Abstract
Total phenols, flavonoids, flavonols, and flavanols of the methanolic extract of the aerial part of Artemisia herba-alba were determined. The extract was analyzed by liquid chromatography with photodiode array coupled with electrospray ionisation mass spectrometry and allowed to identify of 10 phenolic compounds. Apigenin-6-C-glycosyl flavonoids and caffeoylquinic acids were identified. Chlorogenic acid and 1,4 dicaffeoylquinic acid being the major constituents. The essential oil obtained by hydrodistillation was analyzed by gas chromatography–mass spectrometry. Twenty-three compounds, representing 97.8% of the total oil, were identified. The most abundant components were β-thujone (41.9%), α-thujone (18.4%), and camphor (13.2%). Methanolic extract and essential oil exhibited a considerable antioxidant activity as evaluated by 2,2-diphenyl-pycrilhydrazil hydrate scavenging activity, reducing power, β-carotene bleaching test, and chelating ability. The methanolic extract was found to be more efficient, while the essential oil exhibited the highest acetylcholinesterase inhibitory activity. Analysis of the antibacterial activity showed that A. herba-alba methanolic extract and essential oil are efficient against gram positive and gram negative bacteria.
INTRODUCTION
Artemisia herba-alba Asso. (subgen. Seriphidium, Asteraceae family) is widespread in semi-arid and arid steppes of North Africa, Spain, the Middle East, and the Northwest of Himalaya.[Citation1] It is a chaemephyte (30–50 cm in height) with erected stems. Leaves are grey, alternate, 2–3 pinnatisected, ovate, covered with glandular hairs, and sessile at the flowering stage (from September to December in the Mediterranean region). Flower heads are small (3/1.5 mm), ovoid and constituted by two to five yellow hermaphrodite flowers. Involucral bracts are oblong with membranous margin and achenes were obovate.[Citation2] The species is mostly diploid with a chromosome number 2n = 9, and may be tetraploid with 2n = 36.[Citation3] Four subspecies: chitachensis, kabilica, valentina, and herba-alba and three varieties: densiflora Boiss., laxiflora Boiss., and tenuiflora Boiss. had been distinguished within the species.[Citation3,Citation4]
A. herba-alba was extensively used in traditional medicine to treat diabetes, hypertension, colds, intestinal disturbances, scorpion/snake bites, and parasitic infections.[Citation5,Citation6] The plant extracts showed various biological activities such as antibacterial, insecticidal, antioxidant, and neurological activities.[Citation7,Citation8] A high infraspecific variation of essential oils and phenolics within and inter geographical areas was reported.[Citation5,Citation6,Citation9,] This phytochemical polymorphism could be attributed to genetic (i.e., morphological and DNA polymorphisms, varieties) and/or ecological (i.e., altitude, sunlight exposure, type of soil, and amount of rainfall) factors.[Citation10]
In Tunisia the genus Artemisia comprises five species (A. herba-alba Asso., A. arborescens L., A. atlantica Coss. et Dur., A. vulgaris L., and A. campestris L.). A. herba-alba occurs mainly in steppes (covering a surface of 24,400 km2) derived from the degradation of Pinus halepensis and Juniperus phoenicea forests, and expanded from the lower semi-arid to the lower arid bioclimates under an annual rainfall ranging from 100 to 400 mm. Scattered populations according to specific factors (edaphic, climatic, and topographical factors) can be encountered in Saharan (50–100 mm rainfall/year) or upper semi-arid (450–500 mm rainfall/year) bioclimates.[Citation11]
The species grows on calcareous, marneous, sandy clay, and gypseous soils at altitudes ranging from 100 to 1000 m. It is mainly associated with Asteriscus pygmaeus, Ajuga iva, Salvia aegyptiaca, Vella annua, Stipa retorta, Launea nudicaulis, Plantago ovata, and Eruca vesicaria. The anthropogenic pressures (mainly overgrazing and clearing) led to a significant decrease of the size of populations, and the fragmentation of their habitats. A high genetic structure, even at a low geographic distance among the populations, due to genetic drift and low levels of gene flow among them was reported.[Citation12] The loss of populations stemming from the habitat destruction may lead to gradual reduction of individual fitness. Thus, the analysis of the genetic diversity of populations jointly to that of their chemical variation is urgently needed to conceive management, selection, and promoting important genotypes.
The aim of this study was to investigate the essential oil and the phenolic compositions of the Tunisian Artemisia herba-alba collected from the center of the country, and to determine the antioxidant, antiacetylcholinesterase, and antibacterial activities of these extracts. This work is complementary to those previously performed on the same species from the southern part of the country. It gives additional information linked to their phenolic constituents analyzed for the first time by liquid chromatography with photodiode array coupled with electrospray ionisation mass spectrometry (LC-PDA/ESI-MS), as well as to their biological activities in order to increase the source of natural active compounds which recently have attracted considerable interest in food, cosmetic, and pharmaceutical industries instead of using more toxic synthetic compounds.
MATERIALS AND METHODS
Plant Material
Artemisia herba-alba plants was collected in the region of Kairouan (latitude 35°40’N, longititude 10° 05’E, altitude 65 m, upper arid bioclimate). Aerial parts including leaves and flowers were isolated from a random sample of 10 plants. The samples were air dried at room temperature for 2 weeks and then finely powdered in a mortar grinder mill. A voucher specimen was deposited in our laboratory for future reference.
Essential Oil
Essential Oil Isolation
Seventy grams of powder in 700 mL of distilled water were steam stilled for 3 h using a Clevenger-type apparatus. The obtained oil was dried using anhydrous sodium sulphate and then stored at 4°C until analyses.
