Abstract
The sensory, physicochemical, microbiological, and volatiles changes of postmortem turbot during 20 days of chilled storage at 4°C were studied. No significant changes were found in pH value and whiteness during the storage, while total viable counts, total volatile base nitrogen, and K-values increased significantly with storage time. The textures profile, springiness and resilience, and sensory attributes changed significantly during storage. A total of 50 volatile compounds including aldehydes, ketones, esters, alcohols, alkenes, alkanes, aromatics, amines, and others were identified in turbot muscle during storage by the solid-phase microextraction and gas chromatography–mass spectrometry methods. Among these volatile components, the relative content of some decreased significantly with time, while others increased significantly. The results suggested that hardness, springiness, resilience, sensory attributes, total volatile base nitrogen, K-value, and total viable counts combined with some volatile compounds could more perfectly reflect the quality changes of turbot during storage. The study indicated that turbot fillets stored at 4°C maintain better quality for 16 days during storage.
INTRODUCTION
Turbot (Psetta maxima), a valuable flatfish species is an economically important fish species along the northern coast of Turkey. It is popular in the market because of its white and flavorful flesh, special nutritive value, and delicious taste.[Citation1] Currently, turbot has been the most important marine farming fish species along the northern coast and had large population among marine cultured fish. However, fish are perishable foods, which generally deteriorate faster than other muscle foods.
Freshness is one of the key attributes for fish quality assessment. It indicates the degree of various physicochemical, biochemical, and microbiological changes in fish. Traditionally, fish freshness assessment has been based on sensory, physical, chemical, and microbiological methods.[Citation2] Sensory characteristics of whole fish are clearly visible to consumers and sensory analysis has been used to evaluate the loss of freshness.[Citation3] The quality of fish decreases after death due to microbiological spoilage and chemical reactions such as changes in protein and lipid fractions, the formation of biogenic amines.[Citation1] Quite a few spoilage indicators have been used to assess the quality of fish, such as total volatile base nitrogen (TVB-N), trimethylamine (TMA) and biogenic amine composition which can be performed by microbiological count and identification.[Citation4] However, prior to bacterial spoilage, there is a stage of autolytic changes which could be evaluated by Hypoxanthine and the K-value.[Citation3] The K-value reflects the early freshness changes of fish and has been widely applied to evaluating the freshness of fish and shellfish.[Citation5] Given the fact that the results of these analyses do not always show good correlations, it is important to use all these conventional methods of assessing fish quality when the purpose is to adequately define the freshness of a particular species of fish.[Citation6]
Odor is one of the main quality parameters because the smell of fish changes rapidly according to the degree of freshness.[Citation7] Therefore, the key volatiles that contribute to this characteristic odor can be used as quality indicators. These volatiles in fish can be produced by enzymatic reactions, lipid oxidation, or microbial actions during storage, and can also be developed as a consequence of environmental contaminations. The spoilage of seafood is usually accompanied by the change of volatile profiles. Besides, the change of seafood volatile characteristics during spoilage is always earlier than that of flesh property.[Citation2] The volatile profile characteristics of seafood during storage could provide much more useful bio-information than the single volatile such as TMA.[Citation8] As a result, the volatile characteristics and the key volatile compounds can be measured and indicate the effective information for seafood freshness evaluation during their shelf life.[Citation2] The method of headspace solid-phase microextraction (HS-SPME) is appropriated for sampling volatile organic compounds (VOCs) in seafood and considered as a good choice for sample preparation in the trace fragrance-and-aroma analysis.[Citation9] The technique of gas chromatography–mass spectrometry (GC–MS) which has been widely used in flavor analysis of aquatic products is successfully regarded as a sensitively analytical technique for the biological VOCs.[Citation10] By now, the technique of HS-SPME combined with GC–MS has been widely used to identify volatile compounds in fishes and other seafood.[Citation11]
Turbot is a high nutritive and economic fish in China. So far, correlative studies on turbot have focused on dietary,[Citation12] immunology,[Citation13] and genomics.[Citation14] However, there are few studies on postmortem changes during refrigerated storage. The objective of our study was to give relevant information for freshness and spoilage evaluation of postmortem quality changes of turbot during storage. The fish quality indices were assessed by determining sensory, microbiological, physicochemical parameters, and analyzing the change of the entire volatile profile characteristics over a storage period of 20 days. The present study was to set up better processing and marketing strategies for domestic consumption and for export.
