Abstract
The nanoprecipitation method has been investigated to obtain particles in a nanometric and micrometric scale. The particles obtained by this method have been applied in recent years in the food and agricultural industries. Variations of the method as flash nanoprecipitation and two-step nanoprecipitation are explained in this article, besides its relation with the ouzo effect. This work presents an overview of the nanoprecipitation method, its advantages, and the potential applications in the food and agricultural industries for improving the quality of food products.
INTRODUCTION
The application of new technologies in the food industry has been highly researched in the last few decades to obtain benefits in terms of safety, health, and products with high quality.[Citation1] Nanotechnology is a science that offers favorable conditions and qualities for applications in this specific industry and other industries, providing a good alternative for control and food production.[Citation2] The nanoprecipitation method is a good option for the development of nanoparticles as nanospheres, having several advantages—the procedure takes only a short time, only a small amount of raw material is required, and it consumes a low amount of energy.[Citation3–Citation5] Nanoprecipitation was first described by Fessi et al.[Citation6] in 1989. Over the years, several important method variations have resulted as flash nanoprecipitation (FNP) and two-step nanoprecipitation.[Citation7,Citation8] In recent years, the increasing world population requires an increasing quantity and quality of foodstuffs. Accordingly, the exploitation of agricultural fields and the application of fertilizers and pesticides have increased, resulting in soil depletion and environmental contamination. For these reasons, the implementation of nanomaterials developed from natural and biodegradable sources has been important as a viable option for improving conditions and quality of food from agricultural fields. Also, the nanoscale systems are an alternative option for encapsulation of fertilizers, nutrients, and bactericidal compounds.[Citation9,Citation10] In this review, we explore the remarkable importance of the nanoprecipitation method for developing novel nanoparticles for application within the food industry and agricultural fields.
NANOPARTICLES AND MICROPARTICLES
Nanoparticles and microparticles have always been present as part of all materials on this planet. Nanoparticles are defined as particles that are smaller than 1000 nm in diameter, and variation in size will depend on the obtention method and raw material.[Citation11–Citation14] Currently, the literature has become more stringent, attesting that nanoparticles should not exceed 200 nm.[Citation15]
The size of nanoparticles could affect physicochemical stability, biological activity, and especially the characteristics of encapsulation and release of active compounds.[Citation16] Moreover, nanoparticles are defined as sub-micro sized solids that act as carriers of drugs, components of electrical appliances, construction materials, and as metal bound compounds, catalysts, etc., and they can be made from biodegradable or nonbiodegradable materials.[Citation17,Citation18] Thereby, “nanoparticle” is a general term and may include nanospheres and nanocapsules, among others.[Citation19]
During nanoparticle formation, the highly polar particles tend to be solubilized in water, while the particles with nonpolar surfaces tend to interact with other nonpolar particles and form aggregates in an aqueous medium. Whereby, it can be ascertained that nanoparticles can have a positive, negative, or neutral charge, depending on the materials used to develop them and their environment.[Citation16]
Nanomaterials can be classified as nanotubes, nanofibers, nanowhiskers, nanosheets, and nanospheres, where nanotubes and nanofibers have a diameter ratio of 3:1. Nanofibers are solid structures, while nanotubes are hollowed structures, and nanowhiskers are very fine nanofibers, ranging up to 20 nm in cross-section, and length can reach several micrometers. Nanosheets only have a dimension of the material in nanoscale.[Citation20] Nanospheres contain a structural matrix, and the drugs can be linked to the surface by groups that favor binding of compounds, such as COOH, NH2, OH, etc.[Citation21] Moreover, they may be encapsulated within the particle, which can be defined as a vesicular drug system bordering a central cavity (core) and coated by a polymeric membrane (shell).[Citation22–Citation32]
Furthermore, microparticles are defined as structures with dimensions less than 1000 µm and greater than 1 µm, which can also be obtained from biodegradable and non-biodegradable materials.[Citation33] A variety of materials can be used to prepare nanoparticles and microparticles using different methods, e.g., nanoprecipitation, emulsion diffusion, double emulsion, etc.[Citation34–Citation40] The polymers most commonly used for preparing microparticles are polylactic acid (PLA), poly (D, L-lactic-co-glycolic acid, PLGA), polystyrene, and polyacrylamide.[Citation41–Citation44] Also, some inorganic materials for producing microspheres can be used as zeolites, silicas, and ceramics.[Citation45]
Nanoparticles have been booming in recent years because of their advantage as drug carriers in treating different diseases. Since this was the first application in the pharmaceutical industry, they are considered very important in biomedical and pharmaceutical research,[Citation46] and interest has been increasing through developing nanoparticles that have been used to target cancer tumors.[Citation47]
Nanoparticles have also been developed by using not only synthetic materials but also natural sources, such as proteins, gelatin, gums, chitosan, hyaluronic acid, some polysaccharides, cellulose, agarose, dextran, and starch.[Citation48–Citation51] Materials based on nanoparticles and microparticles are now used in the food industry because of their various applications and possible uses they may have.
APPLICATIONS OF NANOPARTICLES AND MICROPARTICLES IN THE FOOD INDUSTRY
Agriculture has always been a common practice and is a primary source of food. Better agronomic practices are always required to meet the needs of the world population.[Citation52] Using land to produce agricultural products results in soil degradation and pollution of the product and the environment by fertilizers and pesticides, causing an alteration of the nitrogen and phosphorus cycles.