Gas Chromatography Analysis
The composition of the essential oil was assessed by gas chromatography–mass spectrometry (GC–MS). The Agilent 7890A gas chromatograph is equipped with a HP-5MS fused silica column (30 m × 0.25 mm; 0.25 μm film thickness), interfaced with an Agilent mass selective detector 5975 C inter MSD. Oven temperature program was from 60 to 240°C at 4°C/min; injector temperature was 250°C; helium at 0.8 mL/min was used as carrier gas and interface temperature was 280°C; MS source temperature was 230°C; MS quadrupole temperature was 150°C; mass scan range from 50 to 550 amu at 70 eV; scan velocity was 2.91 scans/s. One microliter of sample was injected.
Identification of essential oil compounds
The identification of compounds was based on comparison of their relative retention times determined in relation to a homologous series of n-alkanes (C9-C24) under the same operating conditions. The identification of volatile components was also confirmed by comparison of their mass spectra with those recorded in NIST08 and W8N08 libraries.
Preparation of methanolic extract
Two grams of powder were extracted with 20 mL of 80% methanol at ambient temperature for 24 h. The extract was filtrated and stored at 4°C.
Determination of Total Phenolic, Flavonoid, Flavonols, and Flavanols Contents
The Folin–Ciocalteu reagent was used to quantify total phenols according to Singleton and Rossi’s method.[Citation13] Total phenols was expressed as mg gallic acid equivalent per gram of dry weight (mg GAE/g DW). The flavonoid content was determined according to Djeridane et al.[Citation14] The result was expressed as mg of rutin equivalent per gram of dry weight (mg RE/DW). Flavonols in the extracts were estimated using the method reported by Adedapo et al.[Citation15] The content was expressed as mg of quercetin equivalents per g of dry weight (mg EQ/g DW). The flavanol contents, determined using the p-dimethylaminocinnamaldehyde (DMACA) method[Citation16] were expressed as mg catechin equivalent per gram of dry weight (mg CE/g DW).
LC-PDA/ESI-MS Analysis of Methanolic Extract
LC-PAD/ESI-MS analysis was conducted in negative mode electrospray ionisation on an Agilent 1100 series HPLC systems (Agilent Technologies, Palo Alto, CA, USA) equipped with a photodiode array detector (PDA) and a triple quadrupole mass spectrometer type Micromass Autospec Ultima Pt (Kelso, UK). The separation was achieved using a reversed-phase Uptisphere C18 (Interchim; 2 mm × 100 mm, 5 μm particle size) column with a rate flow of 0.25 mL/min at 40°C.
The methanolic extract (20 µL) was eluted through the column with a gradient mobile phases consisting of A (0.1% acetic acid) and B (acetonitrile). The following multi-step linear solvent gradient was employed: 0–5 min, 2% B, 5–60 min, 40% B, 60–80 min, 100% B, 80–90 min, 2% B, and 90–110 min, 2% B. The ultraviolet (UV) spectra were recorded from 210 to 550 nm and the mass spectra were recorded in negative ionization mode, under the following operating conditions: capillary voltage, 3.2 kV; cone voltage, 40 V; probe temperature, 350°C; ion source temperature, 120 C. The spectra were acquired in the m/z range of 150–1000 amu. The compounds were tentatively identified on the basis of their λ max and tandem mass spectra. Data from Han et al.[Citation17] reporting phenolic constituents for A. annua was also used to corroborate our results.
Antioxidant Capacity
The antioxidant activity of methanolic extract and essential oil was tested using 2,2-diphenyl-pycrilhydrazil hydrate (DPPH) radical scavenging activity (RAS), inhibition of β-carotene bleaching test, ferric reducing power (FRAP) assay, and ferrous ion chelating ability.
Free RAS
DPPH assay was performed as reported by Brand et al.[Citation18] Different concentrations of the sample solution (300 µL) were added to 900 µL of DPPH methanolic solution (4 × 10–5M). The mixture was vortexed and left to stand for 60 min at room temperature before measuring absorbance at 517 nm. The RAS was calculated using the equation: RAS (%) = 100 × [(A0 – A1)/A0]; where A0 and A1 are the absorbance of the control and the absorbance of the sample, respectively. Results were determined as IC50 (concentration required to inhibit 50% of DPPH). Butylated hydroxytolune (BHT) was used as a positive control.
Inhibition of β Carotene Bleaching
The β-carotene bleaching test was carried out as described by Messaoud et al.[Citation19] Two milligrams of β-carotene was dissolved in 20 mL of chloroform. Four milliliters of this solution were mixed with 40 mg of linoleic acid and 400 mg of Tween 40. After evaporation of the chloroform under vacuum at 40°C, 100 mL of oxygenated ultra-pure water were added and the emulsion was vigorously vortexed. At 750 µL of this emulsion, 50 µL of different concentrations of extracts or essential oils previously diluted in methanol were added. The zero time absorbance was measured at 470 nm and the test emulsion was incubated at 50°C for 120 min, when the absorbance was measured again. The percentage of inhibition was determined according to the formula: Inhibition (%) = 100 × [(At – Ct)/(C0 – Ct)]; where At and Ct are the absorbance values measured, respectively, for each test sample and for the control after 120 min. C0 is the absorbance value measured for the control at zero time of incubation. The results were expressed as IC50 (the concentration required to inhibit 50% of the β-carotene bleaching). BHT was used as a positive control.
