ABSTRACT
The phytochemicals content and radical scavenging activity of pistachio (Pistacia vera L.) hull extract obtained by different solvents (water, ethanol, and butanol) were measured and compared. Water was selected as superior solvent. Ultrasound-assisted aqueous extraction of the hull by power ultrasound (35 kHz) was more efficient in ascending the phytochemicals content than the sonochemical ultrasonication (130 kHz). High-performance liquid chromatography-mass spectrometry showed increased amounts of vanillic acid, p-coumaric acid, naringenin, and catechin in ultrasound-assisted extracts. Post-extraction sonication declined significantly the phenolics amount and antioxidant property of the aqueous extract. Microwave-assisted extraction increased the phenolics and flavonoids content at extract in a power-dependent trend.
Introduction
Bioactive compounds and natural antioxidants have become a popular category in human nutrition and are increasingly sought by consumers in recent years.[Citation1] Therefore, extensive research is currently being conducted in this field. The low incidence of cardiovascular diseases in societies and individuals consuming diets rich in fruits, vegetables, and their derivatives is ascribed to the health-promoting impact of certain phytochemicals including phenolic compounds.[Citation2] These plant metabolites are also regarded as alternatives for synthetic antioxidants at production of “all natural” food commodities. According to the Food and Agriculture Organization,[Citation3] Iran ranks among the top seven countries in production of 22 important agricultural products so that it ranks first for pistachio, berberis (Zereshk), saffron, stone fruits, and berries worldwide.
Various compartments of pistachio represent good sources for bioactive compounds with diverse functionalities including anti-inflammatory, anticarcinogenic, antiangiogenic, antimicrobial, and antioxidant activities. The hull of pistachio contains higher amounts of phenolics compared to the skin and nuts much comparable with those present in already recognized phenolic sources.[Citation4,Citation5] At present, pistachio dehulling units generate large quantities of wastes and byproducts which are often discharged to orchards and cultivation fields causing serious environmental concerns.[Citation6] These wastes, however, contain substantial amounts of phenolic compounds with documented antioxidant properties.[Citation7] Phenolics extraction and recovery from dehulling wastes would, therefore, confer a dual-benefit to both environment and nutraceutical ingredients sector. Indeed, extraction with aqueous solutions has been selected as the most appropriate tool for the extraction of phenolic compounds from plant compartments due to their food grade nature and consideration regarding green chemistry.[Citation8]
Recently, ultrasound- and microwave-assisted extractions (MAEs) of bioactive compounds from different plant tissues were investigated by several researchers due to the accelerating and shortening extraction times, efficient extraction, automation, and reduction of organic solvent consumption.[Citation9–Citation11] By understanding the effects of various extraction methods on the bioactive compounds, food formulations containing these ingredients can be interpreted more appropriately.[Citation12] In addition to extraction procedures, post-extraction (PE) processing such as microwave heating may influence the quality indices and nutritional value of nutraceutical-enriched functional foods. To the best of authors knowledge, there was no comprehensive report in the literature about the influence of ultrasound- and MAEs on phenolic content and profile of pistachio hull extract, as well as, their changes upon subsequent sonic and microwave processing.[Citation10,Citation11] The objective of the present study was to follow up the changes in phytochemicals profile of pistachio hull as influenced by ultrasound and microwave either during extraction or PE processing.
Materials and Methods
Standards and Reagents
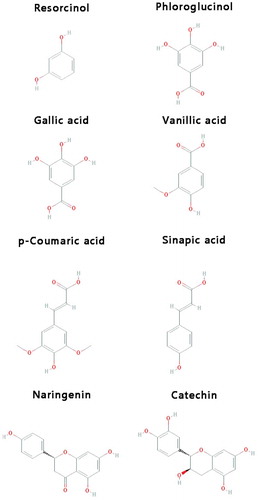
Absolute butanol and ethanol, sodium carbonate, 2,2-diphenyl-1-picrylhydrazyl (DPPH) and Folin–Ciocalteu reagent were purchased from Merck (Darmstadt, Germany). Whatman® filter paper No.1 was obtained from Whatman International Ltd. (Maidstone, England). Resorcinol and phloroglucinol (simple phenolics), gallic acid and vanillic acid (phenolic acids), sinapic acid and p-coumaric acid (cinnamic acids), naringenin and catechin (flavonoids), as well as methanol and formic acid high-performance liquid chromatography ([HPLC] grade) were obtained from Sigma-Aldrich (Milan, Italy). All other chemicals used in this study were of analytical grade quality.
