Abstract
Antioxidant and DNA damage protection activity of duck gizzard peptides were evaluated using chemiluminescence method. The duck gizzard peptides exhibited free radical scavenging activity against superoxide anions, hydroxide radicals, and hydrogen peroxide, as well as DNA damage protection in a dose-dependent manner. The duck gizzard peptides had varying degrees of antioxidant activity for the inhibition of different radical species as well as DNA damage, with half-inhibition concentration (IC50) values of 14.58, 0.0163, 19.80, and 1.93 mg/mL, respectively. The duck gizzard peptides had the strongest inhibition of hydroxyl radical activity, and the weakest inhibition of hydrogen peroxide radicals. The results clearly indicated that duck gizzard peptides were good sources of natural antioxidants for future applications in the food industry.
INTRODUCTION
The meat sector consistently produces large quantities of waste including bones, blood, skin, horns, hoofs, and offal. Disposing of this waste represents both a cost for the meat processing sector as well as an environmental pollution problem.[Citation1] Duck gizzard can be converted into value-added products by enzymatic hydrolysis, which is widely applied to improve and upgrade the functional and nutritional properties of proteins.[Citation2] Presently, some studies report the utilization of enzymatic hydrolysis for the recovery of valuable components from fish by-products.[Citation3] However, there have been no reports of the enzymatic hydrolysis of duck gizzard.
TABLE 1 The ability of antioxidation of duck gizzard peptide
Oxidation in foods is one of the major causes of food deterioration, where undesirable off-flavors from lipid peroxidation affect food quality.[Citation4] What’s more, free radical reactions in the human body cause the degradation of biological molecules throughout the cells and tissues of the body[Citation5] and are associated with various diseases.[Citation6] Antioxidants were added into food products to decrease the adverse effects of reactive species, such as reactive oxygen and nitrogen, on normal physiological functions.[Citation7] Although some synthetic antioxidants including butylated hydroxyanisole (BHA) and butylated hydroxyltoluene (BHT) have been applied in food preservation, they have potential risks to human health.[Citation8] Many natural antioxidant activities have been studied such as eggs, duck skin, mango, and Malaysian wild edible plants.[Citation9–Citation12] What’s more, Bacillus subtilis fermentation was used to improve the antioxidant capacity of fermented peanut meal and prolong shelf life.[Citation13] The purpose of this study was to find new antioxidant peptides from duck gizzard.
MATERIALS
Materials and Chemicals
The duck gizzard was stored at –20°C. Calf thymus DNA and luminal were purchased from Sigma. Pyrogallic acid, phenanthroline, and ascorbic acid were chromatographically pure. Papain was purchased from Nanning Pangbo Biological Engineering Co., Ltd. The ultrafiltration centrifuge tubes (<3 kDa) were purchased from Millipore. BPCL-2-KGC was from the Institute of Biophysics of Academia Sinica in China.
METHODS
Preparation of Duck Gizzard Peptides
Enzymatic hydrolysis with papain was performed to obtain duck gizzard peptides, under the optimal conditions (pH 6.0; temperature 50°C). The duck gizzard (100 g) and enzyme (0.2 g) were mixed in a 1 L reactor. The mixture was incubated and stirred for 3 h at 50°C, before heating in boiling water for 5 min to inactivate the enzyme. After cooling, the hydrolysates were filtered using an ultrafiltration centrifuge tube (<3 kDa).Then the hydrolysates were lyophilized and stored at −18°C.
Scavenging Capacity of Superoxide Anion Free Radicals
Based on the pyrogallol-luminol chemiluminescence system,[Citation14] luminol was mixed with 0.05 mol/L NaOH to a concentration of 0.05 mmol/L. Pyrogallol was dissolved in 1 mmol/L HCl to a concentration of 6.25×10–4 mol/L. Various concentrations of 10 μL duck gizzard peptides solution, 50 μL pyrogallol, and 940 μL luminol solution were added into the sample cell in that order. The luminescence intensity was tested every 2 s and the total test time was 120 s at 20°C with 900 V.[Citation15] The control was detected without sample peptides and the background was detected without pyrogallol.