The issues that have arisen while developing agricultural products demanded by global requirements have resulted in implementing new technologies to generate an advantage and get good land productivity.[Citation52] Using materials at the nanometric and micrometric scale is a technology that has been studied extensively in recent years, which can help implement alternative fertilization in agriculture to improve yields and product quality.[Citation53,Citation54] It is convenient, however, to use nanoparticles because of the greater area of contact (reaction) when compared with the microparticles.[Citation55,Citation56] Nanomaterials are being investigated for their wide applications worldwide and already have several functions in the areas of food safety and sanitation.[Citation57] In this sense, Kayaci et al.[Citation58] generated nanofibers from a complex of cyclodextrin and encapsulated eugenol. The results showed that these nanofibers have thermal stability and release eugenol, which is a natural compound extracted from plants and used as a fragrance, flavoring, and natural preservative in the food industry because of its antibacterial and antifungal properties and antioxidant activity.[Citation58] On the other hand, Antiochia et al.[Citation59] developed single-walled carbon nanotube paste, in which they immobilized two enzymes, invertase, and fructose dehydrogenase, with application as biosensors for the detection of sucrose and fructose in some commercial fruit juices. As a result, they had a low detection limit, high sensitivity, high reproducibility, and fast response compared with a commercial spectrophotometric enzymatic kit. Finally, they concluded that development and improvement of such biosensors are important for application in food quality and sanitary control.[Citation59] Moreover, Zhang et al.[Citation60] developed nanorods as sanity indicators for perishable products. They used the reaction of epitaxial overgrowth of Ag-Au shell nanorods to reveal product quality based on an indicator in the package that measures product temperature, which is directly related to the organisms that might be developed. They concluded that this method can greatly affect the food, pharmaceutical, and cosmetic industries.[Citation60] For these applications, using nanomaterials in the food and agriculture industries is not discarded, and both could have been used to combat bacterial contamination as slow and controlled release systems.[Citation61–Citation63]
NANOPRECIPITATION
Nanoprecipitation, also known as antisolvent precipitation, desolvation, solvent displacement, and solvent shifting,[Citation64] was described by Fessi et al.[Citation6] in 1989 and is a method for developing nanoparticles and microparticles. This technique has several advantages compared with other methods, among which stand out the facility to develop nanoparticlesin in one step, not much expense is involved, low electric power is required, and it is fast.[Citation3,Citation4] On the other hand, emulsion-diffusion methods, emulsion-evaporation, and precipitation by salting-out need a precursor emulsion, while nanoprecipitation does not.[Citation65]
The nanoprecipitation technique can also usually produce nanoparticles in the range of 50 to 300 nm, which is an advantage because the smaller particle size generates greater contact area. This characteristic is important for its application in adsorption and desorption systems.[Citation66–Citation68] The size of the nanoparticles depends, however, on the raw material, the concentration of the polymer used, the miscibility of the solvent, the type of agitation, and in general terms, the applied methodology.[Citation66] Furthermore, this method according Bilati et al.[Citation3] should not use an excessive amount of or prominent stirring, involve high temperatures, and not to create oil-water interfaces.[Citation69] A few years later, Lucas et al.[Citation70] reported that the use of surfactant in the process is not necessary, providing an advantage for obtaining surfactant-free particles.[Citation70] This method can develop particles of about 170 nm, with this size increasing the opportunities of applications and efficiency.
Development of Nanoparticles through Simple Nanoprecipitation
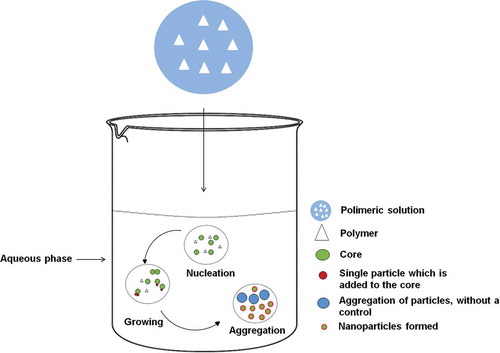
Developing nanoparticles is explained by nucleation theory (), which involves several steps: particle nucleation, growth, and aggregation.[Citation71] Nucleation occurs when the concentration of polymer reaches the critical limit of saturation, i.e., when the breaking of the interface between the polymer and solvent is carried out through the addition of the aqueous phase. Growth occurs with a release of energy, the particles being added to the core by growth through condensation or coagulation. During the aggregation, a release of energy is also present. In this step, it is important to maintain control by some type of stir, which will help homogenize the nanoparticles and achieve uniformity. The temperature also directly affects the rate aggregation. Furthermore, polymer and stabilizer concentrations must be controlled because high concentrations of both can induce excessive aggregation.[Citation72–Citation76]
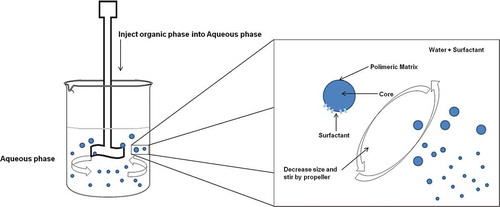
The method described by Fessi et al.[Citation6] has been modified several times. It creates an organic phase and an aqueous phase. The organic phase contains a solvent that must be miscible or partially miscible in the aqueous phase; the polymer (synthetic or natural), which will be used to create the polymer matrix of the nanoparticles, must be soluble in the solvent and therefore insoluble in the aqueous phase. The active ingredient used must be soluble in the solvent, and it must have some interaction with the polymeric matrix to be formed, and the aqueous phase will be constituted solely of water and a surfactant (stabilizer, tensoactive). During the process, an organic phase is add by dropwise to the aqueous phase under moderate magnetic stirring.[Citation77,Citation78] Another option is to quickly add the aqueous phase to the organic phase, inducing an instant precipitation, mainly in nanoscale.[Citation79]
The basis of this technique involves an organic phase (solvent mix) being added into the aqueous phase. The solvent phase tends to have an effect of diffusion, while the polymer automatically tends to collapse forming nanoparticles or microparticles that can encapsulate an active ingredient that is contained in the organic phase (). Some important factors to be considered are the characteristics of the raw material, the solvent that has to dissolve the active ingredient and the polymer, and also the effect of diffusion in the aqueous phase must be present. The polymer must be insoluble in the aqueous phase with the objective of showing aggregation.[Citation80–Citation91] The precipitation of the polymer is given by the increase of diffusion of the solvent, adding a larger amount of non-solvent, or by evaporating the solvent.[Citation21] The characteristics of the nanoparticles formed by the nanoprecipitation technique will vary, depending on the modifications to be made to the organic phase and aqueous phase as well as physical variables to be applied to the technique.[Citation92–Citation95] Among the variations involved in this method, we can mention the following: FNP, two-step nanoprecipitation, and the ouzo effect.
