Abstract
Cereal mixed linkages (1 → 3) (1 → 4)-β-D-glucan is a linear polysaccharide composed of glucose units. Oat β-glucan is a natural polymer. The main products of β-glucanase are oligosaccharides with DP3 and DP4, i.e., 3-Ob-cellobiosyl-D-glucose and 3-Ob-cellotriosyl-D-glucose, which represent over 90% of the molecule. Keeping in mind all the benefits of oat bran, the present study was planned to investigate the structural properties of oat bran, high-performance anion exchange chromatography with pulsed amperometric detection was used to examine these oligosaccharides. The structural analysis of oat bran of two oat varieties revealed that the ratio of soluble and insoluble triose to tetraose in β-glucan fraction was 1.44 and 1.78, respectively, for Avon variety; while the ratio of soluble and insoluble triose to tetraose in β-glucan fraction for Sargodha-81 was 1.49 and 1.77. The major units determined were cellotriose and cellotetraose. Other units cellopentaose and hexaoses were also existed but in minor fractions. Lichenase hydrolysis high-performance anion exchange chromatography with pulsed amperometric detection appeared to be the best choice for structural analysis of purified samples of mixed-linkage β-glucan.
INTRODUCTION
The occurrence of bioactive molecules in plants provides them with an imperative position in diet-based regimes. In addition, some of the bioactive elements hold multifaceted positions for their utilization that they add high value to the foods.[Citation1] Cereals, especially oat, are one example in this abatement as it contains several nutrients of great worth and and many phytochemicals as well. In this perspective, presence of bioactive ingredient in oat are of significance value, e.g., β-glucan. The β-glucans in the oat groat are more concentrated in the bran fraction than in the endosperm fraction. Oat bran possesses 4.5% β-glucans.[Citation2] The β-glucan contains both soluble and insoluble components of dietary fiber but oat β-glucan is classified as soluble fiber.[Citation3]
The functionality and physical properties of β-glucan depends on its fine structure, molecular size, molecular weight distribution, and extractability. It is important to know that the source (cereal species, cultivar), processing methods (milling, temperature, pH-shear effects, etc.), and their interaction with other constituents are likely to affect the concentration, structural features, and solubility of β-glucan.[Citation4] Oat β-glucan is a natural polymer composed of glucose molecules individually which are inter-connected by a series of β-(1-3) and β-(1-4) linkages.
Cereal mixed linkages (1 → 3) (1 → 4)-β-D-glucan is a linear polysaccharide composed of glucose units. The sequences of (1 → 4)-linked D-glucopyranosyl units are separated by a (1 → 3)-β-linked units. Mostly (1 → 4)-linked sequences are essentially three or four glucose units long, but the sequences up to 13 glucose units in water-soluble and up to 20 in water-insoluble β-glucan have been found.[Citation5] It is believed that long cellulose-like sequences of (1 → 4)-linked β-D-glucose residues may form strong internal or external aggregation through hydrogen bonding with adjacent chain and make β-glucan less soluble. The ratio of trisaccharides (DP3) to tetrasaccharides (DP4) forms the fingerprint of a particular cereal and is different for each. This ratio is highest for wheat and reported to have the ranges from 3.7–4.8 followed by barley/rye (2.7–3.6), and oats (1.7–2.4).[Citation6] Higher molar proportion of the trisaccharides, i.e., higher ratio of DP3/DP4, offers greater chances of consecutive cellotriosyl units, which result in a more regular structure of β-glucan, thus making it less soluble. Further differences in DP3/DP4 ratio can be found in different grain tissues.[Citation7] Keeping in mind the factors discussed above, the present study was designed to study the structure of oat bran β-glucan.
