Abstract
The physicochemical properties and volatile profiles of cold-pressed Trichosanthes kirilowii Maxim (T. kirilowii) seed oils from four regions in China were determined in this study. The total oil content and cold-pressed oil yield of the four different sourced seeds were 38.06–44.33% and 15.17–30.97%, respectively. All the cold-pressed oil samples were found to be rich in polyunsaturated fatty acids, with content ranging from 45.41 to 75.32% of the total fatty acids. Punicic, α-eleostearic and catalpic acids were the main conjugated linolenic acid isomers in the cold-pressed T. kirilowii seed oils. The results of melting and crystallization profiles indicated that each oil sample exhibited different transitions steps due to its triacylglycerol composition, crystal structure and total unsaturation. Analysis of volatile profiles showed that 2,4-nonadienal was one of the most important aldehydes in the cold-pressed T. kirilowii seed oils, and less short chain acids (0.20%) but more esters (5.48%) were found in the sample with high content of punicic acid (Hebei sample). Results of oil quality indices indicated that cold-pressed T. kirilowii seed oils were liable to be oxidized, and their stabilities reduced with the increase of acid values. In general, more attention should be paid to improve the oxidative stability of cold-pressed T. kirilowii seed oils in their further application in food industry.
INTRODUCTION
Trichosanthes kirilowii Maxim (T. kirilowii), belonging to the Cucurbitaceae family, has been cultivated in China since ancient times.[Citation1] It is considered as one of the 50 fundamental herbs in China, and the seeds of the plant have been commonly used in traditional Chinese medicine to treat cough, inflammation, and constipation.[Citation2,Citation3] Recent studies showed that T. kirilowii seeds could be an excellent nutritional source of amino acids, essential mineral elements, and triacylglycerols.[Citation1,Citation4]
T. kirilowii seed oil, as a byproduct during the processing of T. kirilowii seed, has already gained much attention for its high content of conjugated linolenic acids (CLnAs).[Citation5] It has been reported that the isomers of CLnAs have many beneficial physiological effects, such as anticarcinogenic and antiatherogenic actions and regulation of body fat and lipid metabolism.[Citation6–Citation8] In addition, a recent study found that T. kirilowii seed oil consisted of high amounts of punicic acid (33.09–39.15%).[Citation1] Structurally, punicic acid is a conjugated octadecatrienoic acid, containing cis-9, trans-11, and cis-13 double bonds.[Citation9,Citation10] For these reasons, T. kirilowii seed oil with abundant natural CLnAs could be a valuable dietary source in the food industry.
In recent years, with the rapid development of the food industry, nutrition, and other related disciplines, consumers are more willing to choose the least-processed foods and avoid synthetic additives. This makes cold-pressed oils an appealing choice, since no solvents are involved during the whole process of oil extraction.[Citation11,Citation12] Many minor components are preserved in the cold-pressed oils, and these minor constituents can have either prooxidative (free fatty acids [FAs], hydroperoxides, chlorophylls, carotenoids) or antioxidative (tocopherols, phenols, phospholipids) effects.[Citation11] Besides, it has been reported that high amounts of polyunsaturated fatty acids (PUFAs) contained in cold-pressed oils are susceptible to lipid oxidation.[Citation13] Hence, the quality of cold-pressed oils needs to be evaluated before widespread consumption.
Until now, little research has focused on the quality of cold-pressed T. kirilowii seed oil, and its characteristics relating to regional differences are unknown. The objective of this study was to provide basic information of the cold-pressed T. kirilowii seed oils, and evaluated the differences in physicochemical properties and volatile profiles of the T. kirilowii seed oils from four different regions in China.
MATERIALS AND METHODS
Materials
Raw T. kirilowii seeds from Sichuan, Hebei, Guangdong, and Zhejiang provinces in China were uniformly harvested by local farmers when they were mature. Seeds from each geographical region were randomly separated into two groups, and immediately transported to our laboratory. Kernels were separated from the seeds and stored at 4ºC for further analysis. Standard mixtures of fatty acid methyl esters (FAMEs) and standards of CLnA isomers (α-eleostearic, catalpic, and punicic acids) were purchased from Nu-Chek-Prep Inc. (Elysian, MN, USA) and Larodan Fine Chemicals (Malmo, Sweden), respectively. All other chemicals and reagents of analytical grade were purchased from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China).