MATERIALS AND METHODS
Chemicals and Materials
All chemicals used were of analytical grade. Standards of n-alkanes (C8–C20) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). The manual SPME device and 50/30 µm divinylbenzene-carboxen-polydimethylsiloxane (DVB/CAR/PDMS) fiber were obtained from Supelco (Bellefonte, PA, USA), and the fiber was conditioned in the GC injection port prior to use according to the manufacturer’s prescriptions.
Fish Sample Preparation
Live turbot samples with an average weight of 750 ± 50 g were obtained from a local retail market called Linxi Aquatic Market in Jinzhou, Liaoning Province, China. They were delivered to the Food Processing Laboratory of Bohai University in water within 20 min and kept alive before being processed. Upon arrival, the fish were killed by immersion in a 1:2 (w/v) ice-water mixture, kept whole, and packaged by polyethylene bags, and then stored at 4 ± 0.5°C with approximately 67% relative humidity until testing. Fish samples were withdrawn at 4-day intervals over a storage period of 20 days and analyzed at different levels of quality (days 0, 4, 8, 12, 16, and 20).
Sensory Evaluation
The quality index method (QIM) was used for sensory evaluation of raw fish by six trained panelists from the laboratory members. The QIM is based on changes in the sensory characteristics of fish during spoilage.[Citation15] Samples of the whole turbot were evaluated for overall acceptability according to the smell, texture, skin appearance, outer slime, eyes, and gills. For each parameter, numerical scores for sensory analysis were assessed from 0 to a maximum of 3, in which 0 represented the freshest quality and the score increased gradually up to 3 was considered to be the lower limit of acceptability (). The sum of the scores for each parameter gave a comprehensive sensory score.
TABLE 1 QIM scheme for sensory evaluation of turbot during refrigerated storage
pH and TVB-N Analysis
The pH values were determined using a digital FE20 pH meter (Mettler Toledo, Zurich, Switzerland). TVB-N values were estimated using the FOSS method.[Citation16] TVB-N values were measured with a Kjeltec 8400 (FOSS, Sweden) and TVB-N content was expressed in mg N/100 g muscle.
Determination of K-Value
Adenosine triphosphate (ATP) and its breakdown products were determined by the method of Li et al.[Citation5] ATP in fish muscle could be decomposed after early death of fish. Breakdown products also called ATP related compounds including ADP, AMP, IMP, HxR and Hx. Among them, HxR and Hx are the final breakdown products. The identification of nucleotides, nucleosides, and bases was performed by comparing their retention times with those of commercially obtained standards, which was obtained from Sigma Chemical Co. (St. Louis, MO). The equation was defined as follows:
Instrumental Texture Analysis
Texture profile analyses (TPA) were carried out using a TA-XT2i Texture Analyzer (Stable Micro Systems®). Turbot flesh samples were cut into small cubes (2.0 × 2.0 × 1.0 cmCitation3) and kept in refrigerator prior to texture analysis. Each sample was compressed (double compression) with a flat aluminium plunger of 50 mm in diameter (P/50) at a constant test speed of 1 mm s–Citation1 until it reached 50% deformation. The compression force was perpendicular to the muscle fiber orientation. Texture variables (hardness, springiness, cohesiveness, gumminess, chewiness, and resilience) were calculated as reported by Bourne.[Citation17]
Whiteness Measurement
A CR-400 chroma meter (Konica Minolta Sensing, Inc., Tokyo, Japan) was used to measure the color of turbot flesh. Color analysis was expressed according to Commission International d’ Eclairage (CIE) L*a*b* color space. In this system, L* variable represents lightness (L* = 0 for black, L* = 100 for white), while the a* scale represents the red/green dimension, with positive values for red and negative ones for green. The b* scale represents the yellow/blue dimension, with positive values for yellow and negative ones for blue. Whiteness was calculated using the following equation:[Citation18]
Microbiological Analysis
Total viable counts (TVC) were determined using aerobic plate counts (APCs) according to the recommended national standards (GB/T) (2010; GB/T 4789.2-2010). Twenty-five grams of representative fish sample was transferred aseptically into a polyethylene bag containing 225 mL of 8.5 g L–Citation1 sterile NaCl water and homogenized for 1 min. Appropriate decimal dilutions were prepared using the same diluents, and then 1 mL of each dilution was pipetted onto the surface of plate count agar (Aoboxing Bio-Tech, Beijing, China) plates in triplicate. They were then incubated for 3 days at 30°C. The results were reported as colony-forming units per gram (CFU/g).