Scavenging Capacity of Hydroxyl Free Radicals
The scavenging ability of hydroxyl free radicals can be determined according to the copper sulfate-luminol-vitamin C-hydrogen peroxide system.[Citation16] Reagents were added into the sample cell in the following order: 50 μL sample, 50 μL CuSO4 (1.0 mmol/L), 50 μL 1,10-phenanthroline (1 mmol/L), 700 μL borate buffer (0.05 mol/L, pH 9.0), 100 μL ascorbate solution (1 mmol/L), and 50 μL H2O2 (0.15%). The luminescence intensity was tested every 2 s and the total test time was 300 s at 20°C with 900 V. The control, reaction solution without sample peptides, was performed in the same manner, and the background was detected without H2O2 addition.
Scavenging Capacity of Hydrogen Peroxide Free Radicals
Scavenging ability was measured using the hydrogen peroxide-luminol system.[Citation17] First, luminol (0.1 mmol) was mixed with carbonate buffer (0.05 mol/L, pH 9.4).The following reagents were then added into the sample cell including 50 μL duck gizzard peptides, 50 μL hydrogen peroxide (0.15 mol/L), and 900 μL luminol-carbonate buffer. The luminescence intensity was tested every 3 s and the total test time was 120 s, at 20°C with 900 V. The control, reaction solution without sample peptides, was performed in the same manner, and the background was conducted without H2O2.
Determination of the Capacity of Protection of DNA Damage
DNA damage protection capacity of antioxidants in gizzard peptide solution was based on the Copper sulfate-Phen-Vc-H2O2–DNA system.[Citation18] Copper sulfate and 1,10-phenanthroline were dissolved in phosphate buffer (0.1 mol/L, pH 5.5) to 7.5×10–5 and 5.25×10–4 mol/L, respectively. Then 3 μg/mL DNA was incubated with phen-Cu solution. The following reagents were added into the sample cell in order: 100 μL duck gizzard peptides solution, 800 μL Phen-Cu2+-DNA, 100 μL Vc (4.2×10–3 mol/L) and 200 μL H2O2 (6%). The luminescence intensity was tested every 3 s and the total test time was 540 s at 20°C with 900V. The blank was performed in the same manner without sample peptides. The background was detected without H2O2 addition.
Statistical Data Processing
All the tests were conducted in triplicate. The antioxidant activities of the samples were calculated based on the equation:[Citation19]
RESULTS AND DISCUSSION
Scavenging Capacity of Superoxide Anion Free Radicals
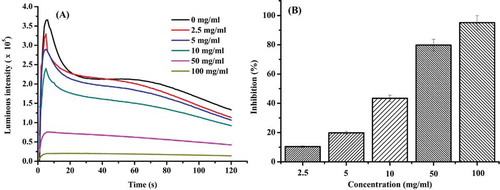
In the pyrogallol-luminol chemiluminescence system, pyrogallol autoxidzes and produces superoxide anion, which excites luminol. This excitation returns to the ground state accompanied by light emission.[Citation20] When antioxidants are added to the system, this eliminates superoxide anions, and therefore, reduces the chemiluminescence intensity. Reduced chemiluminescence intensity was associated with increased antioxidant scavenging due to increasing concentrations of duck gizzard peptide, with chemiluminescence intensity reaching a maximum within 3 s (). The duck gizzard peptides exhibited scavenging activity on superoxide anion in a dose-dependent manner. The duck gizzard peptides scavenged superoxide anion effectively with an IC50 of 14.58 mg/mL.
