Abstract
The effects of ultrasound-assisted, supercritical CO2 and solvent extraction methods on antioxidant activity of Ziziphus mauritiana (Lam.) extracts were investigated using 2,2-diphenyl-1-picrylhydrazyl and β-carotene assays. Ethanol-water extract of the jujube that was obtained with ultrasound-assisted extraction had the highest antioxidant activity. Ultrasound-assisted extraction was the most effective method on extraction of phenolic compounds. The effect of ultrasound extract in stability of soybean oil was compared to synthetic antioxidants by measuring total polar compounds, carbonyl value, peroxide value, free fatty acids, oxidative stability index, and conjugated dienes and trienes values. Results showed that ultrasound ethanol-water extract at 600 ppm had a higher stabilization efficiency than commercial antioxidants butylated hydroxyanisole, butylated hydroxytoluene, and tertiary butylhydroquinone.
INTRODUCTION
Fried foods are major part of home foods and in fast-food restaurants. So, deep frying is one of the most important unit operations in the food processing industry.[Citation1] Deep frying causes changes in the flavor, color, texture, and nutritional quality of fried foods. The type of oil, frying time, and temperature have significant effects on the quality of fried foods.[Citation2] Deep frying at high temperatures (160–200°C) and in the presence of oxygen and moisture causes important chemical reactions, such as hydrolysis, oxidation, cyclization, and polymerization.[Citation3] Volatile and non-volatile compounds are formed in vegetable oils during deep frying. Volatile compounds are removed from oil and non-volatile compounds accumulate in the oil. Non-volatile compounds are produced primarily by thermal oxidation and polymerization of unsaturated fatty acids.[Citation4] Non-volatile compounds are often polar and of high molecular weight which have toxic effects on humans and animals.[Citation5]
The synthetic antioxidants such as butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA), and tertiary butylhydroquinone (TBHQ) are often used to increase the oxidation stability and shelf life of oils.[Citation6] BHT and BHA have been reported to have toxic and carcinogenic effects.[Citation7] However, despite the high antioxidant activity of TBHQ, its use has been prohibited in some countries, such as Japan and Canada. Studies have shown that plants are rich sources of antioxidant compounds, such as flavonoids, phenolic compounds, tocopherols, carotenoids, and tannins.[Citation8] Therefore, research on natural antioxidants as alternatives to the synthetics has been increasing in recent years.
Jujube (Ziziphus mauritiana Lam.) is a member of the Rhamnaceae family. The tree (lotus) grows in arid and semi-arid regions.[Citation9] The lotus originated from Central Asia and is widely cultivated in Zimbabwe, Thailand, India, and Malaysia.[Citation10] Lotus height is about 10 meters and its leaves are small and heart-shaped, and are also painted with three prominent veins. Its fruit is round with dark green skin color in young stage which turns to light green or yellow–green in the mature stage. The flesh is white to yellow–white and turns red with skin shrinkage in the ripe stage.[Citation11] This fruit has pharmaceutical properties and is a traditional medicine to help improve blood circulation, the nervous system, as well as a relief to insomnia. Ziziphus mauritiana fruit has good antioxidant property due to the presence of phenolic, flavonoid, and tocopherol compounds.[Citation9]
Therefore, the aim of this work was to compare the effects of solvent extraction, ultrasound-assisted, and supercritical CO2 extraction techniques on antioxidant activity of jujube (Ziziphus mauritiana) fruit extracts. The study also attempted to evaluate the antioxidant potential of jujube fruit extracts during oxidation of soybean oil by measuring both the primary and secondary oxidation products and to compare its antioxidant activity with commonly employed synthetic antioxidants.
MATERIALS AND METHODS
Materials
Jujube (Ziziphus mauritiana) fruits were collected from fields in Bandar Abbas in the Hormozgan province, Iran. Refined, bleached, and deodorized soybean oil with no added antioxidant was supplied by Rana (Gorgan, Iran) and was stored at –20°C until analysis. All reagents used in the experiments were of analytical grade and obtained mostly from Sigma Chemical Co. (St. Louis, MO, USA). Also, solvents were purchased from Merck (Darmstadt, Germany).
Natural Antioxidant Preparations
The jujube fruits (10 kg) were sun-dried and powdered in a grinder to reach 40-mesh and then were packed and stored at –20°C until the extraction of antioxidant. In solvent extraction, dried powders of sample (20 g) were mixed with 100 mL of solvents (ethanol-water [1:1], water, and ethanol). The mixtures were stirred in a shaker at 160 rpm away from light at room temperature for 48 h. After extraction, the extracts were filtered and solvents evaporated using a rotary evaporator (Heidolph, Schwabach, Germany) at 50°C. The concentrated extracts were stored until testing at –20°C.[Citation12]
A Suprex MPS/225 Multipurpose system (Roth Scientic, Basingstoke, Hampshire, UK) in the supercritical CO2 extraction mode was used for the extraction of phenolic compounds. In this method, extractions of 20 g of dried powders of jujube were accomplished with 100 mL of solvent at 35°C, 100 bar, for 30 min. The extract was filtered and solvent evaporated. The concentrated extract was stored at –20°C until testing.[Citation13]
The extraction of phenolic compounds from mixture of solvents (100 mL) and powdered sample (20 g) by ultrasonic treatment was performed with a power of 100 W ultrasonic bath (KQ2200E, Kunshan Ultrasonic Instrument Co., Jiangsu, China). The mixtures were sonicated for 30 min at 35°C. The extracts were filtered and solvents evaporated. The concentrated extracts were stored in a freezer.[Citation14]
Total Phenolic Content
The content of phenolic compounds in the extracts was determined with Folin-Ciocalteu reagent following the colorimetric method adapted by Taghvaei et al.[Citation15] Measurements were carried out in triplicate and calculations were based on a calibration curve obtained with gallic acid. The contents of total phenols were expressed as mg of gallic acid equivalents per g of dry weight ([DW] (mg GAE.g–1 DW).