Ferrous Ion Chelating Capacity
The ferrous ion chelating activity of extract and essential oil was measured according to Yan et al.[Citation20] Briefly, 0.5 mL of different concentrations of extract or essential oil were added to 0.5 mL of FeSO4 solution (0.125 mM), and left for incubation at room temperature for 5 min. Then, the reaction was initiated by adding 0.5 mL of ferrozine (0.3125 mM). The mixture was shaken vigorously and left standing at room temperature for 10 min. Absorbance of the solution was measured at 562 nm. The ability to chelate ferrous ion was calculated using the following formula: Chelating effect (%) = 100 × [(AC – AS)/AC]; where AC is the absorbance of the control and AS represents the absorbance of the tested sample. Results were expressed as IC50 (efficient concentration corresponding to 50% ferrous iron chelating). Ethylenediaminetetraacetic acid (EDTA) was used as a positive control.
FRAP Assay
The FRAP assay was carried out according to Messaoud et al.[Citation19] The FRAP reagent was freshly prepared by mixing acetate buffer (300 mM, pH 3.6), TPTZ solution (10 mM TPTZ in 40 mM HCl) and FeCl3-6H2O (20 mM) in a ratio of 10:1:1. To perform the assay, 900 µL of FRAP reagent, 90 μL distilled water, and 30 μL of methanolic extract or essential oil diluted in methanol were mixed and incubated at 37°C for 30 min. The absorbance was measured at 593 nm, using FRAP working solution as a blank. The antioxidant potential of samples was determined from a standard curve plotted using the FeSO4.7H2O linear regression equation. The results were corrected for dilution and expressed as µmol Fe2+/g DW or essential oil.
Acetylcholinesterase (AChE) Inhibitory Activity
Inhibition of AChE activity was determined according to Eldeen et al.[Citation21] with some modifications. Buffers used in the assay were buffer A (50 mM Tris-HCl, pH 8.0), buffer B (50 mM Tris-HCl, pH 8.0, containing 0.1% bovine serum albumin), and buffer C (50 mM Tris-HCl, pH 8.0, containing 0.1 M NaCl, 0.02 M MgCl2-6H2O). Lyophilized AChE (from the electric eel, type VI-S) was dissolved in buffer A to make 500 U/mL stock solution, and further diluted with buffer B to get 0.28 U/mL. Tested essential oil and extract were dissolved in 5% methanol (in buffer B) and buffer B, respectively. Essential oil or extract (20 µL, at different concentrations) was mixed with 25 μL of AChE (0.28 U/mL) and incubated during 15 min at 37°C. Subsequently, 100 μL of 0.15 mM ATCI in water, 500 μL of 0.3 mM DTNB in Buffer C and 355 μL of Buffer B were added and further incubated for 30 min at 37°C. The absorbance of the reaction mixture was then measured at 405nm. Percentage of inhibition of AChE enzyme was determined by comparison of reaction rates of samples relative to blank sample (5% methanol or buffer B) using the formula:
where Bs is the activity of enzyme without test sample, and Ts is the activity of enzyme with test sample. Results were also expressed as IC50.
Antibacterial Activity
The antibacterial activity of A. herba-alba essential oil and methanolic extract was determined by the diffusion method in a solid medium according to Sacchetti et al.[Citation22] The used bacteria were Staphylococcus aureus (ATCC6538), Escherichia coli (ATCC10536), Pseudomonas aeruginosa (ATCC9027), and Bacillus cereus (ATCC11778). The methanolic extracts were first concentrated by using a rotary evaporator (40°C). The residue was dissolved in DMSO (10%) to obtain 100 mg/mL. Wells (6 mm in diameter) were made in the Petri dishes and 100 µL (equivalent to 10 mg of extract per well) of the filter-sterilized extract was dropped into each well. For essential oil, sterile discs of Whatman filter paper (6 mm in diameter) were impregnated with 10 µL (equivalent to 9.2 mg per disc). Petri dishes were incubated at 4°C for 1 h followed by incubation at 37°C for 24 h before measuring the diameter of the inhibition zone around discs or wells. Gentamycine (30 µg/disc) was used as positive control, and DMSO (10%) was used as negative control.
Statistical Analysis
All tests were performed in triplicate and results were expressed as mean ± standard deviation. Antioxidant, AChE inhibitory, and antibacterial activities of essential oil and extract were statistically compared by analysis of variance (ANOVA) using SAS v. 9.1.3 program.[Citation23]
RESULTS AND DISCUSSION
Essential Oil Composition
Several studies have reported the therapeutic proprieties of A. herba-alba essential oil as an antileshmanial, anthelmintic, and antispasmodic agent.[Citation24] The oil exhibited also antimutagenic activity against carinogen benzopyrene.[Citation25] However, the biological activities of essential oils varied significantly according to the chemical composition and chemotypes.[Citation24,Citation26,Citation27]
In our study, a total of 23 compounds accounting for 97.8% of the oil were identified (). The essential oil was characterized by a high content of oxygenated monoterpenes (85.4%), mainly represented by β-thujone (41.9%), α-thujone (18.4%), camphor (13.2%), 1,8 cineole (3.4%), borneol (3.3%), and chrysanthenone (2.3%). The sesquiterpene hydrocarbon fraction (6.6%) was found dominated by germacrene D (4.8%). Monoterpene hydrocarbons, representing 5.7% of the essential oil, mainly included camphene (2.5%) and α-pinene (1.5%).