HS-SPME and GC–MS Analysis
According to previous studies,[Citation19] the manual SPME device equipped with the fiber was used for extraction of volatiles from the fish. Three grams of minced fish muscle and 6 mL NaCl solution (0.36 g mL–Citation1) were placed into a 20 mL vial containing a micro stirring bar. Samples were equilibrated for 15 min in a water bath at 50°C, and then 50/30 µm DVB/CAR/PDMS fiber was exposed to the headspace at the same temperature for 40 min under stirring (500 rpm). After extraction, desorption was carried out in the GC injector at 250°C for 5 min in splitless mode.
Volatile compounds were analyzed using an Agilent 7890 gas chromatography-5975C mass-selective spectrometry (Agilent Technologies, USA) equipped with an HP-5MS capillary column (30 m length × 0.25 mm I.D. × 0.25 µm film thickness). The carrier gas was helium at a flow rate of 1 mL min–Citation1. The analysis was performed in the splitless mode and injector temperature was 250°C. The column temperature program was: initial 40°C for 3 min, then raised to 100°C at 3°C min–Citation1, and finally increased to 230°C at 5°C min–Citation1 and final temperature held for 5 min. Mass spectrometer conditions were: electron impact voltage 70 eV, interface temperature 280°C, ion source temperature 230°C, and quadropole 150°C. Full scan mass spectra was collected for all samples in the range of 30–550 amu. Identification of compounds detected by GC–MS analysis was based on computer matching with the reference mass spectra of the MS library of NIST 11 and Wiley 7.0. RI was calculated using a mixture of n-paraffin C8–C20 as standards. The amounts of volatile compounds in the samples were calculated by comparing the peak area of each compound with that of total peak area of all volatiles.
Statistical Analysis
All experiments were performed in triplicate. Presented data were expressed as mean ± standard deviation of replicated measurements. The data were subjected to the analysis of variance (ANOVA). The relationship between variables was assessed by the principal component analysis (PCA). Statistical analysis was performed using the SPSS statistic program (Version 19.0, SPSS Inc., Chicago, Illinois, USA) and significant differences were defined at p < 0.05.
RESULTS AND DISSCUSION
Sensory Evaluation
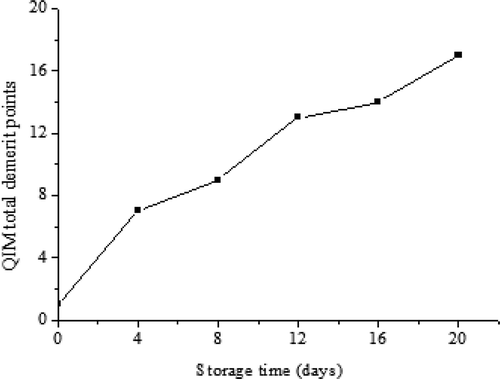
Sensory assessment is the best way to evaluate the freshness. Moreover, it is widely accepted by consumers and is indispensable for marketing.[Citation20] The results of the sensory evaluation of turbot during refrigerated storage are presented in . The turbot samples, as a whole, had better sensory scores in the initial stages, and the scores of this parameter increased with the prolong of the storage time until the samples could not be accepted. On day 4 and day 12, the demerit points increased significantly. Through the agreement of six trained panelists, on day 8, the fish maintained in the refrigerator still had acceptable appearance, gill, eye color, and flesh odor, while those stored over 8 days did not, which might be due to ammoniac odor, acidic taste, and darker color.[Citation21]
pH and TVB-N
shows pH changes of turbot muscle during 20 days of storage. It can be noticed that pH did not show significant changes (p > 0.05) with the time. The pH values decreased initially and then increased during storage and similar changes have also been found by Li et al.[Citation5] The initial decrease of pH value might be associated with the generation of lactic acid by anaerobic glycolysis and the liberation of inorganic phosphate by the degradation of ATP, while the increase may be attributed to the accumulation of alkaline compounds such as ammonia and TMA resulting from autolytic and microbial reactions.[Citation22]
FIGURE 2 Changes in pH, TVB-N, K-value, and total viable counts (TVC) of turbot during refrigerated storage for 20 days.