2,2-diphenyl-1-picrylhydrazyl (DPPH)• Radical Scavenging Activity
DPPH radical scavenging activity of extracts was measured according to the method of Xu and Chang[Citation16] with little modifications. The DPPH• solution was prepared by dissolving 5.9 mg of DPPH• in ethanol (100 mL). Accurately, 3.8 mL of DPPH• ethanolic solution was added to 0.2 mL of fruit extracts. The mixture was shaken vigorously for 1 min and left to stand at room temperature in the dark for 30 min. Absorbance was measured against the blank reagent at 517 nm. All determinations were carried out in triplicate. The radical scavenging activity was calculated according to Eq. (1) below:
A0 and A1 are the absorbance of blank and sample, respectively.
Inhibition of β-Carotene Bleaching
Lipid peroxidation inhibition activity was determined using the β-carotene bleaching assay as described by Lagha-Benamrouche and Madani.[Citation17] The extract (0.2 mL) was added to 5 mL of β-carotene/linoleic acid solution. The absorbance of the samples was measured at 470 nm at initial time (T = 0) against a blank, consisting of an emulsion without β-carotene. The remaining samples were placed in dark for 24 h. Then, the absorbance of each sample was measured at 470 nm. The radical inhibition activity was calculated according to Eq. (2) below:
where the Abss24 is the absorbance of the antioxidant after 24 h, Absc24 is the absorbance of control after 24 h, Abss0 is the absorbance of the antioxidant at t = 0 and Absc0 is the absorbance of the control at t = 0.
Frying Process
The jujube fruit extract in levels of 600 and 800 ppm, BHA, BHT, and TBHQ in level of 100 ppm were added to soybean oil. Soybean oil without antioxidant addition was used as a negative control. Oil sample (2.5 L) was placed in a fryer oven of 2.5 L capacity (Tefal model 1250, France) and heated at 185 ± 5°C for 24 h. A batch of 20 g of potato pieces (7.0 cm × 0.5 cm × 0.3 cm) was fried for 7 min. After time of heating (0, 4, 8, 12, 16, 20, and 24) 20 g of frying oil samples were stored until testing at –20°C.[Citation18]
Total Polar Compounds (TPC), Carbonyl Value (CV), and Peroxide Value (PV)
The TPC were determined according to the method described by Schulte.[Citation19]
The CV of the oil samples was measured according to the method developed by Endo et al.[Citation20] using 2-propanol and 2,4-decadienal as solvent and standard, respectively. The results were expressed in micromols of 2, 4-decadienal per gram of oil.
PV expressed in milliequivalents of active oxygen per kilogram (meq O2/kg of oil) was determined according to Shantha and Decker.[Citation21]
Conjugated Dienes (CD), and Trienes (CT), and Free Fatty Acids (FFAs) Content
The contents of CD and CT were calculated according to the method described by Urbančič, et al.,[Citation22] which is based on the measurement of absorbance solution at 234 and 270 nm for CD and CT, respectively.
The FFA expressed as free oleic acid percentage, was determined by titration of an accurate sample solution, dissolved in ethanol/ether (1:1, v/v), with 0.1 M sodium hydroxide solution, using phenolphthalein as indicator.[Citation23]
Oxidative Stability (Rancimat)
A Metrohm 743 Rancimat instrument (Herisan, Switzerland) was used in the experiment. Air supply was maintained at 15 L/h and the temperature was kept at 110°C.[Citation24]
Statistical Analysis
Each analysis was carried out in triplicate. The data were subjected to analysis of variance (ANOVA) and the significant differences between means were determined by Duncan’s multiple range test (p < 0.05) using SPSS statistical software (version 19; SPSS Inc., Chicago, IL, USA).
RESULTS AND DISCUSSION
Extraction Yield
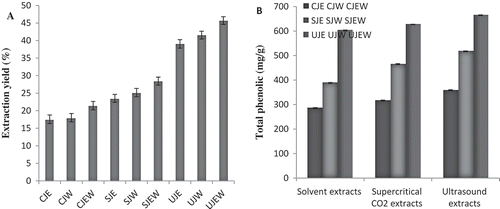
shows the extraction yield of jujube fruit (Ziziphus mauritiana) which range from 17.56 to 45.71%. Results showed that the ethanol-water (1:1) was the most effective solvent for extraction of bioactive compounds compared to water and ethanol. This is in accordance with the findings of Hemwimol et al.[Citation25] The extraction yields of different techniques in descending order were: ultrasound-assisted > solvent extraction > supercritical CO2. This shows that ultrasound-assisted extraction was the best technique for the extraction of bioactive compounds from the Ziziphus mauritiana. Our results concurred with previously published results such as the works by Hemwimol et al.[Citation25] and Rodríguez-Rojo et al.[Citation26] that reported the ultrasound-assisted extraction method was more effective in extraction of plant materials compared to other techniques.