Determination of Total Oil Content in T. kirilowii Kernels
The total oil content of T. kirilowii kernels was determined by solvent extraction according to the standard procedure of GB/T 14488.1-2008 (National Standard of the People’s Republic of China, 2008), and calculated as follows:
where m1 (g) is the mass of the oil obtained by solvent extraction, and m2 (g) is the dry mass of the T. kirilowii kernels.
Preparation of Cold Pressed T. kirilowii Seed Oils
The cold-pressed T. kirilowii seed oils were extracted via pressure by using a screw-type oil expeller (KOMET, Germany, model CA-59-G). The rotating speed of the expeller was 10 rpm, and the diameter of the aperture size was 5 mm. No solvent was used during the whole process, and the oil temperature was kept below 60ºC. The crude T. kirilowii seed oil obtained by cold pressing was centrifuged at 1500 rpm for 15 min, the oily phase was collected and stored at 4ºC for further analysis. The oil yield obtained by cold pressing was calculated as follows:
where m1 (g) is the mass of the oil obtained by cold pressing, and m1 (g) is the dry mass of the T. kirilowii kernels.
Determination of FA Composition
The FAMEs solution of the cold-pressed oil samples was prepared based on the method of Sun et al.[Citation14] One gram of the oil sample was dissolved in 40 mL of n-hexane, and then fully mixed with 40 mL of 0.4 mol/L KOH-CH3OH solution. The mixture was kept at room temperature for 30 min, and 20 mL of saturated NaCl solution was added. The upper layer was collected for gas chromatography (GC) analysis.
The FA composition of the cold-pressed oil samples was analyzed with a GC instrument (Shimadzu, Japan, model GC-2010) equipped with a DB-WAXETR capillary column (30 m × 0.25 mm × 0.25 μm, Agilent, USA), a flame ionization detector (FID) and helium as carrier gas. The FAMEs solution (0.2 μL) was injected and the split mode was performed at a ratio of 1:5. The column temperature program was as follows: 70ºC (5 min), 70–200ºC (10ºC/min), 200–250ºC (5ºC/min), 250ºC (22 min). The injector and detector temperature was 280ºC. The peaks were identified by comparing with the retention times of the standard FAMEs mixtures, and the FA composition was calculated based on the relative FID response areas.
Determination of Oil Quality Indices
The color of the cold-pressed T. kirilowii seed oil samples was measured by Lovibond method (GB/T 22460-2008, National Standard of the People’s Republic of China, 2008). The acid value (AV), peroxide value (PV), p-anisidine value (AnV), and unsaponifiable matter of the oil samples were determined in accordance with GB/T 5530-2005, GB/T 5538-2005, GB/T 24304-2009, and GB/T 5535.1-2008, respectively. The oxidative stability (OSI) of the oil samples was analyzed with a Metrohm 743 Rancimat instrument (Herisau, Switzerland). Samples of 3.0 g were placed in a heating block at 120ºC with a constant air flow of 20 L/h for OSI analysis.[Citation15]
Determination of Melting and Crystallization Properties
Analysis of the melting and crystallization properties of the oil samples was modified based on the method of Zhang et al.[Citation16] by using a differential scanning calorimeter (DSC) instrument (PerkinElmer Corp., USA, model Diamond DSC). Indium was used as a reference for temperature calibration, and nitrogen was used as the purge gas at a flow rate of 20 mL/min. The oil sample (10 mg) was placed in an aluminum pan and then went through the calorimeter program of cooling and heating for three times. The temperature of the calorimeter program was decreased from 30 to –50ºC at a rate of 5ºC/min, and then increased from –50 to 30ºC at the same rate.
Analysis of Volatile Compounds
The solid phase microextraction (SPME) apparatus (Supelco Co., Bellefonte, Pa., USA) consisting of SPME fiber and holder was used to concentrate the volatile compounds of cold-pressed T. kirilowii seed oils.[Citation17,Citation18] Eleven milliliters of the cold-pressed oil sample was weighed into a 22 mL glass vial sealed with an aluminum cover and Teflon septum. The sample was then allowed to reach equilibrium in a 40°C water bath for 5 min under magnetic stirring. After that, the SPME fiber (100 μm polydimethylsiloxane [PDMS]) was exposed to the headspace of the sample with full release so that the volatiles could be adsorbed onto the fiber at 40°C for 30 min. Then the fiber was inserted into the injector port of the gas chromatography-mass spectrometer (GC-MS), and the volatiles were thermally desorbed at 250°C for 4 min.