MATERIALS AND METHODS
Materials
Two oat varieties namely Avon and Sargodha-81 grown commercially were procured from Ayub Agricultural Research Institute (AARI), Faisalabad-Pakistan. The milling process of oats includes three steps. In the first step cleaning and sizing of the material was carried out to remove the dust, chaff, rocks, other grains, and other foreign material from the oats. In the second step dehulling of the oat was carried out by passing each variety of grain through the de-huller. The outer hull from the inner groat of each variety was separated. The lighter oats are separated and groats are taken for further processing. In the final step, the groats obtained after dehulling were milled through Quaderumate Senior Mills. The oat bran was separated from flour in several grinding and sieving operations to a coarse fraction (bran) and fine fraction (endosperm flour).
Extractability of β-Glucan
The extractability of β-glucan was estimated by the method described by McLeary and Glennie-Holmes[Citation8] using the β-glucan enzymatic assay kit (Megazyme Intl.) with some modifications to accommodate the liquid extract. An aliquot (3.0 mL) of the physiological extract was added to 5.0 mL of 20 mM sodium phosphate buffer and pH was adjusted to 6.5 and then mixed. The Lichenase (200 μL, 50 U/mL, Megazyme Intl.) was then added and the solution was incubated at 40°C for 60 min in a water bath. Then the solution was diluted to 15 mL with distilled water, mixed it and an aliquot of each solution was centrifuged at 1000 × g for 10 min. Each sample (100 μL) was then incubated for 15 min at 40°C with 100 μL each of acetate buffer (pH 4.0) and β-glucosidase (2 U/mL, Megazyme Intl. Ltd.). Then glucose oxidase/peroxidase (GOPOD, Megazyme Intl. Ltd.) was added (100 μL), and the solution was incubated for 20 min at 40°C and the absorbance was measured at 510 nm against a reagent blank.
Determination of β-Glucan Content
The oat bran samples were tested for total β-glucan content by employing the Megazyme assay kit as outlined by McCleary and Codd.[Citation9] For this purpose the samples were vortexed by the addition of sodium phosphate buffer and this mixture was boiled for 1 min. Thereafter, the mixture was stirred vigorously, and incubated at 100°C for 2 min and equilibrated at 50°C for 5 min. Then, lichenase (0.2 mL) was added and incubated for 60 min. Then acetate buffer was added and vigorously mixed the tube contents on a vortex mixer. Allowed the tube to equilibrate at room temperature (5 min), and then centrifuged (1000 × g) for 10 min. Dispensed aliquots (0.1 mL), and β-glucosidase (0.1 mL) were added. Incubation was carried out at 50°C for 10 min. Finally, GOPOD reagents were added and incubated at 50°C for 20 min and measured the absorbance on a spectrophotometer (IREMCO, Model 2020, Germany) at 510 nm within one hour.
Structural Analysis of β-Glucan
The enzymatic hydrolysis with lichenase was carried out to determine the amount of oligosaccharides and structure of oat bran β-glucan. The hydrolysis was performed according to method of Johansson et al.[Citation10] The β-glucan was dissolved in phosphate buffer (4.0 mL, 20 mM, pH 6.5) and incubated with lichenase (100 U) at 60°C for 2 h. The resulting solution was again incubated in a boiling-water bath for 10 min to inactivate the lichenase. The oligosaccharides produced were analyzed through high-performance anion exchange chromatography with pulsed amperometric detection (HPAEC-PAD).
RESULTS AND DISCUSSIONS
Extractablity of β-D-Glucans and Its Contents in Oat Bran
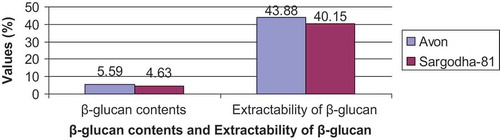
The results regarding β-glucan extractability of oat bran exhibited differences between the two analyzed varieties (, ). The higher value for β-glucan extractability of oat variety Avon (43.88%) was observed as compared to variety Sargodha-81 (40.15%). The results of the present study are supported by the findings of Bhatty[Citation11] who reported that extractability of β-glucan in the bran of oat varieties varies from 28 to 73%. Similarly, Jaskari et al.[Citation12] observed that extractibility of β-glucan varies from 40.67 to 44.71%. However, Immerstrand et al.[Citation13] reported relatively higher extractibility of β-glucan which varied from 42 to 45.87% in bran of different oat varieties. It is well-known that β-glucan extractability is controlled by extraction conditions such as temperature, time, presence of enzymes, pH, and agitation. Further, differences can be attributed to genetic and environmental variabilitiies.