FIGURE 1 (a) Extraction yield (%) of jujube extracts; (b) Total phenolic contents of jujube extracts. Solvent extracts of jujube by ethanol (SJE), water (SJW) and ethanol-water (SJEW). Ultrasound-assisted extracts of jujube by ethanol (UJE), water (UJW), and ethanol-water (UJEW). Supercritical CO2 extracts of jujube by ethanol (CJE), water (CJW), and ethanol-water (CJEW).
Total Phenolic Content
Polyphenolic compounds are widely distributed in different parts of plants[Citation27] and reports showed that there is a positive relation between total phenolic content and antioxidant activity in many plant species.[Citation28] There were significant differences (p < 0.05) between phenolic compounds of extraction techniques with different solvents (). There were various phenolic contents in the jujube fruit extracts ranging from 285.74 to 664.72 mg GAE/g. Jujube extract was previously reported to have total phenolic compounds in the range of 1321.98 mg[Citation29] to 2352.5 mg GAE/100 g.[Citation30] The composition of fruits and phenolic content depend on the genetic and environmental factors as well as post-harvest processing conditions.[Citation31] Our results indicated that the ethanol-water (1:1) had better effects on extraction of phenolic compounds compared to other solvents in the same extraction conditions. Also, we found that UJEW had a greater effect on the extraction of phenolic compounds. Goli et al.[Citation32] and Yasoubi et al.[Citation33] reported similar results indicating that the ultrasound-assisted extraction was the most effective method in extraction of phenolic compounds in comparison with supercritical CO2 and solvent extraction techniques.
DPPH• Radical Scavenging Activity
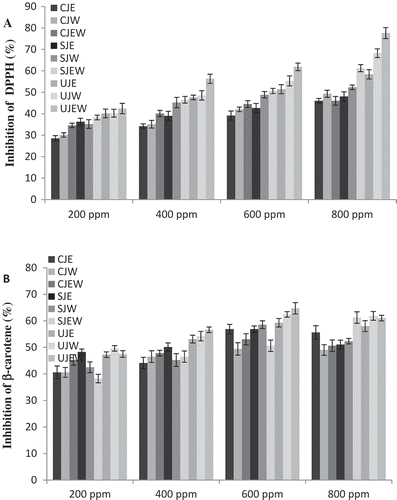
In the reaction of free radical DPPH• with antioxidant species, antioxidant inhibits and stabilizes the free radical which causes color to change from purple to yellow, and decreases the absorbance which was monitored using a spectrophotometer at 517 nm.[Citation34] shows the ability of jujube fruit extracts to scavenge DPPH• radical as inhibition percentage at concentrations of 200 to 800 ppm. In this assay similar to previously published results, such as the works by Chirinos et al.[Citation35] and Akkol et al.,[Citation36] the results indicated that scavenging free radicals increased as concentrations of the extracts increased, which was due to the increasing amount of phenolic compounds at higher concentrations of the extracts. With increasing concentrations of phenolic compounds the number of hydroxyl groups available in the reaction medium increased. Therefore, the possibility of hydrogen donation to free radicals increased.[Citation37] The results showed UJEW at concentrations of 200 to 800 ppm had the highest DPPH• radical-scavenging capacity between other extracts. This was in agreement with González-Montelongo et al.[Citation38] who mentioned that ethanol-water (1:1) extract was the best to scavenge DPPH• free radical. Also, our results indicated that the ultrasound had better performance on antioxidant activity of jujube in scavenging of free radicals. These results concurred with previously published results, such as the work by Rodríguez-Rojo et al.,[Citation26] in which they evaluated effects of different extraction methods on antioxidant activity of rosemary extract.
FIGURE 2 (a) Scavenging activities of jujube extracts against DPPH• radicals; (b) Antioxidant activities of jujube extracts in β-carotene/linoleic acid system. Solvent extracts of jujube by ethanol (SJE), water (SJW) and ethanol-water (SJEW). Ultrasound-assisted extracts of jujube by ethanol (UJE), water (UJW), and ethanol-water (UJEW). Supercritical CO2 extracts of jujube by ethanol (CJE), water (CJW), and ethanol-water (CJEW).