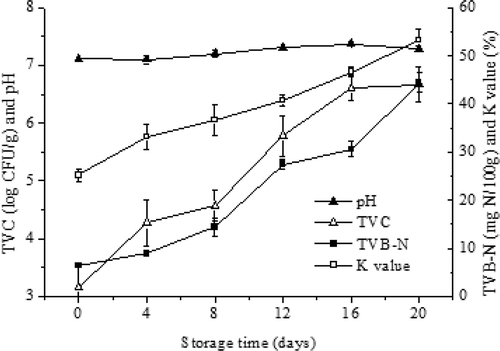
TVB-N is one of the most widely used indicators for fish spoilage. It is mainly consisted of ammonia, methylamine, dimethylamine, and TMA which were caused by the microbial degradation of protein and non-protein nitrogenous compounds, such as amino acids and nucleotide catabolites.[Citation23] Changes in TVB-N concentrations of turbot samples during refrigerated storage are shown in . The results showed that TVB-N content increased gradually during the 20-day storage. The average values of TVB-N content significantly increased from an initial value of 6.36 ± 0.04 to 44.34 ± 2.04 mg N/100 g of fish muscle at day 0 and day 20 of storage at 4°C, respectively. The similar results were proposed by Ocaño-Higuera et al.[Citation24] and Bhatta et al.[Citation25] For several fish species, TVB-N values were reported to increase linearly or curvilinearly with the storage time, and a level of 30 mg TVB-N/100 g of muscle has been considered the upper limit of acceptability and unfits for human consumption.[Citation26] Therefore, in this study, the fillet of turbot was not acceptable for human consumption after 16 days of storage, since it reached a value of 30.23 ± 1.58 mg of TVB-N/100 g of muscle, which is above the maximum allowance.
K-Value
ATP degradation is one of the most important postmortem chemical changes in the muscle of marine organism and its degradation products have been used to monitor freshness and shelf life of fish muscle. The ATP in fish decomposes following the sequence: ATP–ADP–AMP–IMP–HxR–Hx,[Citation24] and its nucleotide degradation compounds have been utilized as the basis to calculate the K-value. shows the changes of K-values in the muscle of turbot stored for 20 days in refrigerator at 4°C. The results indicated that K-values of turbot increased significantly with the storage time, from a value of 25.15 ± 1.36% (day 0) to a final value of 55.33 ± 2.28% (day 20). Saito et al.[Citation27] presented K-values of fishing products lower than 20% as very fresh ones, less than 50% as moderately fresh, and higher than 70% as not fresh. According to these K-value categories, the turbot muscle under the experimental conditions of this study could be considered moderately fresh up to day 16 and slightly tainted at the end of the storage period.
Texture and Whiteness
The texture of fish flesh is an available indicator for freshness evaluation.[Citation23] Fish muscle texture is based on many intrinsic biological factors such as muscle style, collagen, and fat content.[Citation28] Some autolytic enzymes and microbiological actions that made muscle less elastic and softer were activated after the death of fish. Additionally, the loss of texture of fishery products during storage is also associated with enzymatic degradation of muscle proteins.[Citation28] Changes in the muscle texture of turbot measured instrumentally are shown in . The average values for hardness, springiness, and resilience () decreased during storage and displayed significant variations (p < 0.05) on the different sampling days and correlated significantly with storage time (r = –0.65, –0.64, and –0.68, respectively). Similar trends have been found in frozen common carp fish reported by Vacha et al.[Citation29]
TABLE 2 Instrumental texture analyses and whiteness for turbot during refrigerated storage for 20 days
Ocaño-Higuera et al.[Citation24] reported that color is one of the most important parameters used to assess the quality of fishing products. The development of opaque white color for turbot muscle without peel during storage was quantified as changes in whiteness (). The minimum value for whiteness occurred on day 0, and the maximum on day 20. The parameter of whiteness gently increased over the whole storage period (r = 0.28). Similar results have been reported by Liu et al.[Citation23] The flesh color has a bearing on heme-based pigments and tissue structure which can affect light scattering and reflection.[Citation30]
Microbiological Analysis
Changes of TVC of turbot during the refrigerated storage are presented in . In our study, a significant increment (p < 0.05) in microbiological growth with the storage time was observed. The initial TVC was 3.15 log CFU/g, indicating that the turbot studied was of good quality. The fish flesh is sterile when caught, but quickly contaminated by surface as well as intestinal bacteria, along with contamination and storage conditions. The value of TVC increased slightly at the initial days, and then increased significantly after 8 days of storage. Similar trends have been found in cooked edible crab reported by Anacleto et al.[Citation21] It has been reported that for raw fish, the value of 7 log CFU/g for TVC was regarded as the upper acceptability limit.[Citation31] In this study, the value of TVC was 6.67 log CFU/g at the end of the storage period which approached to the TVC limit and the fish flesh was close to spoilage.