TABLE 1 β-glucan contents (%) and extractability of β-glucan
The oat bran of Avon variety possessed higher (5.59%) β-glucan content as compared to Sargodha-81 (4.63%; ). These results are also in close relation to those reported by Immerstrand et al.[Citation13] who found 7.2% β-glucan content in bran of oat variety. The results of present study are also supported by Luhaloo et al.[Citation14] who reported β-glucan content in different commercial oat bran ranges from 4.7 to 8.3%.
Structure Analysis of Oat Bran β-D-Glucans of Two Oat Varieties
The structural features of β-glucan are considered important for their functional properties.[Citation15] The interpretation of structural features determined through lichenase hydrolysis of β-glucan has shown diversity in oat bran of two oat varieties studied in the present research. Lichenase hydrolysis of water soluble β-glucan in oats yields fragments of degree of polymerization (DP) up to 13,[Citation5] while the water-insoluble part of β-glucan may yield much longer cellulose-like sequences up to DP 20. However, in the present experiment, the β-glucan was analyzed up to DP6. Among the oligomers released DP3 and DP4 were most predominant.[Citation15]
The results regarding structural analysis of oat bran β-glucan are given in and . It is obvious from the results that oligosaccharides with DP 3–6 were obtained in enzymatic hydrolyses of the β-glucans of brans of two oat varieties analyzed using HPAEC-PAD. Differences in the amounts of the oligosaccharides were recorded between soluble and insoluble fractions of oat bran β-glucan but no difference was observed between these two oat varieties. The difference between soluble and insoluble β-glucans suggested that soluble β-glucans have less cellotriose units than insoluble β-glucans. The ratio of the amount of soluble and insoluble triose to tetraose was 1.44 and 1.78 for the Avon variety () and exhibited 1.49 and 1.79 in Sorgadhda-81 () in the case of soluble and insoluble β-glucan fractions, respectively. The major units were cellotriose and cellotetraose, cellopentaose, and hexaoses also existsted but in minor fractions.
FIGURE 2 Yields of oligosaccharides DP3-DP6 produced with lichenase hydrolysis as analyzed with HPAEC-PAD.
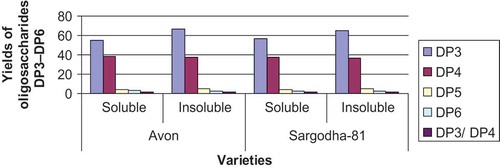
TABLE 2 Yields of oligosaccharides DP3-DP6 produced with lichenase hydrolysis as analyzed with HPAEC-PAD
β-glucan is a significant constituent of soluble dietary fiber in oats. β-glucan is generally considered a branched semi-flexible and linear polymer. It is stitched up of glucose units linked by D-(1-4) with a casual glycoside (1-3)-glycosidic. The configuration of the chain depends on the relative amount of (1-4)- and (1-3)-glycosidic bond between replicating units of glucose.[Citation10] Oat β-glucan is a linear polysaccharide consisting of only β-D-glucopyranosyl units. Structural sequence analysis is often performed by breaking the β-glucan oligosaccharides. The best way is the use of enzyme glucanohydrolase, i.e., lichenase, which specifically cleaves the (1 → 4)-side connection at the reducing end. The products are (1 → 4)-linked oligosaccharides with a glucose unit (1 → 3)-linked as terminal group at the reducing end. Oligosaccharides with DP3, is the main product and DP4, comes second. Both are more than 90% of total β-glucan.[Citation16]