Scavenging Capacity of Hydroxyl Free Radicals
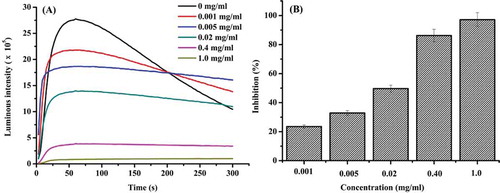
With the CuSO4-Phen-Vc-H2O2 CL system employed, Cu2+ was reduced to Cu+ by VC. The hydroxyl radical was generated when Cu+ reacted with H2O2 in the Fenton reaction. The Phen was oxidized by OH- leading to Phen excitation, resulting in light emission upon de-excitation. Addition of antioxidant to the system, results in the elimination of OH- and the associated inhibition of Phen excitation. All the kinetics of the reaction reached maximal luminescence before 80 s, and then stabilized, where the duck gizzard peptides exhibited scavenging activity on hydroxyl free radicals in a dose-dependent manner (). When the concentration of duck gizzard peptides was 5 mg/mL, the inhibition reached 97.17%. The IC50 of duck gizzard peptides was 0.016 mg/mL. Similar experiments have been carried out on ophiurasaponin extracted from the brittle star species, Ophiopholis mirabilis.[Citation14] The scavenging ability of hydroxyl free radicals by the duck gizzard peptides was stronger than ophiurasaponin extracted from O. mirabilis. This suggests that duck gizzard peptides may contain greater amounts of hydrophobic amino hydrogen which could donate electrons, leading to a more pronounced scavenging effect. Several amino acid compositions in protein have been reported as contributing to scavenging properties.[Citation21] For example, Phe, His, and Pro containing peptides have been reported to exhibit superior antioxidant activity.[Citation22]
Scavenging Capacity of Hydrogen Peroxide Free Radicals
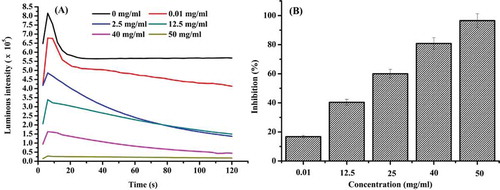
Using the hydrogen peroxide-luminol system, luminol was oxidized due to the co-presence of H2O2 and O2. Hydrogen peroxide is a weak oxidizing agent that inactivates a few enzymes directly, usually by oxidation of essential thiol (–SH) groups.[Citation23] The light emission intensity in the system reached to the maximum in the first 30 s, before dropping sharply and stabilizing at around 50 s (). The IC50 of duck gizzard peptides was 19.8 mg/mL, indicative of effective hydrogen peroxide scavenging activity. In a similar type of experiment, the purified peptide from flounder fish muscle hydrolysate showed strong hydroxyl radical scavenging activity at 50% in the concentration of 163.90 μg/mL.[Citation24] The ability of duck gizzard peptides to scavenge hydrogen peroxide was weaker than others, perhaps due to the absence of a purification process in the preparation of the duck gizzard peptides solution.
DNA Damage Protection in Duck Gizzard Peptides
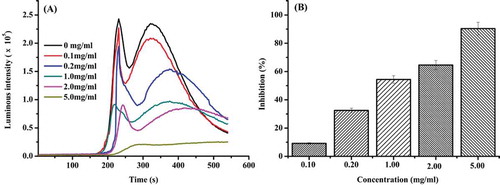
In the CuSO4-Phen-VC-H2O2-DNA system, two peaks appeared on the kinetic curves of chemiluminescence (). The first peak was generated by the Fenton reaction and the second peak was produced by DNA damage caused by OH, where supercoiled plasmid DNA exposed to hydroxyl radicals derived from the Fenton’s reaction can lead to the formation of open circular DNA.[Citation25] When various concentrations of duck gizzard peptides ranging from 0.10 to 5.0 mg/mL were included in the system, an inhibited and delayed light emission was observed (). The IC50 of duck gizzard peptides’ DNA damage protection was 1.93 mg/mL. When the concentration of duck gizzard peptides was 5 mg/mL, the inhibition reached a maximum of 90.04%, indicative of effective prevention of DNA damage.
CONCLUSION
The IC50 of duck gizzard peptides for inhibition of luminescence by superoxide anions, hydroxide radicals, and hydrogen peroxide, as well as DNA damage protection were 14.58, 0.016, 19.80, and 1.93 mg/mL, respectively. The results show that the duck gizzard peptides had the greatest scavenging activity against hydroxyl radicals and weakest activity against hydrogen peroxide radicals. Future research should focus on optimization of duck gizzard peptide purification to further improve upon and evaluate its antioxidant potential. The present study reveals that duck gizzard peptides may provide a plentiful source of natural antioxidants for applications in the food industry.