GC–MS Analysis
lists the changes of volatile compounds identified in turbot during storage at 4°C. In total 50 volatile compounds were obtained by SPME. Most of the detected compounds have been previously found in seafood such as gilthead sea bream[Citation8] and so on. The total level of aldehydes, alcohols, ketones, alkanes, and esters decreased during storage. On the contrary, the content of alkenes and amines increased. Most aldehydes showed a downward trend during storage, whereas the content of 3-methyl-butanal and benzeneacetaldehyde increased. It has been reported that 3-methyl-butanal is linked to the spoilage and could generate from not only Strecker degradation but also microbial activity on leucine and isoleucine.[Citation32] Ketones can be produced from the thermal degradation, fat oxidation, amino acid degradation, or Maillard reaction.[Citation33] There were five ketones detected in turbot flesh during storage. Among them, 2, 3-octanedione was the most abundant, followed by (E, E)-3, 5-octadien-2-one. And the content of them decreased significantly during the storage period. 1-Penten-3-ol and l-octen-3-ol were the major alcohols at the previous storage period, while 3-methyl-1-butanol became the major components at the later storage period. 3-Methyl-1-butanol could be a potential indicator of fish spoilage during ice storage.[Citation32] C8-C19 alkanes have unimportant contribution to flavor because of their high odor threshold values. Among alkenes, D-limonene was the most abundant terpene detected in turbot, which may be formed from the living surrounding. Four aromatics were detected in turbot muscle, which have been reported in different fishes.[Citation8] Total content of amines increased significantly in the later storage period, especially for TMA. TMA is responsible for ammonia odor and unpleasant fishy odor, which is generated from TMA oxide by bacterial enzymes during spoilage and usually used as the fish spoilage marker.[Citation34]
TABLE 3 The GC-MS analysis of the volatile components of turbot samples during storage at 4°C. Values represent as mean ± standard deviation (n = 3)
PCA
It is noted that fish freshness can be evaluated by sensory, physicochemical, and microbiological methods.[Citation1] However, the spoilage of fish is not only related with flesh degradation but also combined with the change of volatile profiles. Moreover, the volatile characteristics could change earlier than that of flesh property during storage.[Citation2] Thus, the individual volatile indicators or conventional indices could not be precisely used for fish freshness assessment. In order to obtain a better understanding between physicochemical (whiteness, texture, and pH, TVB-N, K-value), sensory, microbiological (TVC), and volatile profiles changes of turbot during storage, the systematic structure in their attributes data was studied by PCA (). The distribution difference was caused by the physicochemical, sensory, microbiological, and volatiles data. The results suggested that the PCA could predict quality changes of turbot during storage based on the changes of conventional freshness parameters and volatiles.
FIGURE 3 Bi-plot PCA for all volatile compounds, pH, TVB-N, K, TVC, sensory, whiteness, and texture of turbot during storage. The volatile organic compound (VOC) numbers 1–50 represent the volatile compounds which are shown in .