Sample Preparation and Solvent Extraction
The green pistachio nut (kallequchi variety) was obtained from the Food and Drug Department of Rafsanjan University of Medical Sciences (Rafsanjan, Iran) and then dehulled manually. Soon after, the samples were air-dried at 30°C for 24 h, powdered in a fine grinder (Toosshekan T8500, Iran) and then passed through a 50-mesh sieve. The powdered dry hull with mean particle size of 0.1 mm was stored in sealed plastic bags at –20°C until use.
Ten grams of accurately weighed milled-sieved powder was mixed with 500 mL of the desired solvent (distilled water, ethanol, and butanol) and stirred at 400 rpm for 1, 2, and 3 h at ambient temperature (25 ± 1°C). Subsequently, the extracts were filtered through a series of filter papers, followed by collecting filtrates in reagent bottles covered with aluminum foil to avoid light exposure. Extraction by each solvent for different time periods was performed in three replicates.
Ultrasound-Assisted Extraction (UAE)
UAE of pistachio hull by water was performed using a double frequency ultrasonic bath model TI-H 10 (Elmasonics, Singen, Germany) of internal dimensions of 30 × 24 × 15 cm. The sound could be irradiated at either 130 or 35 kHz by four disc transducers. During sonication, temperature of the reactor vessel jacket was kept constant by tap water circulation around the container. PE (treatment) sonication of the conventionally obtained extract (aqueous extraction at 25°C for 2 h) was also carried out by comparable frequencies. The actual power dissipated to the samples during sonication was calculated according to the following equation:[Citation13]
where P is the power (W), Cp is the specific heat capacity of sample at a constant pressure considered nearly equal to that of water (4.18 J °C–1 g–1), m is mass of sample (g), dT/dt is the temperature difference over the first 60 s of sonication without temperature control. The ultrasonic actual powers dissipated to samples are depicted in . Each experiment carried out in three replicates.
Table 1. Nominal and actual power of ultrasonic and microwave treatments.
MAE
MAE of phenolics and flavonoids from pistachio hull by water was investigated by using a regular household oven with adjustable power output (1000 W, Butan Ltd., Iran). Ground hull in water samples were subjected to radiation at different nominal powers (20, 40, 60, and 80%) for 30 s. After irradiation, the temperature of samples was measured immediately within the oven chamber using a mercury thermometer. A series of PE microwaving treatments were also performed at identical nominal powers for 30 s on the conventionally-obtained aqueous extract (aqueous extraction at 25°C for 2 h). The actual power of microwave dissipated to the solution was calculated again according to the equation presented in previous section.
Determination of Total Phenolic Content
Total phenolics content of extracts was determined by the modified Folin–Ciocalteu assay.[Citation14] A properly diluted aliquot (30 µL) of the extracts (to reach spectrophotometric absorption less than 1.0) was mixed with 150 µL Folin–Ciocalteu reagent and 2.37 mL bi-distilled water. Samples were kept for 7 min at room temperature and then charged with 450 µL sodium carbonate solution. The tubes were vortexed for 30 s and allowed to set in a dark place for 70 min for color outreach. Spectrophotometric measurements were carried out by a UNICO spectrophotometer (New Jersey, USA).The estimation of phenolic compounds was done at 760 nm and calculated by a calibration curve plotted with gallic acid. Total phenolic content was expressed as mg gallic acid per gram dry sample (mg gallic acid extract [GAE]/g dry sample).