FNP
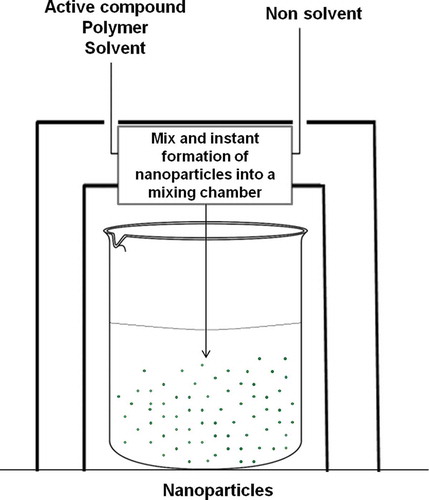
FNP was developed at Princeton University and is a modification of a controlled precipitation that produces small, highly reproducible particles, with a higher load of hydrophobic compounds.[Citation96] Basically, the precipitation of an active compound (organic phase) in a non-solvent phase specifically is mixed in separately or beside the active compound and the polymer in a suitable solvent for both. At the same time, the organic phase and non-solvent phase are added into a mixing chamber. A reaction can be performed in a micro reactor, which carries out the formation of nanoparticles.[Citation73] Moreover, the mix of the polymer with the active compound and the solvent can be added to a central cavity or base. At the same time, the non-solvent phase is introduced, which creates an instantaneous diffusion of the solvent into the non-solvent so that the polymer tends to precipitate and form nanoparticles instantaneously (). This method has the same technical principle as normal nanoprecipitation, but the advantage is that the nanoparticles develop instantly, i.e., within milliseconds or seconds, and the distribution of particle size is better than the original nanoprecipitation. Nanoparticles in the range of 100 to 300 nm are produced compared with FNP, with particles ranging in size from 50 to 150 nm.[Citation97]
Two-Step Nanoprecipitation
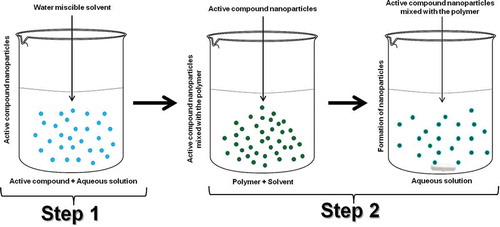
The two-step nanoprecipitation method was designed as a solution to the problem of solvent selection that could dissolve the polymer and the active ingredient. Likewise, this solvent will not affect polymer structure and thus the functionality.[Citation8] This technique consists of two steps: first to induce nanoprecipitation of the active ingredient by action of the solvent, generating a suspension. Then a solvent is used to dissolve the polymer and have a second nanoprecipitation that encapsulates the active ingredient ().[Citation98]
Ouzo Effect
The ouzo effect is a method that can create emulsions of various solutes, such as polymers, lipids, or pharmaceutical compounds, which are added to an aqueous phase to form a simple and spontaneous emulsification. This method allows preparation of microparticles in the form of drops in either a solid or liquid state. Formation of nanoparticles is also possible by a simple modification of the process, either by changing the solute used in the aqueous phase or by combining various solutes.[Citation99]
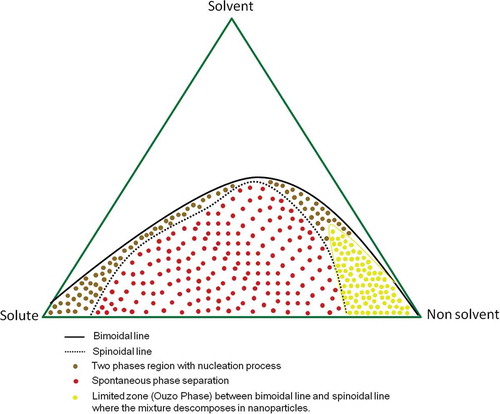
The ouzo effect is also known as spontaneous emulsification, solvent shifting, coacervation with addition of a non-solvent, solvent displacement process, or nanoprecipitation (surfactant free). It is an emulsification process that requires low energy and no surfactant.[Citation70,Citation91,Citation99] The effect can be explained as the quick coupling of the hydrophobic compound in the “ouzo” or metastable region between the spinoidal and binoidal limits shown in a ternary phase diagram. When both the miscibility and stability curves reach their limits, the curves are taken to an oversaturation of the hydrophobic compound which leads to the nucleation process and the end product particle formation ().[Citation66]
POSSIBLE APPLICATIONS OF THE NANOPRECIPITATION METHOD IN AGRICULTURE AND THE FOOD INDUSTRY
The nanoprecipitation technique has been useful in several fields. Nanoparticles have been made from starch and protein because of their physicochemical properties.[Citation100,Citation101] Developing nanoparticles using organic raw material as food, in this case proteins, is a great advantage because these same nanoparticles can be harnessed and applied to the same foods.[Citation53] Protein nanoparticles can be combined with chemical or organic compounds for food application.
Gliadins are wheat gluten proteins that have been used for the production of nanoparticles by various methods, one of which is nanoprecipitation, but implementation has only occurred in the field of pharmacology, although they could also be applied in the food and agriculture industry.[Citation102–Citation107] Development of nanoparticles from natural raw materials is favorable for improving food from agricultural fields and also to avoid environmental damage. The nanoprecipitation method has also been used to develop nanoparticles with possible applications in the food industry.