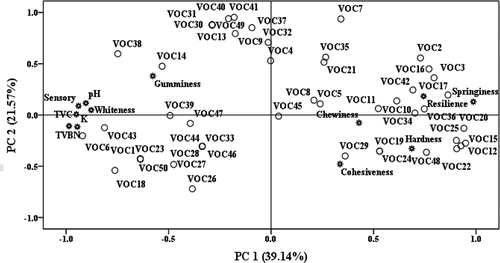
The first and second principal component (PC1 and PC2) accounted for 39.14 and 21.57% of total system variation, respectively. The bi-plot indicated PC1 accounted mainly for the variation (). The most influential features on the first component were springiness, resilience, VOC2, VOC3, VOC12, VOC15, VOC16, VOC17, VOC20, VOC22, VOC25, VOC36, VOC48, with PC1 loadings from 0.75 to 1.0. All of them could reflect the characteristics of turbot samples in early storage time and be potential indicators of turbot freshness. However, characteristics of spoiled turbot samples may be well reflected by sensory attributes, TVB-N, K-value, TVC, VOC6, VOC18, VOC43.
CONCLUSION
The volatile profiles, physicochemical (pH, TVB-N, K-value, texture profiles, whiteness), microbiological (TVC) and sensory changes of turbot during 20 days of storage at 4°C were studied. Characteristics of the relationship between variables were interpreted by PCA. And the relatively high influential contributing indices to the differences of characteristics were extracted from the PCA result. The parameters that proved to be the most sensitive to variations over storage time were sensory attributes, textures profile (springiness and resilience), TVB-N, K-value, TVC, and some volatile compounds. They may, therefore, be proposed as good indicators for evaluating spoilage of turbot. The results suggested that the different volatile profiles during storage coupled with traditional freshness evaluation indices would provide more accurate information to evaluate the quality loss and freshness of turbot during refrigerated storage.
ACKNOWLEDGMENT
We gratefully acknowledge the Food Safety Key Lab of Liaoning Province for providing experimental conditions.
FUNDING
The present work was supported by the National Key Technologies R&D Program of China during the 12th 5-Year Plan Period (No. 2012BAD29B06), Open Foundation of Food Safety Key Laboratory of Liaoning Province and Engineering and Technology Research Center of Food preservation, Processing and Safety Control of Liaoning Province (LNSAKF2011023). We would also like to thank Jianrong Li for helpful suggestions and financial support on this study.
Additional information
Funding
REFERENCES
- Özogul, Y.; Özogul, F.; Kuley, E.; Özkutuk, A.S.; Gökbulut, C.; Köse, S. Biochemical, Sensory and Microbiological Attributes of Wild Turbot (Scophthalmus Maximus), from The Black Sea, During Chilled Storage. Food Chemistry 2006, 99, 752–758.
- Zhang, Z.M.; Li, G.K.; Luo, L.; Chen, G.N. Study on Seafood Volatile Profile Characteristics During Storage and Its Potential Use for Freshness Evaluation by Headspace Solid Phase Microextraction Coupled with Gas Chromatography–Mass Spectrometry. Analytica Chimica Acta 2010, 659, 151–158.
- Calanche, J.; Samayoa, S.; Alonso, V.; Provincial, L.; Roncalés, P.; Beltrán, J.A. Assessing the Effectiveness of a Cold Chain for Fresh Fish Salmon (Salmo Salar) and Sardine (Sardina Pilchardus) in a Food Processing Plant. Food Control 2013, 33, 126–135.
- Hebard, C.E.; Flick, G.J.; Martin, R.E. Occurrence and Significance of Trimethylamine Oxide and Its Derivates in Fish and Shellfish. In Chemistry and Biochemistry of Marine Food Products; Martin, R.E.; Flick, G.P.; Ward, D.R.; Eds.; CRC Press: Florida, 1982; 149–304 pp.
- Li, T.T.; Li, J.R.; Hu, W.Z.; Zhang, X.G.; Li, X.P.; Zhao, J. Shelf-Life Extension of Crucian Carp (Carassius Auratus) Using Natural Preservatives During Chilled Storage. Food Chemistry 2012, 135, 140–145.
- Paleologos, E.K.; Savvaidis, I.N.; Kontominas, M.G. Biogenic Amines Formation and Its Relation to Microbiological and Sensory Attributes in Ice-Stored Whole, Gutted, and Filleted Mediterranean Sea Bass (Dicentrarchus Labrax). Food Microbiology 2004, 21, 549–557.