Determination of Total Flavonoids Content
Total flavonoids content of pistachio hull extracts was measured following the method by Tomaino et al.[Citation5] with minor modification. Briefly, aliquots of the aqueous/ethanol/butanol extracts were diluted in distilled water to a final volume of 50 µL, and 3 mL NaNO2 (50 µg mL–1) solution was added. Subsequently, after 5 min, 6 mL AlCl3 (100 mg mL–1), and ultimately after 6 min, 20 mL sodium hydroxide (0.5 N) and 21 mL distilled water were added to the mixture. The absorbance of samples was measured at 510 nm and total flavonoids content was stated as mg catechin equivalents per gram dry sample (mg CatE/g dry sample).
Free Radical Scavenging Activity
DPPH scavenging capacity of extracts was evaluated according to Ince et al.[Citation10] with some modifications. Briefly, after being diluted in distilled water (1:20), 0.1 mL of the extract was mixed with 3.9 mL methanolic DPPH solution (25 mg L–1). The mixture was vortexed severely and left to stand for 30 min at room temperature. A DPPH solution with no added extract was considered as the control. The percentage of radical scavenging activity (RSA%) was calculated by using the following equation:
where Asample and Acontrol are the absorbance of the extract sample with DPPH and the absorbance of the DPPH solution without extract, respectively, at 515 nm. SC50 values denote the μg GAE/mL required to scavenge 50% DPPH free radicals.
High-Performance–Diode Array Detector–Fluorescence Detector–Mass Spectrometry (HPLC-DAD-FLD-MS) Analysis of Phenolic Compounds
The identification of simple phenolics, phenolic acids, cinnamic acids, and flavonoids () in treated pistachio hull extracts was performed using an Agilent 1200 series HPLC (Agilent Technologies, CA, USA) equipped with an auto-injector, a quaternary pump, a column counterpart, a UV-DAD and FLD. An aliquot (20 µL) of each sample was injected into a C18 column (250 × 4.6 mm, CA, USA) with eluent A (water/formic acid, 95/5, v/v) and eluent B (methanol) as mobile phase under gradient elution as follow: 0–6 min, 0% B; 6–12 min, 5% B; 12–21 min, 10% B; 21–30 min, 25% B; 30–36 min, 30% B; 36–43 min, 40% B; 43–56 min, 50% B; 56–69 min, 65% B; 69–76 min, 80% B. The column temperature was controlled at 30°C and compounds were monitored at 260–500 nm UV-spectra. After injection of standard solutions (at flow rate of 0.5 mL min–1), calibration curves were plotted for identification and quantification of samples. The total run time was 76 min and all measurements were performed in duplicate. For identification of individual compounds, mass spectra were obtained using Bruker (model Esquire 3000+, Germany) mass spectrometer with an electro-spray ionization (ESI) source in the negative mode in the range of m/z 50–1000. Nitrogen was used as nebulizing gas at 45.0 psi pressure at 365°C. The source voltage was set at 4000 V.
Statistical Analysis
The data were subjected to analysis of variance (ANOVA) and the significance of the difference between means was determined by Duncan’s multiple range test (p < 0.05) using SPSS statistical software (version 18; SPSS Inc., Chicago, IL).