β-Carotene Bleaching Assay
In this assay, β-carotene is oxidized by free radicals derived from the oxidation of linoleic acid, which can better simulate the food system compared to DPPH assay.[Citation39] The antioxidant activities of the jujube fruit extracts were measured by bleaching of β-carotene which is illustrated in . The extracts obtained from different methods and solvents, showed different degrees of antioxidant activity. Ethanol-water extract of jujube fruit that extracted by ultrasound showed maximum antioxidant activities with significant differences than other solvents. This result was in agreement with González-Montelongo et al.[Citation38] who reported that banana peel extract obtained by ethanol-water (1:1) showed higher antioxidative activity than water and ethanolic extracts in the β-carotene/linoleic acid system. In general, supercritical CO2 extraction method had comparatively poor on the antioxidant activity of jujube, whereas samples extracted by UJEW technique at different concentrations had the highest inhibitory effects in prevention of β-carotene oxidation. Our results were in agreement with previously published results of Esmaeilzadeh Kenari et al.[Citation40]
From the evaluation of the antioxidant properties of extracts in vivo (DPPH and β-carotene assay) it is realized that ultrasound-assisted extraction of jujube by ethanol-water at 600 and 800 ppm had the highest antioxidant activity. Therefore, UJEW was used to assess its effect on stability of soybean oil during frying process.
Changes in TPC
Determination of TPC is one of the most important tests and a valid criterion for assessing the thermal oxidative degradation of the oils during deep frying (Stier 2013). shows changes in TPC content of the oil samples during deep frying process.[Citation41] In many European countries the TPC value is considered as a major oil degradation indicator and it is acceptable at a maximum of 25–27% for used frying oil.[Citation5] Fresh soybean oil had a TPC content of 5.62%, reflecting the good quality of the oil used, as TPC content of unused oils normally ranges between 0.4 and 6.4%.[Citation42] TPC content increased during frying and had high correlation coefficient with frying time. This result concurred with the results of Matthäus,[Citation43] Bansal et al.,[Citation44] and Osawa et al.[Citation45] The correlation equation and correlation coefficient in soybean oils containing 600 and 800 ppm of extract, BHT, BHA, TBHQ, and SBO (soybean oil as a control) were y = 5.209x – 0.594; R2 = 0.978, y = 6.195x – 2.841; R2 = 0.972, y = 6.726x – 2.32; R2 = 0.981, y = 6.414x – 2.18; R2 = 0.981, y = 6.254x + 2.958; R2 = 0.973, y = 8.116x – 1.698; R2 = 0.995, respectively.
TABLE 1 Changes in total polar compounds (TPC) content, carbonyl value (CV), and peroxide value (PV) of soybean oil with and without jujube fruit antioxidant during deep frying at 180°C
The TPC of the soybean oils containing UJEW of 600 and 800 ppm, BHT, BHA, TBHQ, and SBO after 24 h of frying were 42.02, 44.31, 47.47, 45.53, 42.10, and 56.59%, respectively. These results showed that SBO reached 25% TPC (degradation limit for regulation purposes) after 10.2 h of deep-frying cycles, while the oil with the addition of UJEW 600 and 800 ppm, BHT, BHA, TBHQ reached 25% TPC after 16.01, 15.1, 12.06, 13.8, and 15.12 h deep frying cycles. Therefore, the thermo-oxivative stability of soybean oil increased significantly in the presence of extracts compared to control oil. During frying time observed the rate increase in the amount of TPC in 600 ppm of UJEW was relatively slower than that of other oil samples and later reached the critical limit (25%). Therefore, the rate of discard of oil samples were as follows:
These results were in agreement with previous studies results such as the works by Casarotti and Jorge[Citation46] and Urbančič et al.[Citation22] which indicated that rosemary extract was more effective in reducing polar compounds production in frying oil compared to synthetics antioxidants.
Changes in CV
Changes in CV of the oil samples during frying process are shown in . The CV is an appropriate method for evaluation of frying oil quality because carbonyl compounds are the cause for undesirable flavors and reducing nutritional value of fried foods. CV measures secondary products of oxidation such as aldehydes and ketones.[Citation20] On the basis of the National Standards of Japan frying oils containing more than 50 µmol/g of CV should be discarded, because undesirable changes have been occurred in their flavor.[Citation47] The result showed the CV of a set of oil samples increased and reached a maximum value during frying and then decreased. The result concurred with previously published results of Farhoosh and Kenari[Citation48] and Farhoosh and Tavassoli-Kafrani.[Citation24] According to Farhoosh and Kenari[Citation48] during the prolonged frying period carbonyl compounds may convert to new compounds that are not measurable with CV test. There were no significant differences between the initial CV of the oil samples (13.75 µ mol/g). The CV for soybean oil containing 600 and 800 ppm of UJEW was lower than critical limit (51 µ mol/g) during frying process. Whereas, the oil containing BHT, BHA, and TBHQ after 24 h and SBO after 20 h reached the maximum CV (55.21, 52.08, 51.70, and 64.72 µmol/g, respectively). Therefore, UJEW at 600 ppm showed higher antioxidant activity compared to other oil samples in prevention of carbonyl compounds increase during frying process.
Changes in PV
shows changes in PV of the oil samples during deep frying process at 180°C. PV is used to assess primary products of oxidation (hydroperoxide) in oils and fats.[Citation3] Initial PV for all samples was lower than 2 meq O2/kg. The PV of fresh oil should be less than 2 meq O2/kg.[Citation49] It was generally observed that the extract significantly reduced PV of oils compared to control oil (p < 0.05). There was an initial increase in PV of SBO and BHA from 0 to 12 h, for BHT and TBHQ from 0 to 16 h, for UJEW at 600 and 800 ppm from 0 to 20 h and after that the rate slowed down. Peak values for PV were obtained and are as follows: BHT (5.01 meq O2/kg after 16 h); BHA (5.65 meq O2/kg after 20 h); UJEW at 600 ppm (5.02 meq O2/kg after 20 h); UJEW at 800 ppm (5.38 meq O2/kg after 20 h); TBHQ (5.62 meq O2/kg after 20 h); and SBO (7.44 meq O2/kg after 20 h).