- Leduc, F.; Tournayre, P.; Kondjoyan, N.; Mercier, F.; Malle, P.; Kol, O.; Berdagué, J.L.; Duflos, G. Evolution of Volatile Odorous Compounds During the Storage of European Seabass. Food Chemistry 2012, 131, 1304–1311.
- Iglesias, J.; Medina, I.; Bianchi, F.; Careri, M.; Mangia, A.; Musci, M. Study of the Volatile Compounds Useful for the Characterisation of Fresh and Frozen-Thawed Cultured Gilthead Sea Bream Fish by Solid-Phase Microextraction Gas Chromatography–Mass Spectrometry. Food Chemistry 2009, 115, 1473–1478.
- Augusto, F.; Lopes, A.L.; Zini, C.A. Sampling and sample preparation for analysis of aromas and fragrances. Trac-Trend Analytical Chemistry 2003, 22, 160–169.
- Jaffrès, E.; Lalanne, V.; Macé, S.; Cornet, J.; Cardinal, M.; Sérot, T.; Dousset, X.; Joffraud, J.J. Sensory Characteristics of Spoilage and Volatile Compounds Associated with Bacteria Isolated from Cooked and Peeled Tropical Shrimps Using SPME-GC-MS Analysis. International Journal of Food Microbiology 2011, 147, 195–202.
- Lu, F.; Zhang, J.Y.; Liu, S.L.; Wang, Y.; Ding, Y.T. Chemical, Microbiological, and Sensory Changes of Dried Acetes Chinensis During Accelerated Storage. Food Chemistry 2011, 127, 159–168.
- Peng, M.; Xu, W.; Ai, Q.H.; Mai, K.S.; Liufu, Z.G.; Zhang, K.K. Effects of Nucleotide Supplementation on Growth, Immune Responses, and Intestinal Morphology in Juvenile Turbot Fed Diets with Graded Levels of Soybean Meal (Scophthalmus Maximus L.). Aquaculture 2013, 394, 51–58.
- Zhang, Z.; Wang, Y.G.; Wang, Q.Y.; Deng, N.N.; Liao, M.J.; Qu, J.O.; Li, B.; Wang, L. Study on the Immune Enhancement of Different Immunoadjuvants Used in the Pentavalent Vaccine for Turbots. Fish & Shellfish Immunology 2012, 32, 391–395.
- Haffray, P.; Lebègue, E.; Jeu, S.; Guennoc, M.; Guiguen, Y.; Baroiller, J.F.; Fostier, A. Genetic Determination and Temperature Effects on Turbot Scophthalmus Maximus Sex Differentiation: An Investigation Using Steroid Sex-Inverted Males and Females. Aquaculture 2009, 294, 30–36.
- FOSS. Determination of Total Volatile Basic Nitrogen of Fresh Fish and Frozen Fish. Application Sub Note 2002, 8, 16.
- Duflos, G.; Leduc, F.; N’Guessan, A.; Krzewinski, F.; Kol, O.; Malle, P. Freshness Characterisation of Whiting (Merlangius Merlangus) Using aAn SPME/GC/MS Method and a Statistical Multivariate Approach. Journal of the Science of Food and Agriculture 2010, 90, 2568–2575.
- Bourne, M.C. Texture Profile Analysis. Food Technology 1978, 32, 62–66.
- Benjakul, S.; Visessanguan, W.; Tueksuban, J. Changes in Physico-Chemical Properties and Gel-Forming Ability of Lizardfish (Saurida Tumbil) During Postmortem Storage in Ice. Food Chemistry 2003, 80, 535–544.
- Xu, Y.X.; Liu, Y.; Jiang, C.C.; Zhang, C.M.; Li, X.P.; Zhu, D.S.; Li, J.R. Determination of Volatile Compounds in Turbot (Psetta Maxima) During Refrigerated Storage by Headspace Solid-Phase Microextraction and Gas Chromatography-Mass Spectrometry. Journal of the Science of Food and Agriculture 2014, 94, 2464–2471.
- Sallam, K.I. Antimicrobial and Antioxidant Effects of Sodium Acetate, Sodium Lactate, and Sodium Citrate in Refrigerated Sliced Salmon. Food Control 2007, 18, 566–575.