Results and Discussion
Solvent Selection
Extraction with diverse solvents is abundantly used for the isolation of various bioactive compounds from plant materials due to the capability of different solvents to elicit compounds with dissimilar polarities. The selection of an appropriate solvent is, therefore, a decisive factor owing to the complex structure and composition of plant matrices, in which each matrix-solvent system exhibits an exclusive-unpredictable behavior.[Citation15] Accordingly, different solvents (water, ethanol, and butanol) were assessed for the extraction of pistachio hull and the total phenols content, total flavonoids content, and the antioxidant activity of extracts were compared. reports the results obtained for radical scavenging activity and phytochemical compounds contents of pistachio hull extracts. The highest yield of phytochemicals was obtained by water at any given time of extraction, followed by ethanol and finally by butanol. Total phenolics and total flavonoids content in the extracts of pistachio hull varied from 179 to 76 mg GAE/g dry sample and 42.9 to 20 mg CatE/g dry sample, respectively (). The superiority of water to other solvents for extraction of phenolics and flavonoids is attributed to the high polarity and good water-solubility of such phytochemicals present in the pistachio green hull which supports the principles of green chemistry.[Citation7,Citation16] Fernández-Agulló et al.[Citation15] reported a similar finding for walnut green husk extracts. It has been found that the major contribution on the inhibition of free radicals by plant materials is caused by phenolic compounds.[Citation17]
Table 2. Effect of different solvents on the extraction of total phenols content, total flavonoids, and antioxidant capacity of extracts of pistachio hull at various times.
Prolongation of extraction duration from 1 to 2 h increased (p < 0.05) the extraction yield of phytochemicals and augmented the radical scavenging activity of extracts for all three solvents. However, no further increase was observed upon further prolongation of the extraction duration to 3 h (). Therefore, the aqueous extraction procedure for 2 h was employed for the preparation of samples to be examined in the continue of study.
UAE and PE Sonication
Ultrasonication of the solvent and hull mixture by either power (35 kHz) or sonochemical (130 kHz) ultrasound resulted in increased extraction of phenolics and flavonoids at any given sound power (). The increase in phytochemicals content was more pronounced for the sample extracted by 35 kHz-ultrasound with actual power of 1.4 W (nominal power of 100%). This also provided maximum radical scavenging activity for the extract (). Formation of large cavities with greater maximum expansion radii by the power ultrasound[Citation13] has made it an ideal frequency range for most extraction processes. It has been stated that sonication at lower frequencies could destroy some portions of plant tissues while higher frequencies leave the tissue unaffected.[Citation18,Citation19] The diffusion of solvent into cellular materials and mass transfer rates are facilitated during resonating bubbles collapse, resulting in subsequent disruption of biological membranes.[Citation20] The large oscillating cavities generated by the 35 kHz-ultrasound imposed a more beating power during their life to the plant tissue and their explosion generated higher shear forces causing in overall more destruction of the plant tissue and release of phyto-components into the solvent. Hossain et al.[Citation21] showed that a higher amplitude of ultrasound resulted in increased phenolics solubility, solvent diffusion rate and mass transfer in UAE of marjoram. Attachment of the hydroxyl radicals formed during sonochemical reactions to ortho- and para-positions on aromatic rings of phenolic compounds might also contribute in ascended antioxidant capacity of ultrasound-assisted extractives.[Citation22]
Figure 2. Effect of sonication on bioactive compounds and antioxidant properties of pistachio hull aqueous extract. UAE refers to ultrasound assisted extraction and PE refers to the sonication on post-extraction treatments.
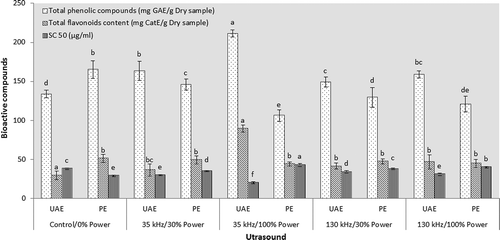
PE sonication of the conventionally obtained extract (evoked by water at non-ultrasound-assisted condition) by various frequency-power combinations indicated that the 35 kHz-ultrasound with actual power of 0.5 W (nominal power of 100%), declined considerably the amount of phytochemicals and radical inhibition activity of the extract compared with other combinations (). This occurred most probably because of the higher heat dissipated to the microenvironment of exploding bubbles by this frequency/power combination than the other sonication conditions. The micro-environmental short-lived hot spots could efficiently degrade the phenolic and flavonoid components.[Citation8] PE sonication by the sonochemical ultrasound (130 kHz) with nominal power of 100% (actual power of 0.1 W) also decreased significantly the amount of phenolics and radical scavenging activity of extract (). Extensive attacks to phenolic molecules by the high number of radicals generated at this frequency range[Citation13] may account for the decreased phenolics content due to the sonochemical sonication.