The PV decreased in oil samples after the peak was reached. The PV results were in agreement with previously published results of Casal et al.[Citation1] and Farhoosh et al.[Citation23] A rapid rise in PV of soybean oils containing BHT, BHA, TBHQ, and SBO showed that they were more sensitive to oxidation degradation. UJEW at 600 ppm indicated greater ability to prevent an increase in PV as compare to the other oil samples. The PV test is not a valid parameter to assess oils’ oxidative changes during deep frying process, because peroxidase under frying conditions are unstable and are converted into other compounds, such as carbonyl and aldehyde, that cause PV abatement.[Citation50]
Changes in CD and CT
Changes in CD which represent the degree of production of primary oxidation products are shown in . The CD values of soybean oil treated with UJEW at 600 and 800 ppm were significantly different from the control oil, and also from the oils with added BHA, BHT, and TBHQ. The CD values in oils containing 800 ppm of UJEW and TBHQ with no significant difference (p > 0.05) at 4 h and in oil with 600 ppm of UJEW at other frying times (8, 12, 16, 20, and 24 h) were lower than other oil samples. At the end of 24 h frying process the CD values for the soybean oil containing 600 and 800 ppm of UJEW, BHT, BHA, and TBHQ were 15.65, 17.08, 17.77, 17.46, and 16.53 (mmol/L), respectively.
TABLE 2 Changes in conjugated diene (CD) and triene (CT) values of soybean oil with and without jujube fruit antioxidant during deep frying at 180°C
The CT values of soybean oils treated with antioxidants were significantly different from the control oil (). The CT value of control oil at the end of 24 h of frying was greater than that of oils treated with jujube fruit extracts and synthetic antioxidants. Generally, CT values for soybean oil containing UJEW at 600 and 800 ppm BHT, BHA, and TBHQ were 5.54, 9.36, 9.76, 9.53, and 8.4 (mmol/L), respectively. Therefore, the UJEW at 600 ppm indicated a greater ability to reduce the production of conjugated compounds compared to other oils treated with antioxidants. These results were approved by previous studies of Casarotti and Jorge[Citation46] and Urbančič et al.[Citation22] In the present study, similar to those reported by Chirinos et al.[Citation35] and Bou et al.,[Citation51] the CD and CT values increased with increasing frying time. Abdulkarim et al.[Citation5] showed that due to the formation of CD and CT absorption increase is proportional to the uptake of oxygen and formation of peroxides during early stages of oxidation. However, the increase of CD was considerably higher compared to the CT, which will be specifically due to the high content of linoleic acid in soybean oil.
Changes in FFA Content
Changes in the FFA content of the oil samples are shown in . The FFA is used as an indicator for assessment of oil deterioration during frying.[Citation5] There was no significant difference between initial FFA (0 h) of oil samples. However, in all samples FFA showed a trend to increase from the beginning of the frying period to the end of experiment, similar to results reported by Kim and Choe[Citation52] and Wang et al.[Citation53] The FFA content at the end of 24 h of frying for soybean oil containing UJEW at 600 and 800 ppm BHT, BHA, TBHQ, and SBO reached to 3.52, 4.22, 4.02, 3.66, 3.73, and 6.41%, respectively. However, the values of oil samples treated with natural and synthetic antioxidants were found to be significantly (p < 0.05) lower than the control oil. These results indicated that natural antioxidants in UJEW of 600 ppm protected soybean oil from hydrolysis better than synthetic antioxidants. Our results concurred with the results of Casarotti and Jorge[Citation46] and Urbančič et al.[Citation22] which examined the antioxidant effect of rosemary extract in soybean and sunflower oil.
TABLE 3 Changes in free fatty acids (FFA) content and oxidative stability index (OSI) of soybean oil with and without jujube fruit antioxidant during deep frying at 180°C
Changes in Oxidative Stability Index (OSI)
Changes in the OSI of oils during deep-frying process are shown in . The Rancimat method is often used for predicting oxidative stabilities of oils under deep frying conditions. The oxidation stability of soybean oil was greatly improved in the presence of extracts. Similar to results reported by Nor et al.[Citation3] and Casal et al.[Citation1] it was observed that stability of oil samples decreased gradually during frying process. Change percentage of oils containing BHA from 4 to 8 h (3.55%), 16 to 20 (6.4%), and UJEW at 600 ppm from 0 to 4 (2.07%), 8 to 12 (4.92%), 12 to 16 (7.84%), and 20 to 24 (5.14%) was lower than that of other oil samples, respectively. The rate of oxidation of oil samples was as follows:
Therefore, results indicated that the maximum stability in soybean oil was obtained by addition of 600 ppm of UJEW. OSI results were in agreement with previously published results such as the work of Taghvaei et al.[Citation15] and Aladedunye and Matthäus.[Citation28]
CONCLUSION
In this study, natural antioxidants extracted from jujube (Ziziphus mauritiana) using ultrasound-assisted extraction method with ethanol-water were found to be the most active in DPPH• radical scavenging capacity and β-carotene-linoleic acid assays. In terms of TPC, CV, PV, OSI, FFAs, CD, and CT our results also confirmed that the protection offered by ultrasound-assisted extraction is better than that of widely used synthetic antioxidants such as BHA, BHT, and TBHQ. Therefore, it is suggested that the best method for the extraction of phenolic compounds from jujube is ultrasound-assisted extraction method. Jujube extract showed acceptable oxidation prevention activity in soybean oil and could be used as a natural antioxidant in oil and oil-based products.