The results of the present study are well-supported by the findings reported by Wood et al.[Citation16] who observed 55% of triose and 36% of tetraose, whereas Doublier and Wood[Citation17] found 58.1% for triose and 34.2% for tetarose. The amount of pentose found in the present study is also similar to the findings of Lazaridou et al.,[Citation18] Doublier and Wood,[Citation19] and Roubroekd et al.[Citation20] who found 3.3, 3.0, and 1.6%, pentose, respectively, pentose. The molar ratios of DP3/DP4 for oat bran soluble and insoluble β-glucan was relatively lower in the present study than those reported by several researchers who found 1.6 and 2.1, respectively, and these values are well in accordance with the findings of Aman et al.[Citation21] who reported the molar ratio between 1.4 and 1.8 for water-soluble and insoluble β-glucans depending on the conditions of extraction. The slightly higher values observed in our study are due to the fact that we accounted for oligosaccharides up to DP 6 and normalized the relative area under each peak. The results are consistent with those of Colleoni-Sirghie et al.,[Citation22] who reported a ratio of 1.6 for β-glucans extracted from oat lines, and with Jiang and Vasanthan.[Citation23] Luhaloo et al.[Citation14] reported ratios for oat β-glucan and lichenan to be 1.4 and 1.81, respectively. The value for DP3:DP4 in the present study seems to be higher for insoluble than for soluble oats. It is generally believed that the more β-glucan containing long sequences of (1-4)-bonds, the higher is their tendency to associate, due to the rigidity of these polymer segments.[24]
Conclusion
Structural differences have been reported in the current study to exist between soluble and insoluble β-glucans, the ratio DP3:DP4 being higher for insoluble than for soluble β-glucans. Also the ratio DP3:DP4 was observed higher for Sargodha-81 than Avon variety, which showed that the Avon variety have higher solubility than Sargodha-81. High-performance anion-exchange chromatography with pulse-amperometric detection is a good tool for analyzing the hydrolysis products. It is sensitive and reproducible and derivatization is not required. Together with lichenase hydrolysis HPAEC-PAD appears to be the best choice for structural analysis of purified samples of mixed-linkage β-glucan.
References
- Butt, M.S.; Shahzadi, N.; Sharif, M.K.; Nasir. M. Guar Gum: A Miracle Therapy for Hypercholesterolemia, Hyperglycemia, and Obesity. Critical Reviews in Food Science and Nutrition 2007, 47, 389–396.
- Usman, S.; Ali, S.S.; Nasreen, Z.; Najim, A. Determination of Biochemical Composition of Avena Sativa (Oat) and to Estimate the Effect of High Fiber Diet on Hypercholesterolemic Rats. Bangladesh Research Pubications Journal 2010, 4(4), 312–319.
- AACC. Approved Methods of American Association of Cereal Chemists; The American Association of Cereal Chemists, Inc.: St. Paul., MN, 2000.
- Yun, L.; Perret, J.; Harris, M.; Wilson J.; Haley, S. Antioxidant Properties of Bran Extracts from Akron Wheat Grown at Different Locations. Journal of Agricultural and Food Chemistry 2003, 51, 1566–1570.
- Izydorczyk, M.S.; Macri, L.J.; MacGregor, A.W. Structure and Physicochemical Properties of Barley Non-Starch Polysaccharides—I. Water-Extractable β-Glucans and Arabinoxylans. Carbohydrate Polymers 1998, 35, 249–258.
- Wood, P.J. Guelph Food Research Centre, ON, Canada. Personal Communication; Yokoyama W.H.; Knuckles, B.E.; Stanford, A.; Inglett, G. (1998). Raw and Processed Oat Ingredients Lower Plasma Cholesterol in the Hamster. Journal of Food Science 2010, 63, 713–715.
- Izydorczyk, M.S.; Chornick, T.L.; Paulley, F.G.; Edwards, N.M.; Dexter, J.E. Physiochemical Properties of Hulless Barley Fiber Rich Fractions Varying in Particle Size and Their Potential As Functional Ingredients in Two Layer Flat Bread. Food Chemistry 2008, 108(2), 561–570.
- McCleary, B.V.; Holmes, G.M. Enzymic Quantification of (1-3) (1-4) β-Glucan in Barley and Malt. Journal of the Institute of Brewing 1985, 91, 285–295.
