 59, 680–686.
- Sant’Ana, L.D.; Buarque-Ferreira, A.B.; Lorenzon, M.C.A.; Berbara, R.L.L.; Castro, R.N. Correlation of total phenolic and flavonoid contents of Brazilian honeys with colour and antioxidant capacity. International Journal of Food Properties 2014, 17, 65–76.
- Kahoun, D.; Řezková, S; Veškrnová, K.; Královský, J.; Holčapek, M. Determination of phenolic compounds and hydroxymethylfurfural in meads using high performance liquid chromatography with coulometric-array and UV detection. Journal of Chromatography A 2008, 1202, 19–33.
- Socha, R.; Juszczak, L.; Pietrzyk, S.; Fortuna, T. Antioxidant activity and phenolic composition of herbhoneys. Food Chemistry 2009, 113, 568–574.
- Ferreres, F.; Andrade, P.; Tomás-Barberán, F.A. Flavonoids from Portuguese heather honey. European Food Research and Technology 1994, 199, 32–37.
- Kenjerić, D.; Mandić, M.L.; Primorac, L.; Bubalo, D.; Perl, A. Flavonoid profile of robinia honeys produced in Croatia. Food Chemistry 2007, 102, 683–690.
- Soler, C.; Gil, M.I.; García-Viguera, C.; Tomás-Barberán, F.A. Flavonoid patterns of French honeys with different floral origin. Apidologie 1995, 26, 53–60.
- Yao, L.; Datta, N.; Tomás-Barberán, F.A.; Ferreres, F.A.; Martos, I.; Singanusong, R. Flavonoids, phenolic acids and abscisic acid in Australian and New Zealand Leptospermum Honeys. Food Chemistry 2003, 81, 159–168.
- Zujko, M.E.; Witkowska, A.M. Antioxidant potential and polyphenol content of beverages, chocolates, nuts, and seeds. International Journal of Food Properties 2014, 17, 86–92.
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid, and proline contents in burkina fasan honey, as well as their radical scavenging activity. Food Chemistry 2005, 91, 571–577.
- Socha, R.; Fortuna, T.; Juszczak, L.; Gałkowska, D.; Pietrzyk, S.; Witczak, T. Phenolic profile and antioxidant properties of Polish honeys. International Journal of Food Science and Technology 2011, 46, 528–534.
- Baltrušaitytė, V.; Venskutonis, P.R.; Čeksterytė, V. Radical scavenging activity of different floral origin honey and beebread phenolic extracts. Food Chemistry 2007, 101, 502–514.
- Socha, R.; Gałkowska, D.; Robak, J.; Fortuna, T.; Buksa, K. Characterization of Polish wines produced from the multispecies hybrid and vitis vinifera L. grapes. International Journal of Food Properties 2015, 18 (4), 699–713.
- Bertoncelj, J.; Doberšek, U.; Jamnik, M.; Golob, T. Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chemistry 2007, 105, 822–828.
- Beretta, G.; Granata, P.; Ferrero, M.; Orioli, M.; Facino, R.M. Standarization of antioxidant properties of honey by a combination of spectrophotometric/fluorimetric assays and chemometrics. Analytica Chimica Acta 2005, 533, 189–191.
- Majewska, E.; Myszka, A. Właściwości przeciwutleniające miodów [Antioxidant activity of mead]. Bromatologia i Chemia Toksykologiczna 2009, 3, 875–879.
- Frankel, S.; Robinson, G.E.; Berenbaum, M.R. Antioxidant capacity and correlated characteristics of 14 unifloral honeys. Journal of Apicultural Research 1998, 37 (1), 27–31.
- Perna, A.; Simonetti, A.; Intaglietta, I.; Sofo, A.; Gambacorta, E. Metal content of southern Italy honey of different botanical origins and Its correlation with polyphenol content and antioxidant activity. International Journal of Food Science and Technology 2012, 47 (9), 1909–1917.
- Yaoa, L.; Jiang, Y.; Singanusong, R.; Datta, N.; Raymont, K. Phenolic acids in Australian melaleuca, guioa, lophostemon, banksia, and helianthus honeys and their potential for floral authentication. Food Research International 2005, 38, 651–658.