REFERENCES
- Casal, S.; Malheiro, R.; Sendas, A.; Oliveira, B.P.; Pereira, J.A. Olive Oil Stability Under Deep-Frying Conditions. Food and Chemical Toxicology 2010, 48(10), 2972–2979.
- Xua, T.; Lia, J.; Fana, Y.V.; Zhenga, T.; Deng, Z.Y. Comparison of Oxidative Stability among Edible Oils under Continuous Frying Conditions. International Journal of Food Properties 2014, 18(7), 1478–1490.
- Nor, F.M.; Mohamed, S.; Idris, N.A.; Ismail, R. Antioxidative Properties of Pandanus Amaryllifolius Leaf Extracts in Accelerated Oxidation and Deep Frying Studies. Food Chemistry 2008, 110(2), 319–327.
- Katalinica, V.; Mozinab, S.S.; Generalica, I.; Skrozaa, D.; Ljubenkovc, I.; Klancnik, A. Phenolic Profile, Antioxidant Capacity, and Antimicrobial Activity of Leaf Extracts from Six Vitis Vinifera L. Varieties. International Journal of Food Properties 2013, 16(1), 45–60.
- Abdulkarim, S.; Long, K.; Lai, O.; Muhammad, S.; Ghazali, H. Frying Quality and Stability of High-Oleic Moringa Oleifera Seed Oil in Comparison with Other Vegetable Oils. Food Chemistry 2007, 105(4), 1382–1389.
- Delfanian, M.; Esmaeilzadeh Kenari, R.; Sahari, M.A. Antioxidative Effect of Loquat (Eriobotrya Japonica Lindl.) Fruit Skin Extract in Soybean Oil. Food Science & Nutrition 2014, 3(1), 74–80.
- Rop, O.; Juríková, T.; Sochor, J.; Mlcek, J.; Kramarova, D. Antioxidant Capacity, Scavenging Radical Activity, and Selected Chemical Composition of Native Apple Cultivars from Central Europe. Journal of Food Quality 2011, 34(3), 187–194.
- Suja, K.; Jayalekshmy, A.; Arumughan, C. Antioxidant Activity of Sesame Cake Extract. Food Chemistry 2005, 91(2), 213–219.
- Thanatcha, R.; Pranee, A. Extraction and Characterization of Mucilage in Ziziphus mauritiana Lam. International Food Research Journal 2011, 18(1), 201–212.
- Nyanga, L.K.; Nout, M.J.; Gadaga, T.H.; Theelen, B.; Boekhout, T.; Zwietering, M.H. Yeasts and Lactic Acid Bacteria Microbiota from Masau (Ziziphus Mauritiana) Fruits and Their Fermented Fruit Pulp in Zimbabwe. International Journal of Food Microbiology 2007, 120(1), 159–166.
- Dahiru, D.; Sini, J.; John-Africa, L. Antidiarrhoeal Activity of Ziziphus Mauritiana Root Extract in Rodents. African Journal of Biotechnology 2006, 5(10), 213–218.
- Tachakittirungrod, S.; Okonogi, S.; Chowwanapoonpohn, S. Study on Antioxidant Activity of Certain Plants in Thailand: Mechanism of Antioxidant Action of Guava Leaf Extract. Food Chemistry 2007, 103(2), 381–388.
- Luengthanaphol, S.; Mongkholkhajornsilp, D.; Douglas, S.; Douglas, P.L.; Pengsopa, L.I.; Pongamphai, S. Extraction of Antioxidants from Sweet Thai Tamarind Seed Coat––Preliminary Experiments. Journal of Food Engineering 2004, 63(3), 247–252.
- Albu, S.; Joyce, E.; Paniwnyk, L.; Lorimer, J.; Mason, T. Potential for the Use of Ultrasound in the Extraction of Antioxidants from Rosmarinus Officinalis for the Food and Pharmaceutical Industry. Ultrasonics Sonochemistry 2004, 11(3), 261–265.
- Taghvaei, M.; Jafari, S.M.; Mahoonak, A.S.; Nikoo, A.M.; Rahmanian, N.; Hajitabar, J.; Meshginfar, N. The Effect of Natural Antioxidants Extracted from Plant and Animal Resources on the Oxidative Stability of Soybean Oil. LWT–Food Science and Technology 2014, 56(1), 124–130.