TABLE 1 Essential oil composition (%) of Artemisia herba-alba
Several components such as sabinyl acetate, davanone, chrysanthenyl acetate and isochrysanthenone classically reported as dominant compounds in A. herba-alba have not been detected in our study. The diversity of the chemical composition of A. herba-alba essential oil from different geographical regions (i.e., Morocco, Algeria, Spain, Jordan) was reported. Essential oils with a composition dominated (up to 50%) by one major compound (i.e., α-thujone, β-thujone, camphor, chrysanthenone, chrysanthenyl acetate, or davanone), and oils characterized by the occurrence at noticeable contents (25–50%) of two or more of these compounds were distinguished.[Citation5,Citation9] These compositions were considered as chemotypes. So, in our study, the chemotype of the investigated essential oil was β-thujone/α-thujone/camphor.
In Tunisia, besides the chemotype comparable to that identified in our work, others chemotypes including high amounts of α-thujone (up to 40%), 1,8-cineole (up to 15%), chrysanthenone (up to 50%), sabinyl acetate (up to 20%), and davanone (up to 20%) were observed.[Citation9,Citation28] The distribution of different chemotypes seemed to be linked to local selective force acting on genotypes/chemotypes diversity. In Tunisia, the remarkable variation of A. herba-alba essential oil and its relationship with ecological and genetic factors remain unclear. Further studies, based on combined genetic, ecological, and chemical traits performed on most populations in the whole distribution area of the species may provide additional information linked to the highly chemotypic differentiation observed.
Phenolic Content and Composition
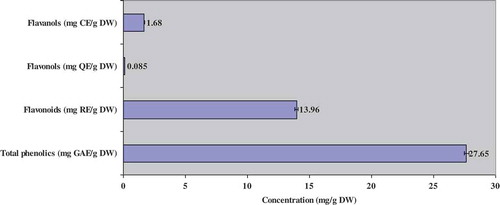
The contents of total phenols, flavonoids, flavonols and flavanols were 27.65 ± 0.08 mg GAE/g DW, 13.96 ± 0.07 mg RE/DW, 0.085 ± 0.001 mg QE/g DW, and 1.68 ± 0.003 mg CE/g DW, respectively (). The content of total phenols and flavonoids, observed in our study, were higher than that reported for samples from the southern part of Tunisia[Citation29,Citation30] or for other Artemisia species such as A. annua, A. arborescens, A. ludoviciana, A. oleandica, A. princeps and A. stelleriana,[Citation31] and Artemisia vulgaris.[Citation32] The contents and the composition of total phenolic and flavonoid of A. herba-alba extracts varied according to plant origin, the solvent of extraction and the analytical method used.[Citation33]
FIGURE 1 Total phenolic, flavonoid, flavanol, and flavonol contents of methanolic extract of Artemisia herba-alba.
The peak chromatogram of the methanolic extract of A. herba-alba resulting from the LC-PDA/ESI-MS analysis was shown in . The characteristics of the detected compounds were summarized in . Of the 15 detected compounds, 10 were identified. Six compounds were identified as C-glycosyl flavonoid (Apigenin-6,8-di-C-glu (3), m/z 593 (vicenin-2); apigenin-6-C-arabinosyl-8-C-glu (5), m/z, 563 (isoschaftoside); apigenin-6-C-glu-8-C-ara (6), m/z 563 (schaftoside); apigenin-6-C-pent-8-C-glu (7), m/z 563; apigenin-6-C-glu-8-C-pent (8), m/z 563 and quercitin-rha-glu (9), m/z 609), and four as phenolic acids (chlorogenic (1), m/z 353; 1,4-dicaffeoylquinic (12), m/z 515; 3,4,5-tricaffeoylquinic (13), m/z 677 and 3,4-dicaffeoylquinic (14), m/z 515, acids). The 1,4-dicaffeoylquinic acid was the dominant compound.
TABLE 2 Identification of phenolic compounds from Artemisia herba-alba methanolic extract
FIGURE 2 LC-PDA-TIC profile of the methanolic extract, and UV spectra and mass spectra of major phenolic compounds of A. herba-alba. The peak assignments are listed in .
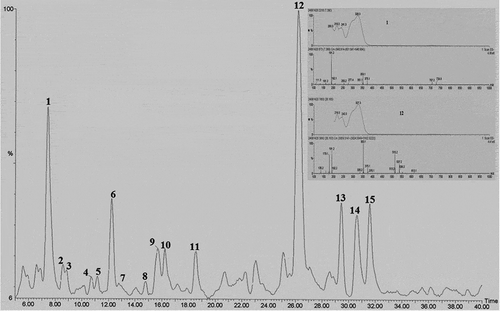
TABLE 3 Antioxidant and antiacetylcholinesterase activities of methanolic extract and essential oil of Artemisia herba-alba
Similar phenolic compounds were identified in limited species of Artemisia such as A. annua,[Citation17] A. rupestris,[Citation34] and rarely reported in A. herba-alba. Different quinic acid derivatives, reported for the genus Artemisia,[Citation17,Citation31,Citation32] have been also observed in A. herba-alba.[Citation35] Thus, the use of LC-ESI-PDA-MS will contribute the increase of the reliability and the accuracy of the chemical methods used to separate polyphenols in the species. The identification of caffeoylquinic acids and C-glycosyl flavonoids suggests that the species could play an important role as inhibitor against mutagens, carcinogens, and diabetes.[Citation36] The potential effects of these compounds as analgesic and inhibitor of human immunodeficiency virus (HIV) infection had been also reported.[Citation37,Citation38]
Antioxidant Activity
In general phenolic contents were correlated with those of flavonoid and biological activities.[Citation31,Citation39,Citation40] The biological efficiency of extracts depends on the experimental method, the nature, and the concentration of extracts.[Citation41] The antioxidant activities of the methanolic extract and the essential oil of A. herba-alba, determined in our study through four complementary methods, were shown in . The methanolic extract exhibited considerable antioxidant activity. IC50 values were 100, 524, and 1720 µg/mL for DPPH, β-carotene bleaching, and ferric chelating assays, respectively. The ferric-reducing power was 372 µmol Fe2+/g. Several glycosyl flavonoids and phenolic acids, mainly caffeoylquinic acids, present in the extract have been identified as dominant antioxidant compounds.[Citation35,Citation42] The presence of these compounds in our extract should be the main cause of its high antioxidant power.