- McCleary, B.V.; Codd, R. Measurement of (1→3) (1→4)-β-d-Glucan in Barley and Oats: A Streamlined Enzymic Procedure. Journal of Science of Food and Agriculture 1991, 55, 303–312.
- Johansson, L.; Virkki, L.; Maunu, S.; Leht, M.; Ekholm, P.; Varo, P. Structural Characterization of Water-Soluble β-Glucan of Oat Bran. Carbohydrate Polymers 2000, 42, 143–148.
- Bhatty, R.S. Laboratory and Pilot Plant Extraction of β-Glucans from Hull-Less Barley and Oat Brans. Journal of Cereal Science 1995, 22, 163–170.
- Jaskari, J.; Henriksson, K.; Nieminen, A.; Suortti, T.; Salovaara, H.; Poutanen, K. Effect of Hydrothermal and Enzymatic Treatments on the Viscous Behaviour of Dry- and Wet-Milled Oat Brans. Cereal Chemistry 1995, 72, 625–631.
- Immerstrand, T.; Bergenstahl, B.; Trägårdh, C.; Nyman, M.; Cui, S.; Oste, R. Extraction of β-Glucan from Oat Bran in Laboratory Scale. Cereal Chemistry 2010, 86(6), 601–608.
- Luhaloo, M.; Mårtensson, A.C.; Andersson, R.; Åman, P. Compositional Analysis and Viscosity Measurements of Commercial Oat Brans. Journal of Science of Food and Agriculture 1998, 76, 142–148.
- Cui, S.; Wang, Q. Cell Wall Polysaccharides in Cereals: Chemical Structures and Functional Properties. Structural Chemistry 2009, 20, 291–297.
- Wood, P.J.; Weisz, J.; Blackwell, B.A. Structural Studies of 1-3;1-4.-β-d-Glucans by 13C-Nuclear Magnetic Resonance Spectroscopy and by Rapid Analysis of Cellulose-Like Regions Using High Performance Anion-Exchange Chromatography of Oligosaccharides Released by Lichenase. Cereal Chemistry 1994, 71, 301–307.
- Wood, P.J.; Braaten, J.A.; Scott, F.D.; Riedel, K.D.; Wolynetz, M.S.; Collins, M.W. Effect of Dose and Modification of Viscous Oat Gum on Plasma Glucose and Insulin Following An Oral Glucose Load. British Jouranl of Nutrition 1995, 72, 731–774.
- Lazaridou, A.; Duta, D.; Papageorgiou, M.; Belc, N.; Biliaderis, C.G. Effects of Hydrocolloids on Dough Rheology and Bread Quality Parameters in Gluten-Free Formulations. Journal of Food Engineering 2007, 79, 1033–1047.
- Doublier, L.L.; Wood, P.J. Rheological Properties of Aqueous Solutions of 1-3 & 1-4-β-Glucan from Oats (Avena Sativa L.). Cereal Chemistry 1995, 72(4), 335–340.
- Roubroeks, J.P.; Andersson, R.; Åman, P. Structural features of (1→3), (1→4)-β-D-Glucan and Arabinoxylan Fractions Isolated from Rye Bran. Carbohydrate Polymers 2000, 42, 3–11.
- Aman, P.; Rimsten, L.; Andersson, R. Molecular Weight Distribution of β-Glucan in Oat-Based Foods. Cereal Chemistry 2004, 81, 356–360.
- Colleoni-Sirghie, M.; Fulton, D.B.; White, P.J. Structural Features of Water Soluble (1,3),(1,4)-β-D-Glucans from High-β-Glucan and Traditional Oat Lines. Carbohydrate Polymers 2003a, 54, 237–249.
- Jiang, G.; Vasanthan, T. MALDI-MS and HPLC Quantification of Oligosaccharides of Lichenase-Hydrolyzed Water-Soluble β-Glucan from Ten Barley Varieties. Journal of Agriculturual and Food Chemistry 2000, 48, 3305–3310.