GC-MS analysis was performed using a GC system (PerkinElmer, Auto System XL GC/Turbo Mass MS, USA) equipped with a nonpolar DB-5 capillary column (30 m × 0.25 mm i.d., 0.25 μm film thickness) and a polar DB-WAX (30 m × 0.25 mm i.d., 0.25 μm film thickness) capillary column, operated in the electron impact ionization mode (70 eV) scanning a mass range (m/z) from 32 to 650 amu. The column temperature program was as follows: 40ºC (2 min), 40–160ºC (3ºC/min), 160–250ºC (10ºC/min), 250ºC (5 min). The injector temperature was set at 250°C, with helium as the carrier gas. The analysis was carried out in splitless mode.
Retention indices were calculated according to the Kovats method using a series of n-alkanes (C6-C28) as external references. The experimental retention indices of the volatile compounds were compared with those from the National Institute of Standards and Technology (NIST) Chemistry WebBook web site (NIST, USA). To identify the peaks in the mass spectra, the mass spectra results obtained in this work were compared with those from the NIST147 library, and also with authentic reference standards when available. The relative concentrations of the volatile compounds were calculated according to the area normalization method.
Statistical Analysis
Unless otherwise stated, all experiments were performed in duplicate and the mean values and standard deviations were calculated. Statistical analysis was performed using Origin 8.0 software (OriginLab Ltd., USA). One-way analysis of variance (ANOVA) was carried out by using Tukey adjustment to determine the significant difference between different oil samples. Significant differences were declared at p < 0.05.
RESULTS AND DISCUSSION
Cold-Pressed Oil Yield of the T. kirilowii Kernels
The four provinces (Sichuan, Hebei, Guangdong, and Zhejiang), respectively, located in the western (Latitude 30º36’N; Longitude 104º04’E), northern (Latitude 38º25’N; Longitude 115º19’E), southern (Latitude 24º48’N; Longitude 113º35’E), and eastern (Latitude 31º43’N; Longitude 119º33’E) parts of China, are the main production areas of T. kirilowii seeds. shows the process of T. kirilowii seed oil extraction, and the results of the moisture content, total oil content and cold-pressed oil yield of the four different sourced kernels are listed in the . It has been reported that the moisture content of the seeds have significant impact on the oil yield obtained during cold-pressing,[Citation19] and each kind of seeds has its own optimum moisture content for extraction.[Citation13,Citation20] As it was impossible to moisture seeds in the laboratory conditions before pressing, so the difference in the cold-pressed oil yield might be attributed to the difference in moisture content. The results of our study showed that there existed no significant relationship between the moisture content, total oil content, and cold-pressed oil yield. For example, the total oil content of the Guangdong sample (43.34%) was a little lower than that of the Zhejiang sample (44.33%). However, the cold-pressed oil yield of the former (19.26%) was much lower than that of the latter sample (30.97%). The moisture contents of the Hebei and Zhejiang samples showed no significant differences (p > 0.05), while the cold-pressed oil yield of the former (15.17%) was significantly lower than that of the latter sample (30.97%). In general, the Zhejiang sample yielded more oil (30.97%) than the Sichuan (23.84%), Hebei (15.17%), and Guangdong (19.26%) samples after cold pressing.
TABLE 1 The moisture content, total oil content, and cold-pressed oil yield of the cold-pressed T. kirilowii kernels
FA Composition of the Cold-Pressed T. kirilowii Seed Oils
shows the DB-WAXETR gas chromatogram of the FA composition for the Hebei and Sichuan oil samples (the gas chromatogram of the other two oil samples was not given). The calculated FA composition of the four different sourced T. kirilowii seed oils are listed in the . The main saturated fatty acids (SFAs) contained in cold-pressed T. kirilowii seed oils were palmitic (C16:0) and stearic (C18:0) acids, which were consistent with the results obtained by solvent extraction.[Citation1,Citation21] Oleic acid (C18:1) was the most abundant monounsaturated fatty acid (MUFA) in T. kirilowii seed oils, accounted for 17.54–32.59% of the total FAs. The Sichuan oil sample contained the highest share of oleic acid (32.59%), while the content of oleic acid in the Hebei sample was the least (17.54%).