The essential oil also exhibited remarkable antioxidant activities but lower than those reported for methanolic extract. The IC50 values determined by DPPH scavenging capacity, β-carotene bleaching test and ion chelating assay were respectively IC50 = 5030 µg/mL; IC50 = 1590 µg/mL and IC50 = 2300 µg/mL. The ferric-reducing power was 79 µmol Fe2+/g. The antioxidant abilities of essential oil of A. herba-alba were attributed to its richness in monoterpenes and/or to the synergistic effect of more than one oil compound.[Citation43–Citation45]
Antiacetylcholinesterase Activity
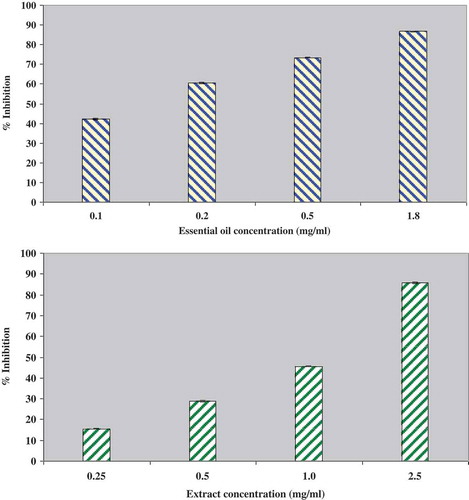
The essential oil and the methanol extract of Artemisia herba-alba were screened for their possible anticholinesterase activity. AChE, the responsible enzyme of the hydrolysis of acetylcholine is involved in the development of Alzheimer’s disease. The AChE inhibitor activity of both extracts was found to increase dose-dependently (). According to our results, the essential oil showed stronger activity than the methanol extract. Furthermore, the IC50 values, calculated from the regression equations obtained from the activity of samples at different concentrations, were 1200 ± 167 µg/mL for methanolic extract and 165 ± 1.1 µg/mL for essential oil ().
FIGURE 3 Dose-dependent anticholinesterase inhibitory activity of essential oil and methanolic extractof A. herba alba.
The methanolic extract activity can be explained, in part, by the presence of caffeoylquinic acid derivatives and C-glycosyl flavonoids including C-glycosyled derivatives of apigenin, which were reported to have strong anti-Alzheimer’s disease.[Citation46,Citation47] In a recent study, acetone and ethanol extracts of A. herba-alba were tested against AChE and butyrylcholinesterase enzymes.[Citation48] Ethanol extract had exhibited antiacetylcholinesterase activity at several concentrations (25, 50, and 100 µg/mL); conversely, acetone extract had been unable to inhibit either enzyme at the same concentrations.
As far as our literature survey could ascertain, little is known about the anticholinesterase activity of essential oil from A. herba-alba. The essential oils obtained from Artemisia absinthium and A. fragrans were tested as AChE inhibitors. At a concentration of 100 µg/mL for each essential oil, the percentage inhibition of AChE ranged from 18.02 to 18.63%.[Citation48] Interestingly, our essential oil exhibited more activity (42.27% of inhibition) at the same tested concentration (100 µg/mL). This activity could be related to the presence of several terpenes, such as α-pinene, p-cymene, 1,8-cineole, ɣ-terpinene, linalool, and camphor, known for their antiacetylcholinesterase activity.[Citation49,Citation50] This suggestion should take account the synergy and antagonism among compounds.
Antibacterial Activity
The effects of A. herba-alba phenolic extract and essential oil the tested bacteria strains were presented in . The diameter of inhibition ranged from 17.0 (Staphylococcus aureus and E. coli) to 20.0 mm (Pseudomonas aeruginosa) for the essential oil. The inhibition activity was slightly less pronounced for phenolic extracts (11.5–15.5 mm), except for Bacillus subtilis (22.5 mm). Gentamycine exhibited more significant inhibition effect than the two sets of extracts (the diameter of inhibition ranged from 20 to 25 mm). These results confirm the substantial antimicrobial activity exhibited by A. herba-alba essential oil and extract. Several major terpenoids or phenolic compounds such as 1,8 cineole, camphor, α-thujone and β-thujone, flavonoid aglycones, and glycosyl flavonoids could be the main responsible of this activity.[Citation51–Citation54] The antibacterial activity of C-glycosyl flavonoids and caffeoylquinic acids remains rarely reported. The bioactivity of these compounds detected in our sample could be suggested. Thus, testing isolate compounds and assessing the relationship between structure-activity should be performed to better understand the physiological function of these components.