- Xu, B.; Chang, S. A Comparative Study on Phenolic Profiles and Antioxidant Activities of Legumes As Affected by Extraction Solvents. Journal of Food Science 2007, 72(2), 159–166.
- Lagha-Benamrouche, S.; Madani, K. Phenolic Contents and Antioxidant Activity of Orange Varieties (Citrus Sinensis L. and Citrus Aurantium L.) Cultivated in Algeria: Peels and Leaves. Industrial Crops and Products 2013, 50(1), 723–730.
- Farhoosh, R.; Moosavi, S.M.R. Carbonyl Value in Monitoring of the Quality of Used Frying Oils. Analytica Chimica Acta 2008, 617(1), 18–21.
- Schulte, E. Economical Micromethod for Determination of Polar Components in Frying Fats. European Journal of Lipid Science and Technology 2004, 106(11), 772–776.
- Endo, Y.; Li, C.M.; Tagiri-Endo, M.; Fujimoto, K. A Modified Method for the Estimation of Total Carbonyl Compounds in Heated and Frying Oils Using 2-Propanol As a Solvent. Journal of the American Oil Chemists’ Society 2001, 78(10), 1021–1024.
- Shantha, N.C.; Decker, E.A. Rapid, Sensitive, Iron-Based Spectrophotometric Methods for Determination of Peroxide Values of Food Lipids. Journal of AOAC International 1993, 77(2), 421–424.
- Urbančič, S.; Kolar, M.H.; Dimitrijević, D.; Demšar, L.; Vidrih, R. Stabilisation of Sunflower Oil and Reduction of Acrylamide Formation of Potato with Rosemary Extract During Deep-Fat Frying. LWT–Food Science and Technology 2014, 57(2), 671–678.
- Farhoosh, R.; Khodaparast, M.H.H.; Sharif, A.; Rafiee, S.A. Olive Oil Oxidation: Rejection Points in Terms of Polar, Conjugated Diene, and Carbonyl Values. Food Chemistry 2012, 131(4), 1385–1390.
- Farhoosh, R.; Tavassoli-Kafrani, M.H. Polar Compounds Distribution of Sunflower Oil As Affected by Unsaponifiable Matters of Bene Hull Oil (BHO) and Tertiary-Butylhydroquinone (TBHQ) During Deep-Frying. Food Chemistry 2010, 122(1), 381–385.
- Hemwimol, S.; Pavasant, P.; Shotipruk, A. Ultrasound-Assisted Extraction of Anthraquinones from Roots of Morinda Citrifolia. Ultrasonics Sonochemistry 2006, 13(6), 543–548.
- Rodríguez-Rojo, S.; Visentin, A.; Maestri, D.; Cocero, M. Assisted Extraction Of Rosemary Antioxidants with Green Solvents. Journal of Food Engineering 2012, 109(1), 98–103.
- Katalinic, V.; Mozina, S.S.; Generalic, I.; Skroza, D.; Ljubenkov, I.; Klancnik, A. Phenolic Profile, Antioxidant Capacity, and Antimicrobial Activity of Leaf Extracts from Six Vitis Vinifera L. Varieties. International Journal of Food Properties 2013, 16(1), 45–60.
- Aladedunye, F.; Matthäus, B. Phenolic Extracts from Sorbus Aucuparia (L.) and Malus Baccata (L.) Berries: Antioxidant Activity and Performance in Rapeseed Oil During Frying and Storage. Food Chemistry 2014, 159, 273–281.
- Ikram, E.H.K.; Eng, K.H.; Jalil, A.M.M.; Ismail, A.; Idris, S.; Azlan, A.; Nazri, H.S.M.; Diton, N.A.M.; Mokhtar, R.A.M. Antioxidant Capacity and Total Phenolic Content of Malaysian Underutilized Fruits. Journal of Food Composition and Analysis 2009, 22(5), 388–393.
- Lamien-Meda, A.; Lamien, C.E.; Compaoré, M.M.; Meda, R.N.; Kiendrebeogo, M.; Zeba, B.; Millogo, J.F.; Nacoulma, O.G. Polyphenol Content and Antioxidant Activity of Fourteen Wild Edible Fruits from Burkina Faso. Molecules 2008, 13(3), 581–594.
- Milivojevic, J.; Slatnar, A.; Mikulic-Petkovsek, M.; Stampar, F.; Nikolic, M.; Veberic, R. The Influence of Early Yield on the Accumulation of Major Taste and Health-Related Compounds in Black and Red Currant Cultivars (Ribes spp.). Journal of Agricultural and Food Chemistry 2012, 60(10), 2682–2691.
- Goli, A.H.; Barzegar, M.; Sahari, M.A. Antioxidant Activity and Total Phenolic Compounds of Pistachio (Pistachia Vera) Hull Extracts. Food Chemistry 2005, 92(3), 521–525.
- Yasoubi, P.; Barzegar, M.; Sahari, M.; Azizi, M. Total Phenolic Contents and Antioxidant Activity of Pomegranate (Punica Granatum L.) Peel Extracts. Journal of Agricultural Science and Technology 2010, 9, 35–42.