FUNDING
The authors would like to thank the Science and Technology Department of Guizhou Province for the financial support under major subject of Guizhou agricultural research project (NY[2010]3038).
Additional information
Funding
REFERENCES
- Di Bernardini, R.; Harnedy, P.; Bolton, D.; Kerry, J.; O’Neill, E.; Mullen, A. M.; Hayes, M. Antioxidant and Antimicrobial Peptidic Hydrolysates from Muscle Protein Sources and By-Products. Food Chemistry 2011, 124, 1296–1307.
- Wang, R.; Xue, X.; Zhen, J.; Guo, C. Antioxidant and Antimicrobial Activity of Ophiurasaponin Extracted from Ophiopholis Mirabilis. Journal of Chemistry 2014, 2014, 1–5.
- Dauksas, E.; Falch, E.; Slizyte, R.; Rustad, T. Composition of Fatty Acids and Lipid Classes in Bulk Products Generated During Enzymic Hydrolysis of Cod (Gadus Morhua) By-Products. Process Biochemistry 2005, 40(8), 2659–2670.
- Antolovich, M.; Prenzler, P.D.; Patsalides, E.; McDonald, S.; Robards, K. Methods for Testing Antioxidant Activity. The Analyst 2002, 127, 183–198.
- Wang, Q.; Li, W.; He, Y.; Ren, D.; Kow, F.; Song, L.; Yu, X. Novel Antioxidative Peptides from the Protein Hydrolysate of Oysters (Crassostrea Talienwhanensis). Food Chemistry 2014, 145, 991–996.
- Achour, A.; Lachgar, A.; Astgen, A.; Chams, V.; Bizzini, B.; Tapiero, H.; Zagury, D. Potentialization of IL-2 Effects on Immune Cells by Oyster Extract (JCOE) in Normal and HIV-Infected Individuals. Biomedicine and Pharmacotherapy = Biomedecine and Pharmacotherapie 1997, 51, 427–429.
- Laguerre, M.; Giraldo, L.J.; Lecomte, J.; Figueroa-Espinoza, M.C.; Barea, B.; Weiss, J.; Decker, E.A.; Villeneuve, P. Chain Length Affects Antioxidant Properties of Chlorogenate Esters in Emulsion: The Cutoff Theory Behind the Polar Paradox. Journal Agriculture of Food Chemistry 2009, 57, 11335–11342.
- Qian, Z.; Jung, W.; Byun, H.; Kim, S. Protective Effect of An Antioxidative Peptide Purified from Gastrointestinal Digests of Oyster, Crassostrea Gigas Against Free Radical Induced DNA Damage. Bioresouce Technology 2008, 99, 3365–3371.
- Lee, S.; Kim, Y.; Hwang, J.; Kim, E.; Moon, S.; Jeon, B.; Jeon, Y.; Kim, J.M.; Park, P. Purification and Characterization of a Novel Antioxidative Peptide from Duck Skin By-Products That Protects Liver Against Oxidative Damage. Food Research International 2012, 49, 285–295.
- Duan, X.; Ocen, D.; Wu, F.; Li, M.; Yang, N.; Xu, J.; Chen, H.; Huang, L.; Jin, Z.; Xu, X. Purification and Characterization of a Natural Antioxidant Peptide from Fertilized Eggs. Food Research International 2014, 56, 18–24.
- Li, L.; Wang, S.; Chen, J.; Xie, J.; Wu, H.; Zhan, R.; Li, W. Major Antioxidants and in Vitro Antioxidant Capacity of Eleven Mango (Mangifera Indica L.) Cultivars. International Journal of Food Properties 2014, 17, 1872–1887.