TABLE 4 Antibacterial activity of methanolic extract and essential oil of Artemisia herba-alba estimated by diameter of inhibition (mm)
CONCLUSION
Our study on the chemical composition of Tunisian Artemisia herba-alba sample confirms the predominance of a common chemotype with high amount of β-thujone. This should not mask the existence of other rare chemotypes reported for local specimens from other areas. The observed infraspecific chemical variation is likely determined by both ecological and genetic factors that should be more clarified in order to conceive improvement programs. This could be achieved by complementary approaches combining the genetic and chemical variations of the species in its whole distribution area. The methanolic extracts, based on LC-ESI/PDA-MS analysis, were mostly represented by C-glycosyl flavonoids and caffeoylquinic acids. The latter phenolic acids have been rarely reported for A. herba-alba. Applying this reliable method to identify phenolic compounds for most Tunisian A. herba-alba populations may enhance our knowledge on their phenolic diversity. Phenols, as well as the essential oil, exhibited considerable antioxidant, AChE inhibitory, and antibacterial activities. These properties associated to those recently reported supporting anti-angiogenic, anti-parasitic, and anti-hyperglycemic effects should highlight the use of the species in a broad health field. At present, the bulk material harvested comes from natural degraded populations with unknown effect on the level of their genetic diversity which determines their in situ maintenance. Our current studies performed on both genetic and chemical structure of the populations via different markers may help to preserve this resource and select genotypes with desirable compounds.
FUNDING
The authors would like to thank the Tunisian Ministry of Scientific Research and Technology and the National Institute of Applied Science and Technology for their financial support (Research grant 99/UR/09-10).
Additional information
Funding
References
- Wang, W.M. On the Origin and Development of Artemisia (Asteraceae) in the Geological Past. Botanical Journal of the Linnean Society 2004, 145, 331–336.
- Pottier-Alapetite, G. Flore de la Tunisie: Angiospermes, Dicotylédones, Gamopétales; Imprimerie Officielle de la République Tunisienne, Tunisia, 1981; 1190 p.
- Vallès, J.; Garcia Giménez, S.; Hidalgo, O.; Martin, J.; Pellicer, J.; Sanz, M.; Garnatje, T. Biology, Genome Evolution, Biotechnological Issues, and Research Including Applied Perspectives in Artemisia (Asteraceae). Advances in Botanical Research 2011, 60, 349–419.
- Nazar, N.; Mahmood, T. Morphological and Molecular Characterization of Selected Artemisia Species from Rawalakot, Azad Jammu, and Kashmir. Acta Physiologiae Plantarum 2011, 33, 625–633.
- Salido, S.; Valenzuela, L.R.; Altarejos, J.; Nogueras, M.; Sánchez, A.; Cano, E. Composition and Infraspecific Variability of Artemisia Herba-Alba from Southern Spain. Biochemical Systematics and Ecology 2004, 32, 265–277.
- Mohamed, A.E.H.; El-Sayed, M.A.; Hegazy, M.E.; Helaly, S.E.; Esmail, A.M.; Mohamed, N.S. Chemical Constituents and Biological Activities of Artemisia Herba Alba. Records of Natural Products 2010, 4, 1–25.
- Kadri, A.; Chobba, I.B.; Zarai, Z.; Békir, A.; Gharsallah, N.; Damak, M.; Gdoura, R. Chemical Constituents and Antioxidant Activity of the Essential Oil from Aerial Parts of Artemisia Herba-Alba Grown in Tunisian Semi-Arid Region. African Journal of Biotechnology 2011, 10, 2923–2929.
- Messaoudene, D.; Belguendouz, H.; Ahmedi, M.-L.; Benabdelkader, T.; Otmani, F.; Terahi, M.; Youinou, P.; Touil-boukoffa, C. Ex Vivo Effects of Flavonoids Extracted from Artemisia Herba Alba on Cytokines and Nitric Oxide Production in Algerian Patients with Adamantiades-Behcet’s Disease. Journal of Inflammation 2011, 8, 35–44.
- Mighri, H.; Akrout, A.; El-Jeni, H.; Zaidi, S.; Tomi, F.; Casanova, J.; Neffati, M. Composition and Intraspecific Chemical Variability of the Essential Oil from Artemisia Herba-Alba Growing Wild in a Tunisian Arid Zone. Chemistry & Biodiversity 2010, 7(11), 2709–2717.
- Al-Ghzawi, A.L.A.; Juma’a, K.A.; Al-Rawashdeh, I.M. Genetic and Chemical Variations Among Wild Populations of a Medicinal Plant (Artemisia Herba-Alba Asso.) Collected from Different Regions in Jordan. Journal of Food, Agriculture, & Environment 2012, 10, 26–31.
- Le Houérou, H.N. Tunisian steppic vegetation (with references to the analogous vegetations of Algeria, Libya and Morocco). Annals of the National Institute of Agronomic Research 1969, 42(5), 1–624.
- Haouari, M.; Firchichi, A. Study of Genetic Polymorphism of Artemisia Herba-Alba from Tunisia Using ISSR Markers. African Journal of Biotechnology 2008, 7, 44–50.
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. American Journal of Enology and Viticulture 1965, 16(3), 144–158.
- Djeridane, A.; Yousfi, M.; Nadjemi, B.; Boutassouna, D.; Stocker, P.; Vidal, N. Antioxidant Activity of Some Algerian Medicinal Plants Extracts Containing Phenolic Compounds. Food Chemistry 2006, 97, 654–660.
- Adedapo, A.A.; Jimoh, F.O.; Koduru, S.; Afolayan, A.J.; Masika, P.J. Antibacterial and Antioxidant Properties of the Methanol Extracts of the Leaves and Stems of Calpurnia Aurea. BMC Complementary and Alternative Medicine 2008, 8(53), 1–8.
- Li, Y.-G.; Tanner, G.; Larkin, P. The DMACA-HCl Protocol and the Threshold Proanthocyanidin Content for Bloat Safety in Forage Legumes. Journal of the Science of Food and Agriculture 1996, 70, 89–101.
- Han, J.; Ye, M.; Qiao, X.; Xu, M.; Wang, B.-R.; Guo, D.A. Characterization of Phenolic Compounds in the Chinese Herbal Drug Artemisia Annua by Liquid Chromatography Coupled to Electrospray Ionization Mass Spectrometry. Journal of Pharmaceutical and Biomedical Analysis 2008, 47, 516–525.