Noronha et al.[Citation63] developed poly ɛ-caprolactone (PCL) nanocapsules loaded with α-tocopherol. PCL is a polyester that is nontoxic, biodegradable, and biocompatible and has been used to release drugs. The nanoprecipitation method was used to prepare and load nanoparticles, and these authors concluded that the method is suitable for developing loaded PCL nanocapsules of α-tocopherol. They have a potential application as food antioxidants and preservatives in food packaging.[Citation63] Later, Noronha et al.[Citation108] developed biodegradable methylcellulose films with α-tocopherol nanocapsules incorporated. Methylcellulose is a polymer used in food packaging. The nanocapsules were developed through the nanoprecipitation technique, obtaining successful encapsulation and films with antioxidant potential because of α-tocopherol nanocapsules. Furthermore, they found an effect of protection versus ultraviolet (UV) and visible light to avoid photooxidation and concluded that the films with α-tocopherol could be beneficial for food preservation.[Citation108]
CONCLUSIONS
Nanoprecipitation is a method that compared with emulsion-diffusion techniques, double emulsification, and others has several advantages. The process is easy, presenting a good result in terms of particle size and encapsulation. Furthermore, using natural polymers as proteins to develop nanomaterials or micromaterials has potential applications for the agri-food industries to improve the quality and yield of products. Implementing new technologies could be very beneficial for improving agricultural products during fertilization using materials in the nano and micro range.
REFERENCES
- Azeem, B.; KuShaari, K.; Man, Z.B.; Basit, A.; Thanh, T.H. Review on Materials and Methods to Produce Controlled Release Coated Urea Fertilizer. Journal of Controlled Release 2014, 181, 11–21.
- Blasco, C.; Pico, Y. Determining Nanomaterials in Food. TrAC Trends in Analytical Chemistry 2011, 30 (1), 84–99.
- Bilati, U.; Allémann, E.; Doelker, E. Development of a Nanoprecipitation Method Intended for the Entrapment of Hydrophilic Drugs into Nanoparticles. European Journal of Pharmaceutical Sciences 2005, 24 (1), 67–75.
- Shakeri, F.; Shakeri, S.; Hojjatoleslami, M. Preparation and Characterization of Carvacrol Loaded Polyhydroxybutyrate Nanoparticles by Nanoprecipitation and Dialysis Methods. Journal of Food Science 2014, 79 (4), N697–N705.
- Mora-Huertas, C.E.; Garrigues, O.; Fessi, H.; Elaissari, A. Nanocapsules Prepared Via Nanoprecipitation and Emulsification–Diffusion Methods: Comparative Study. European Journal of Pharmaceutics and Biopharmaceutics 2012, 80 (1), 235–239.
- Fessi, H.P.F.D.; Puisieux, F.; Devissaguet, J.P.; Ammoury, N.; Benita, S. Nanocapsule Formation by Interfacial Polymer Deposition Following Solvent Displacement. International Journal of Pharmaceutics 1989, 55 (1), R1–R4.
- Zhang, C.; Pansare, V.J.; Prud’Homme, R.K.; Priestley, R.D. Flash Nanoprecipitation of Polystyrene Nanoparticles. Soft Matter 2012, 8 (1), 86–93.
- Morales-Cruz, M.; Flores-Fernández, G.M.; Morales-Cruz, M.; Orellano, E.A.; Rodriguez-Martinez, J.A.; Ruiz, M.; Griebenow, K. Two-Step Nanoprecipitation for the Production of Protein-Loaded PLGA Nanospheres. Results in Pharma Sciences 2012, 2, 79–85.
- Kumari, A.; Yadav, S.K. Nanotechnology in Agri-Food Sector. Critical Reviews in Food Science and Nutrition 2014, 54 (8), 975–984.
- Chen, H.; Seiber, J.N.; Hotze, M. ACS Select on Nanotechnology in Food and Agriculture: A Perspective on Implications and Applications. Journal of Agricultural and Food Chemistry 2014, 62 (6), 1209–1212.
- Joye, I.J.; McClements, D.J. Production of Nanoparticles by Anti-Solvent Precipitation for Use in Food Systems. Trends in Food Science & Technology 2013, 34 (2), 109–123.
- Ezhilarasi, P.N.; Karthik, P.; Chhanwal, N.; Anandharamakrishnan, C. Nanoencapsulation Techniques for Food Bioactive Components: A Review. Food and Bioprocess Technology 2013, 6 (3), 628–647.
- Sripriyalakshmi, S.; Jose, P.; Ravindran, A.; Anjali, C.H. Recent Trends in Drug Delivery System Using Protein Nanoparticles. Cell Biochemistry and Biophysics 2014, 70 (1), 17–26.
- Bonifácio, B.V.; da Silva, P.B.; dos Santos Ramos, M.A.; Negri, K.M.S.; Bauab, T.M.; Chorilli, M. Nanotechnology-Based Drug Delivery Systems and Herbal Medicines: A Review. International Journal of Nanomedicine 2014, 9, 1–15.
- Weiss, J.; Takhistov, P.; McClements, D.J. Functional Materials in Food Nanotechnology. Journal of Food Science 2006, 71 (9), R107–R116.
- Joye, I.J.; Davidov-Pardo, G.; McClements, D.J. Nanotechnology for Increased Micronutrient Bioavailability. Trends in Food Science & Technology 2014, 40 (2), 168–182.
- Kuhlbusch, T.A.; Asbach, C.; Fissan, H.; Göhler, D.; Stintz, M. Nanoparticle Exposure at Nanotechnology Workplaces: A Review. Particle and Fibre Toxicology 2011, 8 (1), 22.
- Sanguansri, P.; Augustin, M.A. Nanoscale Materials Development–A Food Industry Perspective. Trends in Food Science & Technology 2006, 17 (10), 547–556.
- Jyothi, N.V.N.; Prasanna, P.M.; Sakarkar, S.N.; Prabha, K.S.; Ramaiah, P.S.; Srawan, G.Y. Microencapsulation Techniques, Factors Influencing Encapsulation Efficiency. Journal of Microencapsulation 2010, 27 (3), 187–197.
- Cushen, M.; Kerry, J.; Morris, M.; Cruz-Romero, M.; Cummins, E. Nanotechnologies in the Food Industry–Recent Developments, Risks, and Regulation. Trends in Food Science & Technology 2012, 24 (1), 30–46.