- Wong, J.Y.; Matanjun, P.; Ooi, Y.B.H.; Chia, K.F. Evaluation of Antioxidant Activities in Relation to Total Phenolics and Flavonoids Content of Selected Malaysian Wild Edible Plants by Multivariate Analysis. International Journal of Food Properties 2014, 17, 1763–1778.
- Zhang, Y.; Liu, J.; Lu, X.; Zhang, H.; Wang, L.; Guo, X.; Qi, X.; Qian, H. Isolation and Identification of An Antioxidant Peptide Prepared from Fermented Peanut Meal Using Bacillus Subtilis Fermentation. International Journal of Food Properties 2014, 17, 1237–1253.
- Wang, R.; Xue, X.; Zhen, J.; Guo, C. Antioxidant and Antimicrobial Activity of Ophiurasaponin Extracted from Ophiopholis Mirabilis. Journal of Chemistry 2014, 2014, 1–5.
- Triantis, T.M.; Yannakopoulou, E.; Nikokavoura, A.; Dimotikali, D.; Papadopoulos, K. Chemiluminescent Studies on the Antioxidant Activity of Amino Acids. Analytica Chimica Acta 2007, 591, 106–111.
- Yıldız, G.; Demiryürek, A.T. Ferrous Iron-Induced Luminol Chemiluminescence: A Method for Hydroxyl Radical Study. Journal of Pharmacological and Toxicological Methods 1998, 39, 179–184.
- Germano, M.P.; De Pasquale, R.; D’Angelo, V.; Catania, S.; Silvari, V.; Costa, C. Evaluation of Extracts and Isolated Fraction from Capparis Spinosa L. Buds As An Antioxidant Source. Journal of Agricultural and Food Chemistry 2002, 50, 1168–1171.
- Na, W.; Zhida, S. Study on Antioxidant Activity in Vitro and Protection Against DNA Oxidative Damage of Flavonoids of Artemisia Argyi. Chinese Journal of Food Science 2008, 29, 47–50.
- Tian, B.; Wu, Y.; Sheng, D.; Zheng, Z.; Gao, G.; Hua, Y. Chemiluminescence Assay for Reactive Oxygen Species Scavenging Activities and Inhibition on Oxidative Damage of DNA in Deinococcus Radiodurans. Luminescence 2004, 19(2), 78–84.
- Guo, S.; Deng, Q.; Xiao, J.; Xie, B.; Sun, Z. Evaluation of Antioxidant Activity and Preventing DNA Damage Effect of Pomegranate Extracts by Chemiluminescence Method. Journal Agricultural of Food Chemistry 2007, 55(8), 3134–3140.
- Wang, Q.; Li, W.; He, Y.; Ren, D.; Kow, F.; Song, L.; Yu, X. Novel Antioxidative Peptides from the Protein Hydrolysate of Oysters (Crassostrea Talienwhanensis). Food Chemistry 2014, 145, 991–996.
- Di Bernardini, R.; Harnedy, P.; Bolton, D.; Kerry, J.; O Neill, E.; Mullen, A.M.; Hayes, M. Antioxidant and Antimicrobial Peptidic Hydrolysates from Muscle Protein Sources and By-Products. Food Chemistry 2011, 124(4), 1296–1307.
- Golla, U.; Bhimathati, S.S. Evaluation of Antioxidant and DNA Damage Protection Activity of the Hydroalcoholic Extract of Desmostachya Bipinnata L. Stapf. Scientific World Journal 2014, 21, 84–90.
- Ko, J.Y.; Lee, J.H.; Samarakoon, K.; Kim, J.S.; Jeon, Y.J. Purification and Determination of Two Novel Antioxidant Peptides from Flounder Fish (Paralichthys Olivaceus) Using Digestive Proteases. Food Chemistry Toxicology 2013, 52, 113–120.
- Abbas, S.R.; Sabir, S.M.; Ahmad, S.D.; Boligon, A.A.; Athayde, M.L. Phenolic Profile, Antioxidant Potential and DNA Damage Protecting Activity of Sugarcane (Saccharum Officinarum). Food Chemistry 2014, 147, 10–16.