- Brand, W.; Cuvelier, W.; Berset, M.E. Use of a Free Radical Method to Evaluate Antioxidant Activity. Lebensmittel Wissenschaft Technologie 1995, 28, 25–30.
- Messaoud, C.; Laabidi, A.; Boussaid, M. Myrtus Communis L. Infusions: The Effect of Infusion Time on Phytochemical Composition, Antioxidant, and Antimicrobial Activities. Journal of Food Science 2012, 77, 941–947.
- Yan, L.Y.; Teng, L.T.; Jhi, T.J. Antioxidant Properties of Guava Fruits: Comparison with Some Local Fruits. Sunway Academic Journal 2006, 3, 9–20.
- Eldeen, I.M.S.; Elgorashi, E.E.; Van Staden, J. Antibacterial, Anti-Inflammatory, Anticholinesterase, and Mutagenic Effects of Extracts Obtained from Some Trees Used in South African Traditional Medicine. Journal of Ethnopharmacology 2005, 102, 457–464.
- Sacchetti, G.; Maietti, S.; Muzzoli, M.; Scaglianti, M.; Manfredini, S.; Radice, M.; Bruni, R. Comparative Evaluation of 11 Essential Oils of Different Origin As Functional Antioxidants, Antibacterials and Antimicrobials in Foods. Food Chemistry 2005, 91, 621–632.
- SAS Institute. OnlineDoc. Version 9.1.3; Statistical Analysis System Institute: Cary, NC, 2004.
- Yashphie, J.; Feuerstein, I.; Barel, S.; Segal, R. The Antibacterial and Antispasmodic Activity of Artemisia Herba-Alba Asso. Examination of Essential Oils from Various Chemotypes. International Journal of Crude Drug Research 1987, 25(2), 89–96.
- Neffati, A.; Skandrani, I.; Ben Sghaier, M.; Bouhlel, I.; Kilani, S.; Ghedira K.; Neffati, M.; Chraief, I.; Hammami, M.; Chekir-Ghedira, L. Chemical Composition, Mutagenic, and Antimutagenic Activities of Essential Oils from (Tunisian) Artemisia Campestris and Artemisia Herba-Alba. Journal of Essential Oil Research 2008, 20(5), 471–477.
- Kazemi, M. Phytochemical and Antioxidant Properties of Achillea Millefolium from the Eastern Region of Iran. International Journal of Food Properties 2015, 18(10), 2187–2192.
- Jaouadi, I.; Koparal, A.T.; Bostancıoğlu, R.B.; Yakoubi, M.T.; El Gazzah, M. The Anti-Angiogenic Activity of Artemisia Herba-Alba’s Essential Oil and Its Relation with the Harvest Period. Australian Journal of Crop Science 2014, 8, 1395–1401.
- Haouari, M.; Ferchichi, A. Essential Oil Composition of Artemisia Herba-Alba from Southern Tunisia. Molecules 2009, 14, 1585–1594.
- Akrout, A.; Mighri, H.; Krid, M.; Thabet, F.; Turki, H.; El-Jani, H.; Neffati, M. Chemical Composition and Antioxidant Activity of Aqueous Extracts of Some Wild Medicinal Plants in Southern Tunisia. International Journal of Life Science and Medical Research 2012, 2, 1–4.
- Khlifi, D.; Sghaier, R.M.; Amouri, S.; Laouinin, D.; Hamdi, M.; Bouajila, J. Composition and Antioxidant, Anti-Cancer, and Anti-Inflammatory Activities of Artemisia Herba-Alba, Ruta Chalpensis L. and Peganum Harmala L. Food and Chemical Toxicology 2013, 55, 202–208.
- Carvalho, I.S.; Cavaco, T.; Brodelius, M. Phenolic Composition and Antioxidant Capacity of Six Artemisia Species. Industrial Crops and Products 2011, 33, 382–388.
- Melguizo-Melguizo, D.; Diaz-de-Cerio, E.; Quirantes-Piné, R.; Švarc-Gajić, J.; Segura-Carretero, A. The Potential of Artemisia Vulgaris Leaves As a Source of Antioxidant Phenolic Compounds. Journal of Functional Foods 2014, 10, 192–200.
- Seddik, K.; Nadjet, I.; Abderrahmane, B.; Daoud, H.; Lekhmici, A. Antioxidant and Antibacterial Activities of Extracts from Artemisia Herba Alba Asso. Leaves and Some Phenolic Compounds. Journal of Medicinal Plants Research 2010, 4(13), 1273–1280.
- Gu, D.; Yang, Y.; Abdulla, R.; Aisa, H.A. Characterization and Identification of Chemical Compositions in the Extract of Artemisia Rupestris L. by Liquid Chromatography Coupled to Quadrupole Time of Flight Tandem Mass Spectrometry. Rapid Communications in Mass Spectrometry 2012, 26, 83–100.
- Dahmani-Hamzaoui, N.; Salido, S.; Linares-Palomino, P.J.; Baaliouamer, A.; Altarejos, J. On-Line Radical Scavenging Detection and Characterization of Antioxidants from Artemisia Herba-Alba. Helvetica Chimica Acta 2012, 95, 564–576.
- Patel, M.B.; Mishra, S.H. Hypoglycemic Activity of C-Glycosyl Flavonoid from Enicostemma Hyssopifolium. Pharmaceutical biology 2011, 49(4), 383–391.