- Aschenbrenner, E.; Bley, K.; Koynov, K.; Makowski, M.; Kappl, M.; Landfester, K.; Weiss, C.K. Using the Polymeric Ouzo Effect for the Preparation of Polysaccharide-Based Nanoparticles. Langmuir 2013, 29 (28), 8845–8855.
- Reis, C.P.; Neufeld, R.J.; Ribeiro, A.J.; Veiga, F. Nanoencapsulation I. Methods for Preparation of Drug-Loaded Polymeric Nanoparticles. Nanomedicine: Nanotechnology, Biology, and Medicine 2006, 2 (1), 8–21.
- Vauthier, C.; Bouchemal, K. Methods for the Preparation and Manufacture of Polymeric Nanoparticles. Pharmaceutical Research 2009, 26 (5), 1025–1058.
- Shi, J.; Votruba, A.R.; Farokhzad, O.C.; Langer, R. Nanotechnology in Drug Delivery and Tissue Engineering: From Discovery to Applications. Nano Letters 2010, 10 (9), 3223–3230.
- Rosas, J.E.; Pedraz, J.L. PLGA Microspheres: A System for the Controlled Release of Molecules with Immunogenic Activity. Colombian Journal of Chemistry-Pharmaceutical Sciences 2007, 36 (2), 134–153.
- Phillips, D.J.; Patterson, J.P.; O’Reilly, R.K.; Gibson, M.I. Glutathione-Triggered Disassembly of Isothermally Responsive Polymer Nanoparticles Obtained by Nanoprecipitation of Hydrophilic Polymers. Polymer Chemistry 2014, 5 (1), 126–131.
- Mishra, D.; Hubenak, J.R.; Mathur, A.B. Nanoparticle Systems As Tools to Improve Drug Delivery and Therapeutic Efficacy. Journal of Biomedical Materials Research Part A 2013, 101 (12), 3646–3660.
- Matalanis, A.; Jones, O.G.; McClements, D.J. Structured Biopolymer-Based Delivery Systems for Encapsulation, Protection, and Release of Lipophilic Compounds. Food Hydrocolloids 2011, 25 (8), 1865–1880.
- Lassalle, V.; Ferreira, M.L. PLA Nano‐and Microparticles for Drug Delivery: An Overview of the Methods of Preparation. Macromolecular Bioscience 2007, 7 (6), 767–783.
- Charcosset, C.; El-Harati, A.; Fessi, H. Preparation of Solid Lipid Nanoparticles Using a Membrane Contactor. Journal of Controlled Release 2005, 108 (1), 112–120.
- Fathi, M.; Mozafari, M.R.; Mohebbi, M. Nanoencapsulation of Food Ingredients Using Lipid Based Delivery Systems. Trends in Food Science & Technology 2012, 23 (1), 13–27.
- Habib, S.M.; Amr, A.S.; Hamadneh, I.M. Nanoencapsulation of Alpha-Linolenic Acid with Modified Emulsion Diffusion Method. Journal of the American Oil Chemists’ Society. 2012, 89 (4), 695–703.
- Ochekpe, N.A.; Olorunfemi, P.O.; Ngwuluka, N.C. Nanotechnology and Drug Delivery Part 2: Nanostructures for Drug Delivery. Tropical Journal of Pharmaceutical Research 2009, 8 (3), 275–287.
- Zambrano-Zaragoza, M.L.; Mercado-Silva, E.; Gutiérrez-Cortez, E.; Castaño-Tostado, E.; Quintanar-Guerrero, D. Optimization of Nanocapsules Preparation by the Emulsion–Diffusion Method for Food Applications. LWT–Food Science and Technology 2011, 44 (6), 1362–1368.
- Souguir, H.; Salaün, F.; Douillet, P.; Vroman, I.; Chatterjee, S. Nanoencapsulation of Curcumin in Polyurethane and Polyurea Shells by An Emulsion Diffusion Method. Chemical Engineering Journal 2013, 221, 133–145.
- Surassmo, S.; Min, S.G.; Bejrapha, P.; Choi, M.J. Effects of Surfactants on the Physical Properties of Capsicum Oleoresin-Loaded Nanocapsules Formulated Through the Emulsion–Diffusion Method. Food Research International 2010, 43 (1), 8–17.
- Quintanar-Guerrero, D.; Ganem-Quintanar, A.; Allémann, E.; Fessi, H.; Doelker, E. Influence of the Stabilizer Coating Layer on the Purification and Freeze-Drying of Poly (D, L-Lactic Acid) Nanoparticles Prepared by An Emulsion-Diffusion Technique. Journal of Microencapsulation 1998, 15 (1), 107–119.
- Steinhilber, D.; Witting, M.; Zhang, X.; Staegemann, M.; Paulus, F.; Friess, W.; Küchler, S.; Haag, R. Surfactant Free Preparation of Biodegradable Dendritic Polyglycerol Nanogels by Inverse Nanoprecipitation for Encapsulation and Release of Pharmaceutical Biomacromolecules. Journal of Controlled Release 2013, 169 (3), 289–295.
- Esmaeili, A.; Bahrami, S. Effects of the Extraction Phase of Citrus L. Growing in Iran, Loaded in Oil-to-Water Nanocapsules Prepared by the Interfacial Polymerization Method. International Journal of Food Properties 2015, 18 (4), 714–724.
- Dasgupta, N.; Ranjan, S.; Mundra, S.; Ramalingam, C.; Kumar, A. Fabrication of Food Grade Vitamin E Nanoemulsion by Low Energy Approach, Characterization, and Its Application. International Journal of Food Properties 2015, 19 (3), 1–21.
- Moshfeghi, A.A.; Peyman, G.A. Micro-and Nanoparticulates. Advanced Drug Delivery Reviews 2005, 57 (14), 2047–2052.
- Yoncheva, K.; Lizarraga, E.; Irache, J.M. Pegylated Nanoparticles Based on Poly (Methyl Vinyl Ether-Co-Maleic Anhydride): Preparation and Evaluation of Their Bioadhesive Properties. European Journal of Pharmaceutical Sciences 2005, 24 (5), 411–419.