MAE and PE Microwaving
MAE and PE microwaving was carried out at actual powers of 3.6–65.5 W (). Short-time radiation (30 s) was used for both assisted extraction and PE treatments in order to avoid severe overheating of the sample and solvent evaporation. Microwave irradiation increased the amount of phenolics and flavonoids in the pistachio hull extract; the higher the microwaving power, the greater was the amount of phytochemical compounds in the extract (). As expected, higher content of phytochemicals granted more radical scavenging activity for the extract. When water is used as solvent of extraction, internal superheating arisen by the absorbed microwave energy causes disruption of cells, migration of dissolved ions, and enhanced penetration of solvent into the matrix which in altogether increase the extraction yield.[Citation23,Citation24] Accordingly, heat-induced mechanisms including accelerated diffusion of solvent into the plant matrix and eventual accumulation of solutes are the key aspects of the enhancing influence of microwave.[Citation25] Wu et al.[Citation26] argued that produced heat due to the MAE is mainly based on the direct influence of microwaves on molecules by dipole rotation and ionic conduction. Microwaves can penetrate into plant tissues and interact with polar molecules including phenolics and water, resulting in pressure rise inside the plant cells. This leads to cell wall disruption and release of phytochemicals, while the conventional heating depends principally on thermal conductivity.
Figure 3. Effect of microwave irradiation on bioactive compounds and antioxidant properties of pistachio hull aqueous extract. MAE refers to microwave assisted extraction and PE refers to the irradiation on post-extraction treatments.
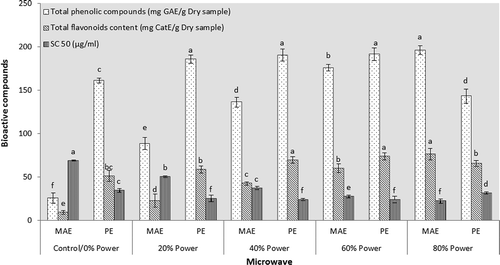
PE microwaving of the pistachio hull water extract increased the phenolics and flavonoids content progressively with increasing wave power expect by the maximum power (65.5 W) which caused a decline in the content of phytochemicals (). Recovery of bioactive compounds from citrus mandarin peels[Citation27] and peanut skin[Citation28] in similarity to our results indicated that microwave is an appropriate method to liberate and activate the bound phenolic compounds and to increase the antioxidant capacity of plant materials. The formation of hot spots in the extract[Citation25] and subsequent degradation of phytochemicals is argued as possible elucidation for decreased content of phyto-components due to microwaving by maximum power. Zhang et al.[Citation29] accentuated that hot spots generated by microwave cavities encourage the convection flow inside the extract which in turn causes the robust boiling of the employed solvent. Rotation of the vessel carousel or mechanical stirring of samples inside the microwave has been proposed by Švarc-Gajic et al.[Citation25] to minimize the arisen drawbacks including non-uniform heating and formation of hot spots.
HPLC-MS Analysis
It is well-known that different treatments could affect the bioactive compounds chemically and contribute in the depletion of natural antioxidant properties.[Citation30] The influence of ultrasound and MAEs, as well as that of PE sonication and microwaving, on the phenolic compounds profile of pistachio hull extract was studied by HPLC-MS technique. By comparing retention times and ultraviolet/visible (UV/Vis) spectral properties of eight selected compounds () in treated samples with those of pure standards, seven phenolic compounds were identified in pistachio hull. reports the quantity of each identified phenolic compound (expressed as µg g–1 dry sample). It is obvious that significantly different profiles for phenolics were obtained through ultrasound and MAE procedures. UAE by the 35 kHz sound with nominal power of 100% (actual power of 1.4 W) led to a considerable liberation of all phenolic compounds especially phenolic acids and flavonoids. The amounts of vanillic acid, p-coumaric acid, naringenin, and catechin were higher significantly (p < 0.05) in ultrasound-assisted extractive in comparison with the control sample (). Similarly, Abid et al.[Citation22] demonstrated that UAE of apple juice increased the amount of a wide range of phenolics (chlorogenic acid, caffeic acid, catechin, epicatechin, and phloridzin) compared to untreated sample. As shown in and , PE sonication of aqueous extract decreased the content of gallic acid and sinapic acid significantly and omitted the phloroglucinol principally. On the other hand, PE sonication of the extract caused a significant drop in all phenolic compounds except for catechin in accordance with results of phyto-components measurements and radical scavenging property.