- dos Santos, M.D.; Gobbo-Neto, L.; Albarella, L.; de Souza, G.E.P.; Lopes, N.P. Analgesic Activity of Di-Caffeoylquinic Acids from Roots of Lychnophora Ericoides (Arnica da Serra). Journal of Ethnopharmacology 2005, 96, 545–549.
- Nakajima, Y.; Shimazawa, M.; Mishima, S.; Hara, H. Water Extract of Propolis and Its Main Constituents, Caffeoylquinic Acid Derivatives, Exert Neuroprotective Effects Via Antioxidant Actions. Life Sciences 2007, 80, 370–377.
- Katalinic, V.; Mozina, S.S.; Generalic, I.; Skroza, D.; Ljubenkov, I.; Klancnik, A. Phenolic Profile, Antioxidant Capacity, and Antimicrobial Activity of Leaf Extracts from Six Vitis Vinifera L. Varieties. International Journal of Food Properties 2013, 16(1), 45–60.
- Sant’Ana, L.D.O.; Buarque Ferreira, A.B.; Lorenzon, M.C.A.; Berbara, R.L.L.; Castro, R.N. Correlation of Total Phenolic and Flavonoid Contents of Brazilian Honeys with Colour and Antioxidant Capacity. International Journal of Food Properties 2014, 17(1), 65–76.
- Wong, J.Y.; Matanjun, P.; Ooi, Y.B.H.; Chia, K.F. Evaluation of Antioxidant Activities in Relation to Total Phenolics and Flavonoids Content of Selected Malaysian Wild Edible Plants by Multivariate Analysis. International Journal of Food Properties 2014, 17(8), 1763–1778.
- Lee, Y.J.; Thiruvengadam, M.; Chung, I.M.; Nagella, P. Polyphenol Composition and Antioxidant Activity from the Vegetable Plant Artemisia Absinthium L. Australian Journal of Crop Science 2013, 7(12), 1921–1926.
- Kelen, M.; Tepe, B. Chemical Composition, Antioxidant, and Antimicrobial Properties of the Essential Oils of Three Salvia Species from Turkish Flora. Bioresource Technology 2008, 99(10), 4096–4104.
- Kazemi, M. Gas Chromatography-Mass Spectrometry Analyses for Detection and Identification of Antioxidant Constituents of Achillea Tenuifolia Essential Oil. International Journal of Food Properties 2015, 18(9), 1936–1941.
- Amiri, H. Chemical Composition and Antioxidant Activity of Essential Oil and Methanolic Extracts of Ferula Microcolea (Boiss.) Boiss (Apiaceae). International Journal of Food Properties 2014, 17(4), 722–730.
- Orhan, I.; Kartal, M.; Tosun, F.; Sener, B. Screening of Various Phenolic Acids and Flavonoid Derivatives for Their Anticholinesterase Potential. Zeitschrift für Naturforschung. C, A Journal of Biosciences 2007, 62(11), 829.
- Choi, J.S.; Islam, M.N.; Ali, M.Y.; Kim, E.J.; Kim, Y.M.; Jung, H.A. Effects of C-Glycosylation on Anti-Diabetic, Anti-Alzheimer’s Disease, and Anti-Inflammatory Potential of Apigenin. Food and Chemical Toxicology 2014, 64, 27–33.
- Erdogan Orhan, I.; Belhattab, R.; Senol, F.S.; Gülpinar, A.R.; Hosbas, S.; Kartal, M. Profiling of Cholinesterase Inhibitory and Antioxidant Activities of Artemisia Absinthium, A. Herba-Alba, A. Fragrans, Marrubium Vulgare, M. Astranicum, Origanum Vulgare Subsp. Glandulossum, and Essential Oil Analysis of Two Artemisia Species. Industrial Crops and Products 2010, 32, 566–571.
- Savelev, S.; Okello, E.; Perry, N.S.L.; Wilkins, R.M.; Perry, E.K. Synergistic and Antagonistic Interactions of Anticholinesterase Terpenoids in Salvia Lavandulaefolia Essential Oil. Pharmacology Biochemistry and Behavior 2003, 75(3), 661–668.
- Öztürk, M. Anticholinesterase and Antioxidant Activities of Savoury (Satureja Thymbra L.) with Identified Major Terpenes of the Essential Oil. Food Chemistry 2012, 134(1), 48–54.
- Liu, X.T.; Winkler, A.L.; Schwan, W.R.; Volk, T.J.; Rott, M.; Monte, A. Antibacterial Compounds from Mushrooms II: Lanostane Triterpenoids and An Ergostane Steroid with Activity Against Bacillus Cereus Isolated from Fomitopsis Pinicola. Planta Medica 2010, 76, 464–466.
- Sbayou, H.; Ababou, B.; Boukachabine, K.; Manresa, A.; Zerouali, K.; Amghar, S. Chemical Composition and Antibacterial Activity of Artemisia Herba-Alba and Mentha Pulegium Essential Oils. Journal of Life Sciences 2014, 8(1), 35–41.
- Ahmadizadeh, C.; Monadi, A.; Rezaie, A.; Pashazadeh, M.; Jafari, B. Antibacterial Activity of Methanolic Extract and Essence of Sagebrush (Artemisia Vulgaris) Against Pathogenic Bacteria. Bulletin of Environment, Pharmacology, and Life Sciences 2014, 3, 121–125.
- Pirbalouti, A.G.; Neshat, S.H.; Rahimi, E.; Hamedi, B.; Malekpoor, F. Chemical Composition and Antibacterial Activity of Essential Oils of Iranian Herbs Against Staphylococcus Aureus Isolated from Milk. International Journal of Food Properties 2014, 17(9), 2063–2071.