- Chin, S.F.; Pang, S.C.; Tay, S.H. Size Controlled Synthesis of Starch Nanoparticles by a Simple Nanoprecipitation Method. Carbohydrate Polymers 2011, 86 (4), 1817–1819.
- Gavory, C.; Durand, A.; Six, J.L.; Nouvel, C.; Marie, E.; Leonard, M. Polysaccharide-Covered Nanoparticles Prepared by Nanoprecipitation. Carbohydrate Polymers 2011, 84 (1), 133–140.
- Roy, A.; Singh, S.K.; Bajpai, J.; Bajpai, A.K. Controlled Pesticide Release from Biodegradable Polymers. Central European Journal of Chemistry 2014, 12 (4), 453–469.
- Kingsley, J.D.; Dou, H.; Morehead, J.; Rabinow, B.; Gendelman, H.E.; Destache, C.J. Nanotechnology: A Focus on Nanoparticles As a Drug Delivery System. Journal of Neuroimmune Pharmacology 2006, 1 (3), 340–350.
- Gulfam, M.; Kim, J.E.; Lee, J.M.; Ku, B.; Chung, B.H.; Chung, B.G. Anticancer Drug-Loaded Gliadin Nanoparticles Induce Apoptosis in Breast Cancer Cells. Langmuir 2012, 28 (21), 8216–8223.
- van Vlerken, L.E.; Vyas, T.K.; Amiji, M.M. Poly (Ethylene Glycol)-Modified Nanocarriers for Tumor-Targeted and Intracellular Delivery. Pharmaceutical Research 2007, 24 (8), 1405–1414.
- Kumar, V.D.; Verma, P.R.P.; Singh, S.K. Development and Evaluation of Biodegradable Polymeric Nanoparticles for the Effective Delivery of Quercetin Using a Quality by Design Approach. LWT–Food Science and Technology 2015, 61 (2), 330–338.
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable Polymeric Nanoparticles Based Drug Delivery Systems. Colloids and Surfaces B: Biointerfaces 2010, 75 (1), 1–18.
- Mozafari, M.R.; Khosravi-Darani, K.; Borazan, G.G.; Cui, J.; Pardakhty, A.; Yurdugul, S. Encapsulation of Food Ingredients Using Nanoliposome Technology. International Journal of Food Properties 2008, 11 (4), 833–844.
- Kastner, T.; Rivas, M.J.I.; Koch, W.; Nonhebel, S. Global Changes in Diets and the Consequences for Land Requirements for Food. Proceedings of the National Academy of Sciences 2012, 109 (18), 6868–6872.
- Castro-Enríquez, D.D.; Rodríguez-Félix, F.; Ramírez-Wong, B.; Torres-Chávez, P.I.; Castillo-Ortega, M.M.; Rodríguez-Félix, D.E.; Armenta-Villegas, L.; Ledesma-Osuna, A.I. Preparation, Characterization, and Release of Urea from Wheat Gluten Electrospun Membranes. Materials 2012, 5 (12), 2903–2916.
- Ghormade, V.; Deshpande, M.V.; Paknikar, K.M. Perspectives for Nano-Biotechnology Enabled Protection and Nutrition of Plants. Biotechnology Advances 2011, 29 (6), 792–803.
- Blanco-Padilla, A.; Soto, K.M.; Hernández Iturriaga, M.; Mendoza, S. Food Antimicrobials Nanocarriers. The Scientific World Journal 2014, 1–11.
- Berti, F.; Todros, S.; Lakshmi, D.; Whitcombe, M.J.; Chianella, I.; Ferroni, M.; Piletsky, S.A.; Turner, A.P.F.; Marrazza, G. Quasi-Monodimensional Polyaniline Nanostructures for Enhanced Molecularly Imprinted Polymer-Based Sensing. Biosensors and Bioelectronics 2010, 26 (2), 497–503.
- Pérez-López, B.; Merkoçi, A. Nanomaterials Based Biosensors for Food Analysis Applications. Trends in Food Science & Technology 2011, 22 (11), 625–639.
- Kayaci, F.; Ertas, Y.; Uyar, T. Enhanced Thermal Stability of Eugenol by Cyclodextrin Inclusion Complex Encapsulated in Electrospun Polymeric Nanofibers. Journal of Agricultural and Food Chemistry 2013, 61 (34), 8156–8165.
- Antiochia, R.; Gorton, L.; Mannina, L. Rapid Determination of Sucrose in Fruit Juices: A New Sensitive Carbon Nanotube Paste Osmium-Polymer Mediated Biosensor. Journal of Food Research 2014, 3 (4), 101–112.
- Zhang, C.; Yin, A.X.; Jiang, R.; Rong, J.; Dong, L.; Zhao, T.; Sun, L.D.; Wang, J.; Chen, X.; Yan, C.H. Time–Temperature Indicator for Perishable Products Based on Kinetically Programmable Ag Overgrowth on Au Nanorods. ACS Nano 2013, 7 (5), 4561–4568.
- Peters, R.; ten Dam, G.; Bouwmeester, H.; Helsper, H.; Allmaier, G.; vd Kammer, F.; Ramsch, R.; Solans, C.; Tomaniová, M.; Hajslova, J.; Weigel, S. Identification and Characterization of Organic Nanoparticles in Food. TrAC Trends in Analytical Chemistry 2011, 30 (1), 100–112.
- Sozer, N.; Kokini, J.L. Nanotechnology and Its Applications in the Food Sector. Trends in Biotechnology 2009, 27 (2), 82–89.
- Noronha, C.M.; Granada, A.F.; de Carvalho, S.M.; Lino, R.C.; de O.B. Maciel, M.V.; Barreto, P.L.M. Optimization of α-Tocopherol Loaded Nanocapsules by the Nanoprecipitation Method. Industrial Crops and Products 2013, 50, 896–903.
- Aubry, J.; Ganachaud, F.; Cohen Addad, J.P.; Cabane, B. Nanoprecipitation of Polymethylmethacrylate by Solvent Shifting: 1. Boundaries. Langmuir 2009, 25 (4), 1970–1979.