TABLE 2 The FA composition of the cold-pressed T. kirilowii seed oils
FIGURE 2 The DB-WAXETR gas chromatogram of the FA composition for the Hebei (Red) and Sichuan (Black) oil samples.1: Palmitic acid (C16:0); 2: stearic acid (C18:0); 3: oleic acid (C18:1); 4: linoleic acid (C18:2); 5: punicic acid (9c, 11t, 13c, C18:3); 6: α-eleostearic acid (9c, 11t, 13t, C18:3); 7: catalpic acid (9t, 11t, 13c, C18:3); 8: eicosenoic acid (C20:1).
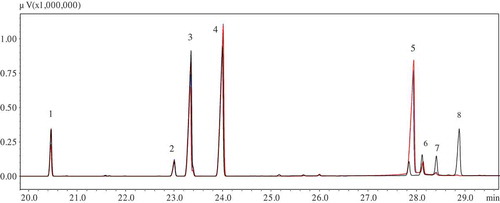
The cold-pressed T. kirilowii seed oils were found to be rich in PUFAs, and the linoleic acid (C18:2) content of the four oil samples ranged from 33.85 to 37.01%. Punicic, α-eleostearic and catalpic acids were the three isomers of CLnAs detected in T. kirilowii seed oils, and the results were consistent with those obtained by Joh et al.[Citation5] The punicic acid contained in Hebei, Guangdong, and Zhejiang oil samples (27.55–33.54%) was significantly higher than that of the Sichuan oil sample (3.01%), which might be attributed to the different geographical features. Compared to the other kinds of edible oils such as soybean, rapeseed, and sunflower oils, T. kirilowii seed oils had higher amount of punicic acid, and could potentially serve as a dietary source of CLnAs to reduce risks of cancer and obesity.[Citation6–Citation8]
Quality Indices of the Cold-Pressed T. kirilowii Seed Oils
The quality indices (color, unsaponifiable matter, AV, PV, AnV, and OSI) of the four cold-pressed T. kirilowii seed oils are given in . The color of the four oil samples was described as dark yellow and orange, and the Guangdong oil sample had the deepest color (R = 11.1; Y = 20.0; B = 2.6) among the four oil samples. The values of unsaponifiable matter among the four cold-pressed T. kirilowii seed oil samples (1.08–4.25 g/100 g) were much higher than those of other kinds of cold-pressed oils such as hemp, flax, and canola seed oils.[Citation12] Besides, the unsaponifiable matter values varied significantly among the four oil samples (p < 0.05), which indicated that the amounts of long chain fatty alcohols, sterols, and pigments contained in cold-pressed T. kirilowii seed oils differed significantly.[Citation22]
TABLE 3 The quality indices of the cold-pressed T. kirilowii seed oils
The AV is an indicator of free FAs which are produced as a result of triacylglycerols hydrolysis by lipases. The results of AV showed that the Sichuan and Guangdong oil samples contained more free FAs than the Hebei and Zhejiang oil samples (p < 0.05), indicating higher hydrolysis degree in the first two oil samples before cold pressing. The cause may be relevant to the different geographical features.
The PV indicates the level of the formed hydroperoxides, which are the primary oxidation products of the oils.[Citation23] In general, the PVs of the cold-pressed T. kirilowii seed oils from four different regions were very high (15.01–48.98 mmol/kg). The lowest PV of 15.01 mmol/kg (Zhejiang sample) was still much higher than those of other kinds of cold-pressed oils such as rapeseed, hemp, and flax seed oils. But it has been reported that the cold-pressed olive oils could also have quite high PV values (6.7–27 mmol/kg).[Citation11] Therefore, other indices, such as AnV and OSI, were used to further evaluate the OSI of the cold-pressed T. kirilowii seed oils.