Table 3. Identified and quantified selected phenolic compounds from treated pistachio hull extracts.
Figure 4. HPLC chromatogram of phenolic compounds for pistachio hull aqueous extract. A: US-control: the control sample without any sonication; B: MV-control: the control sample without any microwave irradiation; C: UAE: extract evoked via ultrasound-assisted extraction, 35 kHz with nominal power of 100%; D: PES: extract subjected to post-extraction sonication, 35 kHz with nominal power of 100%; E: MAE: extract evoked via microwave-assisted extraction with nominal power of 80%; F: PEM: extract subjected to post-extraction microwave irradiation with nominal power of 80%. Peak no: 1: phloroglucinol; 2: gallic acid; 3 vanillic acid; 4: p-coumaric acid; 5: sinapic acid; 6: naringenin; 7: catechin.
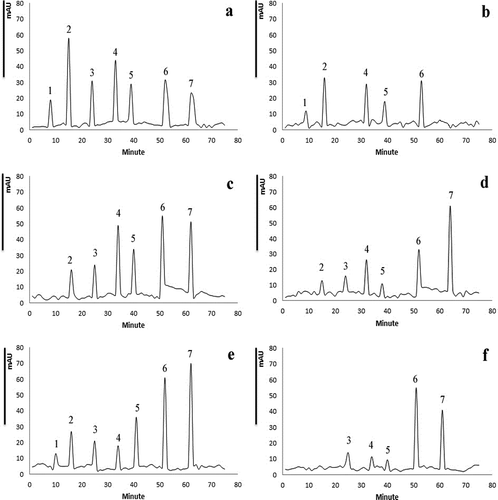
MAE of the pistachio hull in comparison to the control un-waved sample liberated simple phenolic compounds, phenolic acids, and flavonoids, in particular naringnin and catechin ( and ). PE microwave irradiation of the hull extract resulted in considerable degradation of phenolic compounds so that simple phenolics and gallic acid eradicated completely. The amount of phenolic acids and cinnamic acids decreased significantly (p < 0.05) due to the PE microwave irradiation but flavonoids (catechin and naringenin) content showed a partial decrease. Irradiated microwaves could cause a significant development of oxidative decomposition of phenolic compounds, and degrade phenolic compounds considerably.[Citation30] Resorcinol was not detected in any of the pistachio hull extracts examined in the present study, while phloroglucinol and sinapic acid were reported for first time for pistachio hull at the present communication.
Conclusion
Water was chosen as the best solvent for extraction of phyto-components from pistachio hull amongst the three examined cases. Ultrasound and MAE of pistachio hull increased the amounts of phenolic compounds but PE sonication or microwaving of filtered extract caused degradation of bioactive compounds. PE treatments resulted in losses of simple phenolic compounds and phenolic acids but no considerable change was observed in flavonoids. The phloroglucinol and sinapic acid were found for first time in pistachio hull.
References
- Adnan, L.; Osman, A.; Abdul Hamid, A. Antioxidant Activity of Different Extracts of Red Pitaya (Hylocereus Polyrhizus) Seed. International Journal of Food Properties 2011, 14, 1171–1181.
- Chirinos, R.; Pedreschi, R.; Rogez, H.; Larondelle, Y.; Campos, D. Phenolic Compound Contents and Antioxidant Activity in Plants with Nutritional and/or Medicinal Properties from the Peruvian Andean Region. Industrial Crops and Products 2013, 47, 145–152.