- Lepeltier, E.; Bourgaux, C.; Couvreur, P. Nanoprecipitation and the “Ouzo Effect:” Application to Drug Delivery Devices. Advanced Drug Delivery Reviews 2014, 71, 86–97.
- Beck-Broichsitter, M.; Rytting, E.; Lebhardt, T.; Wang, X.; Kissel, T. Preparation of Nanoparticles by Solvent Displacement for Drug Delivery: A Shift in the “Ouzo Region” Upon Drug Loading. European Journal of Pharmaceutical Sciences 2010, 41 (2), 244–253.
- Mugheirbi, N.A.; Paluch, K.J.; Tajber, L. Heat Induced Evaporative Antisolvent Nanoprecipitation (HIEAN) of Itraconazole. International Journal of Pharmaceutics 2014, 471 (1), 400–411.
- Roger, K.; Eissa, M.; Elaissari, A.; Cabane, B. Surface Charge of Polymer Particles in Water: The Role of Ionic End-Groups. Langmuir 2013, 29 (36), 11244–11250.
- Chidambaram, M.; Krishnasamy, K. Modifications to the Conventional Nanoprecipitation Technique: An Approach to Fabricate Narrow Sized Polymeric Nanoparticles. Advanced Pharmaceutical Bulletin 2014, 4 (2), 205.
- Lucas, P.; Vaysse, M.; Aubry, J.; Mariot, D.; Sonnier, R.; Ganachaud, F. Finest Nanocomposite Films from Carbon Nanotube-Loaded Poly (Methyl Methacrylate) Nanoparticles Obtained by the Ouzo Effect. Soft Matter 2011, 7 (12), 5528–5531.
- Luo, C.J.; Okubo, T.; Nangrejo, M.; Edirisinghe, M. Preparation of Polymeric Nanoparticles by Novel Electrospray Nanoprecipitation. Polymer International 2015, 64 (2), 183–187.
- Liu, Y.; Lu, Y.C.; Luo, G.S. Modified Nanoprecipitation Method for Polysulfone Nanoparticles Preparation. Soft Matter 2014, 10 (19), 3414–3420.
- Cheng, J.C.; Vigil, R.D.; Fox, R.O. A Competitive Aggregation Model for Flash Nanoprecipitation. Journal of Colloid and Interface Science 2010, 351 (2), 330–342.
- Legrand, P.; Lesieur, S.; Bochot, A.; Gref, R.; Raatjes, W.; Barratt, G.; Vauthier, C. Influence of Polymer Behaviour in Organic Solution on the Production of Polylactide Nanoparticles by Nanoprecipitation. International Journal of Pharmaceutics 2007, 344 (1), 33–43.
- Rao, J.P.; Geckeler, K.E. Polymer Nanoparticles: Preparation Techniques and Size-Control Parameters. Progress in Polymer Science 2011, 36 (7), 887–913.
- de Oliveira, A.M.; Jäger, E.; Jäger, A.; Stepánek, P.; Giacomelli, F.C. Physicochemical Aspects Behind the Size of Biodegradable Polymeric Nanoparticles: A Step Forward. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2013, 436, 1092–1102.
- Galindo-Rodriguez, S.; Allemann, E.; Fessi, H., Doelker, E. Physicochemical Parameters Associated with Nanoparticle Formation in the Salting-Out, Emulsification-Diffusion, and Nanoprecipitation Methods. Pharmaceutical Research 2004, 21 (8), 1428–1439.
- Govender, T.; Stolnik, S.; Garnett, M.C.; Illum, L.; Davis, S.S. PLGA Nanoparticles Prepared by Nanoprecipitation: Drug Loading and Release Studies of a Water Soluble Drug. Journal of Controlled Release 1999, 57 (2), 171–185.
- Chambon, S.; Schatz, C.; Sébire, V.; Pavageau, B.; Wantz, G.; Hirsch, L. Organic Semiconductor Core–Shell Nanoparticles Designed Through Successive Solvent Displacements. Materials Horizons 2014, 1 (4), 431–438.
- Velázquez, D.L.A.; Chávez, M.A.; Castro, R.R.; Gutiérrez, P.Y.; Galindo, R.S.A. Encapsulation of Clotrimazole in Polymeric Nanoparticles by Nanoprecipitation Technique. Congreso Internacional de QFB. Revista de Salud Pública y Nutrición 2009, 1, 1–16.
- Peltonen, L.; Aitta, J.; Hyvönen, S.; Karjalainen, M.; Hirvonen, J. Improved Entrapment Efficiency of Hydrophilic Drug Substance During Nanoprecipitation of Poly (I) Lactide Nanoparticles. AAPS PharmSciTech 2004, 5 (1), 115–120.
- Álvarez Román, R.; Cavazos Rodríguez, M.R.; Chavéz Montes, A.; Castro Ríos, R.; Waksman de Torres, N.; Salazar Cavazos, M.D.L.L.; Galindo Rodríguez, S.A. Formulation and Characterization of Nanocapsules with a Natural Antioxidant for Cutaneous Application. Química Hoy Chemistry Sciences 2012, 1 (4), 29–35.
- Shi, W.; Zhang, Z.J.; Yuan, Y.; Xing, E.M.; Qin, Y.; Peng, Z.J.; Zhang, Z.P.; Yang, K.Y. Optimization of Parameters for Preparation of Docetaxel-Loaded PLGA Nanoparticles by Nanoprecipitation Method. Journal of Huazhong University of Science and Technology [Medical Sciences] 2013, 33, 754–758.
- Teng, Z.; Luo, Y.; Wang, T.; Zhang, B.; Wang, Q. Development and Application of Nanoparticles Synthesized with Folic Acid Conjugated Soy Protein. Journal of Agricultural and Food Chemistry 2013, 61 (10), 2556–2564.
- Kim, S.; Kim, Y.S. Production of Gliadin-Poly (Ethyl Cyanoacrylate) Nanoparticles for Hydrophilic Coating. Journal of Nanoparticle Research 2014, 16 (2), 1–10.