- Anacleto, P.; Teixeira, B.; Marques, P.; Pedro, S.; Nunes, M.L; Marques, A. Shelf-Life of Cooked Edible Crab (Cancer Pagurus) Stored under Refrigerated Conditions. LWT–Food Science Technology 2011, 44, 1376–1382.
- Delbarre-Ladrat, C.; Chéret, R.; Taylor, R.; Verrez-Bagnis, V. Trends in Postmortem Aging in Fish: Understanding of Proteolysis and Disorganization of the Myofibrillar Structure. Critical Reviews in Food Science and Nutrition 2006, 46, 409–421.
- Liu, D.S.; Liang, L.; Xia, W.S.; Regenstein, J.M.; Zhou, P. Biochemical and Physical Changes of Grass Carp (Ctenopharyngodon Idella) Fillets Stored at –3 and 0°C. Food Chemistry 2013, 140, 105–114.
- Ocaño-Higuera, V.M.; Maeda-Martínez, A.N.; Marquez-Ríos, E.; Canizales-Rodríguez, D.F.; Castillo-Yáñez, F.J.; Ruíz-Bustos, E.; Graciano-Verdugo, A.Z.; Plascencia-Jatomea, M. Freshness Assessment of Ray Fish Stored in Ice By Biochemical, Chemical, and Physical Methods. Food Chemistry 2011, 125, 49–54.
- Bhattaa, B.U.; Prabhua, R.M.; Reddya, A.M.; Elavarasan, K. Biochemical Changes in Dressed Priacanthus Hamrur (Bull’s Eye) During Frozen Storage and Its Effect on Physical and Sensory Quality of Fish Sausage. International Journal of Food Properties 2015, 18, 897–908.
- Gökodlu, N.; Özden, Ö.; Erkan, N. Physical, chemical and sensory analyses of freshly harvested sardines (Sardina pilchardus) stored at 4°C. Journal of Aquatic Food Product Technology 1998, 7, 5–15.
- Saito, T.; Arai, K.; Matsuyoshi, M. A New Method for Estimating the Freshness of Fish. Bulletin of the Japanese Society of Scientific Fisheries 1959, 24, 749–750.
- Papa, I.; Taylor, R.G.; Astier, C.; Ventre, F.; Lebart, M.C.; Roustan, C.; Ouali, A.; Benyamin, Y. Dystrophin Cleavage and Sarcolemma Detachment Are Early Postmortem Changes on Sea Bass (Dicentrachus Labrax) White Muscle. Journal of Food Science 1997, 62, 917–921.
- Vacha1, F.; Cepak, M.; Urbanek, M.; Vejsada, P.; Hartvich, P.; Rost, M. Impact of Long-Term Storage on the Instrumental Textural Properties of Frozen Common Carp (Cyprinus Carpio, L.) Flesh. International Journal of Food Properties 2013, 16, 241–250.
- Chéret, R.; Chapleau, N.; Delbarre-Ladrat, C.; Verrez-Bagnis, V.; Lamballerie, M.D. Effects of High Pressure on Texture and Microstructure of Sea Bass (Dicentrarchus Labrax L.) Fillets. Journal of Food Science 2005, 70, E477–E483.
- Ojagh, S.M.; Rezaei, M.; Razavi, S.H.; Hosseini, S.M.H. Effect of Chitosan Coatings Enriched with Cinnamon Oil on the Quality of Refrigerated Rainbow Trout. Food Chemistry 2010, 120, 193–198.
- Soncin, S.; Chiesa, L.M.; Panseri, S.; Biondi, P.; Cantoni, C. Determination of Volatile Compounds of Precooked Prawn (Penaeus Vannamei) and Cultured Gilthead Sea Bream (Sparus Aurata) Stored in Ice As Possible Spoilage Markers Using Solid Phase Microextraction and Gas Chromatography/Mass Spectrometry. Journal of the Science of Food and Agriculture 2009, 89, 436–442.
- Fratini, G.; Lois, S.; Pazos, M.; Parisi, G.; Medina, I. Volatile Profile of Atlantic Shellfish Species by HS-SPME GC/MS. Food Research International 2012, 48, 856–865.
- Cha, Y.J.; Baek, H.H.; Hsieh, T.C.Y. Volatile Components in Flavour Concentrates from Crayfish Processing Waste. Journal of the Science of Food and Agriculture 2006, 58, 239–248.