- Faostat.fao.org. Retrieved September 22, 2011.
- Garavand, F.; Madadlou, A. Recovery of Phenolic Compounds from Effluents by a Microemulsion Liquid Membrane (MLM) Extractor. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2014, 443, 303–310.
- Tomaino, A.; Martorana, M.; Arcoraci, T.; Monteleone, D.; Giovinazzo, C.; Saija, A. Antioxidant Activity and Phenolic Profile of Pistachio (Pistacia Vera L., Variety Bronte) Seeds and Skins. Biochimie 2010, 92, 1115–1122.
- Behgar, M.; Ghasemi, S.; Naserian, A.; Borzoie, A.; Fatollahi, H. Gamma Radiation Effects on Phenolics, Antioxidants Activity and in Vitro Digestion of Pistachio (Pistachia Vera) Hull. Radiation Physics and Chemistry 2011, 80, 963–967.
- Rajaei, A.; Barzegar, M.; Mobarez, A.M.; Sahari, M.A.; Esfahani, Z.H. Antioxidant, Anti-Microbial, and Antimutagenicity Activities of Pistachio (Pistachia Vera) Green Hull Extract. Food and Chemical Toxicology 2010, 48, 107–112.
- Rahmanian, N.; Jafari, S.M.; Galanakis, C.M. Recovery and Removal of Phenolic Compounds from Olive Mill Wastewater. Journal of the American Oil Chemists’ Society 2014, 91, 1–18.
- Taghvaei, M.; Jafari, S.M.; Nowrouzieh, S.; Alishah, O. The Influence of Cooking Process on the Microwave-Assisted Extraction of Cottonseed Oil. Journal of Food Science and Technology 2013, 52, 1138–1144.
- Ince, A.E.; Sahin, S.; Sumnu, G. Comparison of Microwave and Ultrasound-Assisted Extraction Techniques for Leaching of Phenolic Compounds from Nettle. Journal of Food Science and Technology 2014, 51, 2776–2782.
- Taghvaei, M.; Jafari, S.M.; Assadpoor, E.; Nowrouzieh, S.; Alishah, O. Optimization of Microwave-Assisted Extraction of Cottonseed Oil and Evaluation of Its Oxidative Stability and Physicochemical Properties. Food Chemistry 2014, 160, 90–97.
- Nicoli, M.C.; Anese, M.; Parpinel, M. Influence of Processing on the Antioxidant Properties of Fruit and Vegetables. Trends in Food Science & Technology 1999, 10, 94–100.
- Madadlou, A.; Mousavi, M.E.; Emam-Djomeh, Z.; Ehsani, M.; Sheehan, D. Comparison of pH-Dependent Sonodisruption of Re-Assembled Casein Micelles by 35 and 130 kHz Ultrasounds. Journal of Food Engineering 2009, 95, 505–509.
- Katalinic, V.; Mozina, S.S.; Generalic, I.; Skroza, D.; Ljubenkov, I.; Klancnik, A. Phenolic Profile, Antioxidant Capacity, and Antimicrobial Activity of Leaf Extracts From Six Vitis Vinifera L. Varieties. International Journal of Food Properties 2013, 16, 45–60.
- Fernández-Agulló, A.; Pereira, E.; Freire, M.S.; Valentao, P.; Andrade, P.B.; González-Álvarez, J.; Pereira, J.A. Influence of Solvent on the Antioxidant and Antimicrobial Properties of Walnut (Juglans Regia L.) Green Husk Extracts. Industrial Crops and Products 2013, 42, 126–132.
- Roby, M.H.H.; Sarhan, M.A.; Selim, K.A.H.; Khalel, K.I. Evaluation of Antioxidant Activity, Total Phenols, and Phenolic Compounds in Thyme (Thymus Vulgaris L.), Sage (Salvia Officinalis L.), and Marjoram (Origanum Majorana L.) Extracts. Industrial Crops and Products 2013, 43, 827–831.