- He, W.; Lu, Y.; Qi, J.; Chen, L.; Hu, F.; Wu, W. Food Proteins As Novel Nanosuspension Stabilizers for Poorly Water-Soluble Drugs. International Journal of Pharmaceutics 2013, 441 (1), 269–278.
- Yan, X.; Delgado, M.; Fu, A.; Alcouffe, P.; Gouin, S.G.; Fleury, E.; Katz, J.L.; Ganachaud, F.; Bernard, J. Simple But Precise Engineering of Functional Nanocapsules Through Nanoprecipitation. Angewandte Chemie 2014, 126 (27), 7030–7033.
- Lebouille, J.G.J.L.; Stepanyan, R.; Slot, J.J.M.; Stuart, M.C.; Tuinier, R. Nanoprecipitation of Polymers in a Bad Solvent. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2014, 460, 225–235.
- Moorthi, C.; Kathiresan, K. Fabrication of Highly Stable Sonication Assisted Curcumin Nanocrystals by Nanoprecipitation Method. Drug Invention Today 2013, 5 (1), 66–69.
- He, Y.; Huang, Y.; Cheng, Y. Structure Evolution of Curcumin Nanoprecipitation from a Micromixer. Crystal Growth & Design 2010, 10 (3), 1021–1024.
- Sah, E.; Sah, H. Recent Trends in Preparation of Poly (Lactide-co-Glycolide) Nanoparticles by Mixing Polymeric Organic Solution with Anti-solvent. Journal of Nanomaterials 2015, 61, 1–22.
- Schubert, S.; Delaney Jr, J.T.; Schubert, U.S. Nanoprecipitation and Nanoformulation of Polymers: From History to Powerful Possibilities Beyond Poly (Lactic Acid). Soft Matter 2011, 7 (5), 1581–1588.
- Xie, H.; Smith, J.W. Fabrication of PLGA Nanoparticles with a Fluidic Nanoprecipitation System. Journal of Nanobiotechnology 2010, 8 (1), 18.
- Hornig, S.; Heinze, T.; RemziáBecer, C.; Schubert, U.S. Synthetic Polymeric Nanoparticles by Nanoprecipitation. Journal of Materials Chemistry 2009, 19 (23), 3838–3840.
- Joye, I.J.; Nelis, V.A.; McClements, D.J. Gliadin-Based Nanoparticles: Stabilization by Post-Production Polysaccharide Coating. Food Hydrocolloids 2015, 43, 236–242.
- Margulis, K.; Magdassi, S.; Lee, H.S.; Macosko, C.W. Formation of Curcumin Nanoparticles by Flash Nanoprecipitation from Emulsions. Journal of Colloid and Interface Science 2014, 434, 65–70.
- Pustulka, K.M.; Wohl, A.R.; Lee, H.S.; Michel, A.R.; Han, J.; Hoye, T.R.; McCormick, A.V.; Panyam, J.; Macosko, C.W. Flash Nanoprecipitation: Particle Structure and Stability. Molecular Pharmaceutics 2013, 10 (11), 4367–4377.
- Khan, S.A.; Schneider, M. Nanoprecipitation Versus Two Step Desolvation Technique for the Preparation of Gelatin Nanoparticles. Proceedings in SPIE BiOS. San Francisco, USA. 85950H-85950H, 2013.
- Botet, R. The “Ouzo Effect,” Recent Developments and Application to Therapeutic Drug Carrying. Journal of Physics-Conference Series 2012, 352 (1), 1–10.
- Santander-Ortega, M.J.; Stauner, T.; Loretz, B.; Ortega-Vinuesa, J.L.; Bastos-González, D.; Wenz, G.; Schaefer, U.F.; Lehr, C.M. Nanoparticles Made from Novel Starch Derivatives for Transdermal Drug Delivery. Journal of Controlled Release 2010, 141 (1), 85–92.
- Campos, E.V.R.; de Oliveira, J.L.; Fraceto, L.F.; Singh, B. Polysaccharides As Safer Release Systems for Agrochemicals. Agronomy for Sustainable Development 2015, 35 (1), 47–66.
- Arangoa, M.A.; Campanero, M.A.; Popineau, Y.; Irache, J.M. Electrophoretic Separation and Characterisation of Gliadin Fractions from Isolates and Nanoparticulate Drug Delivery Systems. Chromatographia 1999, 50 (3–4), 243–246.
- Arangoa, M.A.; Ponchel, G.; Orecchioni, A.M.; Renedo, M.J.; Duchene, D.; Irache, J.M. Bioadhesive Potential of Gliadin Nanoparticulate Systems. European Journal of Pharmaceutical Sciences 2000, 11 (4),333–341.
- Duclairoir, C.; Orecchioni, A.M.; Depraetere, P.; Osterstock, F.; Nakache, E. Evaluation of Gliadins Nanoparticles As Drug Delivery Systems: A Study of Three Different Drugs. International Journal of Pharmaceutics 2003, 253 (1), 133–144.
- Fajardo, P.; Balaguer, M.P.; Gomez-Estaca, J.; Gavara, R.; Hernandez-Munoz, P. Chemically Modified Gliadins As Sustained Release Systems for Lysozyme. Food Hydrocolloids 2014, 41, 53–59.
- Kim, S. Production of Composites by Using Gliadin As a Bonding Material. Journal of Cereal Science 2011, 54 (1),168–172.
- Mauguet, M.C.; Legrand, J.; Brujes, L.; Carnelle, G.; Larre, C.; Popineau, Y. Gliadin Matrices for Microencapsulation Processes by Simple Coacervation Method. Journal of Microencapsulation 2002, 19 (3), 377–384.
- Noronha, C.M.; de Carvalho, S.M.; Lino, R.C.; Barreto, P.L.M. Characterization of Antioxidant Methylcellulose Film Incorporated with α-Tocopherol Nanocapsules. Food Chemistry 2014, 159, 529–535.