- Li, Y.; Guo, C.; Yang, J.; Wei, J.; Xu, J.; Cheng, S. Evaluation of Antioxidant Properties of Pomegranate Peel Extract in Comparison with Pomegranate Pulp Extract. Food Chemistry 2006, 96(2), 254–260.
- Chirinos, R.; Huamán, M.; Betalleluz-Pallardel, I.; Pedreschi, R.; Campos, D. Characterisation of Phenolic Compounds of Inca Muña (Clinopodium Bolivianum) Leaves and the Feasibility of Their Application to Improve the Oxidative Stability of Soybean Oil During Frying. Food Chemistry 2011, 128(3), 711–716.
- Akkol, E.K.; Orhan, I.E.; Yeşilada, E. Anticholinesterase and Antioxidant Effects of the Ethanol Extract, Ethanol Fractions, and Isolated Flavonoids from Cistus Laurifolius L. Leaves. Food Chemistry 2012, 131(2), 626–631.
- Rajaei, A.; Barzegar, M.; Mobarez, A.M.; Sahari, M.A.; Esfahani, Z.H. Antioxidant, Anti-Microbial, and Antimutagenicity Activities of Pistachio (Pistachia Vera) Green Hull Extract. Food and Chemical Toxicology 2010, 48(1), 107–112.
- González-Montelongo, R.; Gloria Lobo, M.; González, M. Antioxidant Activity in Banana Peel Extracts: Testing Extraction Conditions and Related Bioactive Compounds. Food Chemistry 2010, 119(3), 1030–1039.
- Razali, N.; Mat-Junit, S.; Abdul-Muthalib, A.F.; Subramaniam, S.; Abdul-Aziz, A. Effects of Various Solvents on the Extraction of Antioxidant Phenolics from the Leaves, Seeds, Veins, and Skins of Tamarindus Indica L. Food Chemistry 2012, 131(2), 441–448.
- Esmaeilzadeh Kenari, R.; Mohsenzadeh, F.; Amiri, Z.R. Antioxidant Activity and Total Phenolic Compounds of Dezful Sesame Cake Extracts Obtained by Classical and Ultrasound‐Assisted Extraction Methods. Food Science and Nutrition 2014, 2(4), 426–435.
- Stier, R.F. Ensuring the Health and Safety of Fried Foods. European Journal of Lipid Science and Technology 2013, 115(8), 956–964.
- Lumley, I. Polar compounds in Heated Oils. Frying of Food. Principles, Changes, New Approaches 1988, 166–173.
- Matthäus, B. Utilization of High‐Oleic Rapeseed Oil for Deep‐Fat Frying of French Fries Compared to Other Commonly Used Edible Oils. European Journal of Lipid Science and Technology 2006, 108(3), 200–211.
- Bansal, G.; Zhou, W.; Barlow, P.J.; Lo, H.L.; Neo, F.L. Performance of Palm Olein in Repeated Deep Frying and Controlled Heating Processes. Food Chemistry 2010, 121(2), 338–347.
- Osawa, C.C.; Gonçalves, L.A.G.; Gumerato, H.F.; Mendes, F.M. Study of the Effectiveness of Quick Tests Based on Physical Properties for the Evaluation of Used Frying Oil. Food Control 2012, 26(2), 525–530.
- Casarotti, S.N.; Jorge, N. Antioxidant Activity of Rosemary Extract in Soybean Oil Under Thermoxidation. Journal of Food Processing and Preservation 2014, 38(1), 136–145.
- Hara, S.; Ogawa, E.; Totani, Y. Evaluation of Heat-Deteriorated Oils. I. TLC-FID Method For Determining Polar Compounds Content. Journal of Oleo Science 2006, 55(4), 167–172.
- Farhoosh, R.; Kenari, R.E. Anti-Rancidity Effects of Sesame and Rice Bran Oils on Canola Oil During Deep Frying. Journal of the American Oil Chemists’ Society 2009, 86(6), 539–544.
- Ramadan, M.F.; Amer, M.M.A.; Sulieman, A.E.R.M. Correlation Between Physicochemical Analysis and Radical‐Scavenging Activity of Vegetable Oil Blends As Affected by Frying of French Fries. European Journal of Lipid Science and Technology 2006, 108(8), 670–678.
- Sulieman, A.R.M.; EL‐Makhzangy, A.; Ramadan, M.F. Antiradical Performance and Physicochemical Characteristics of Vegetable Oils Upon Frying of French Fries: A Preliminary Comparative Study. Journal of Food Lipids 2006, 13(3), 259–276.
- Bou, R.; Navas, J.; Tres, A.; Codony, R.; Guardiola, F. Quality Assessment of Frying Fats and Fried Snacks During Continuous Deep-Fat Frying at Different Large-Scale Producers. Food Control 2012, 27(1), 254–267.
- Kim, H.; Choe, E. Effects of Egg Yolk Powder Addition to the Flour Dough on the Lipid Oxidation Development During Frying. LWT–Food Science and Technology 2008, 41(5), 845–853.
- Wang, Y.; Chen, X.; Zhang, Y.; Chen, X. Antioxidant activities and major anthocyanins of myrobalan plum (Prunus cerasifera Ehrh.). Journal of Food Science. 2012, 77(4), 388–393.