- Choi, Y.M.; Noh, D.O.; Cho, S.Y.; Suh, H.J.; Kim, K.M.; Kim, J.M. Antioxidant and Antimicrobial Activities of Propolis from Several Regions of Korea. LWT–Food Science and Technology 2006, 39, 756–761.
- Garcia-Salas, P.; Morales-Soto, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Phenolic-Compound-Extraction Systems for Fruit and Vegetable Samples. Molecules 2010, 15, 8813–8826.
- Vinatoru, M. An Overview of the Ultrasonically Assisted Extraction of Bioactive Principles From Herbs. Ultrasonics Sonochemistry 2001, 8, 303–313.
- Orphanides, A.; Goulas, V.; Gekas, V. Introducing the Concept of Sono-Chemical Potential: A Phenomenological Model for Ultrasound Assisted Extraction. Journal of Food Engineering 2014, 120, 191–196.
- Hossain, M.B.; Brunton, N.P.; Patras, A.; Tiwari, B.; O’Donnell, C.P.; Martin-Diana, A.B.; Barry-Ryan, C. Optimization of Ultrasound Assisted Extraction of Antioxidant Compounds from Marjoram (Origanum Majorana L.) Using Response Surface Methodology. Ultrasonics Sonochemistry 2012, 19, 582–590.
- Abid, M.; Jabbar, S.; Wu, T.; Hashim, M.M.; Hu, B.; Lei, S.; Zeng, X. Sonication Enhances Polyphenolic Compounds, Sugars, Carotenoids, and Mineral Elements of Apple Juice. Ultrasonics Sonochemistry 2014, 21, 93–97.
- Rafiee, Z.; Jafari, S.M.; Alami, M.; Khomeiri, M. Microwave-Assisted Extraction of Phenolic Compounds from Olive Leaves: A Comparison with Maceration. Journal of Animal & Plant Sciences 2011, 21, 46–51.
- Garofulić, I.E.; Dragović-Uzelac, V.; Jambrak, A.R.; Jukić, M. The Effect of Microwave Assisted Extraction on the Isolation of Anthocyanins and Phenolic Acids from Sour Cherry Marasca (Prunus Cerasus var. Marasca). Journal of Food Engineering 2013, 117, 437–442.
- Švarc-Gajić, J.; Stojanović, Z.; Carretero, A.S.; Román, D.A.; Borrás, I.; Vasiljević, I. Development of a Microwave-Assisted Extraction for the Analysis of Phenolic Compounds from Rosmarinus Officinalis. Journal of Food Engineering 2013, 119, 525–532.
- Wu, T.; Yan, J.; Liu, R.; Marcone, M.F.; Aisa, H.A.; Tsao, R. Optimization of Microwave-Assisted Extraction of Phenolics from Potato and Its Downstream Waste Using Orthogonal Array Design. Food Chemistry 2012, 133, 1292–1298.
- Hayat, K.; Zhang, X.; Chen, H.; Xia, S.; Jia, C.; Zhong, F. Liberation and Separation of Phenolic Compounds from Citrus Mandarin Peels by Microwave Heating and Its Effect on Antioxidant Activity. Separation and Purification Technology 2010, 73, 371–376.
- Ballard, T.S.; Mallikarjunan, P.; Zhou, K.; O’Keefe, S. Microwave-Assisted Extraction of Phenolic Antioxidant Compounds from Peanut Skins. Food Chemistry 2010, 120, 1185–1192.
- Zhang, Q.; Jackson, T.H.; Ungan, A. Numerical Modeling of Microwave Induced Natural Convection. International Journal of Heat and Mass Transfer 2000, 43, 2141–2154.
- Wu, Z.L.; Ondruschka, B.; Cravotto, G. Degradation of Phenol Under Combined Irradiation of Microwaves and Ultrasound. Environmental Science & Technology 2008, 42, 8083–8087.