The AnV characterizes the secondary oxidation products, which are the compounds formed from the decomposition of hydroperoxides.[Citation12] All the oil samples in this study had high AnV values (17.78–134.12), indicating the formation of large amounts of secondary oxidation products. The AnV values of the Sichuan and Guangdong oil samples were significantly higher than that of the other two oil samples (p < 0.05), which meant that the cold-pressed oil samples from Sichuan and Guangdong provinces were more susceptible to oxidation. It should be noted that the AV values for these two samples were also very high as was previously mentioned.
The results of OSI determination further showed that, compared to the cold-pressed olive or rapeseed oils,[Citation11] the OSI of the cold-pressed T. kirilowii seed oils was much lower, and the highest OSI value among the four oil samples was only 0.58 h (Hebei sample). The OSI values of Hebei and Zhejiang oil samples were significantly higher (p < 0.05) than those of the Sichuan and Guangdong oil samples. In conclusion, the results of AV, AnV, and OSI showed that the T. kirilowii seed oils from Hebei and Zhejiang provinces in China had less AV values and were more stable. Therefore, the AV values seemed to contribute to the relative OSI of cold-pressed T. kirilowii seed oils.
Melting and Crystallization Properties of the Cold-Pressed T. kirilowii Seed Oils
The melting and crystallization properties of the four cold-pressed T. kirilowii seed oils analyzed by DSC are listed in . The results of the melting profiles showed that all the four oil samples exhibited two major endothermic peaks with different transition steps. The endothermic peaks for the Sichuan, Hebei, Guangdong, and Zhejiang oil samples were at –38.4 and –22.2°C; –38.2 and –21.9°C; –38.1 and –21.0°C; –37.2 and –20.0°C, respectively.
TABLE 4 The melting and crystallization properties of the cold-pressed T. kirilowii seed oils
For the crystallization profiles of the four cold-pressed seed oils, the Zhejiang oil sample had the lowest transition point at –40.7°C, followed by the Guangdong (–38.7°C), Hebei (–35.3°C), and Sichuan (–31.6°C) oil samples. The difference in the DSC profiles among the four cold-pressed T. kirilowii seed oils might be attributed to the difference in triacylglycerol composition, crystal structure, and total unsaturation of the oil samples.[Citation24] This aspect needs to be further studied.
Volatile Compounds of the Cold-Pressed T. kirilowii Seed Oils
According to the results of sensory evaluation (data was not given), the Hebei, Guangdong, and Zhejiang oil samples had similar and fragrant smell, while the smell of the Sichuan oil sample was found to be fattier. In order to find the difference in the two kinds of smell, two typical oil samples (Sichuan and Hebei) were selected and the volatile compounds analyzed by SPME-GC-MS are listed in the . The main volatile species in the two oil samples were aldehydes and hydrocarbons. Compared to the Sichuan oil sample, less short chain acids (0.20%) but more esters (5.48%) were found in the Hebei oil sample.
TABLE 5 The volatile components of the Sichuan and Hebei oil samples
2, 4-Nonadienal was one of the most important aldehydes in both oil samples. The contents of 2, 4-nonadienal for the Sichuan and Hebei oil samples were 14.44 and 11.09%, respectively. It has been reported that 2, 4-nonadienal has characteristics of lipid and flower aromas, and is mainly found in natural foods such as tomato, tea, and sunflower oil.[Citation25] Hexanal at low concentration has been shown to have fruity aroma, while at high concentration would have the odor of lipid oxidation. In addition, hexanal is considered as one of the secondary oxidation products during the process of lipid oxidation, so the content of hexanal can be used to indicate the degree of lipid oxidation.[Citation26] According to our results, the higher content of hexanal in the Sichuan oil sample (8.20%) indicated its higher oxidation liability in comparison to the Hebei oil sample (hexanal content 2.39%). Besides, the Sichuan oil sample contained more volatile short chain acids () and free FAs (), which may contribute to the odor of lipid oxidation in this sample. In addition, the Hebei oil sample contained more esters (5.48%) than the Sichuan oil sample (1.89%), which may contribute to the slight smell of fragrance in the Hebei sample. However, the smell of the Sichuan sample was found to be fattier.
CONCLUSIONS
The cold-pressed T. kirilowii seed oils from four different regions in China were found to be rich in PUFAs and punicic, α-eleostearic and catalpic acids were the main CLnAs contained in the oils. The results of our study showed that the cold-pressed T. kirilowii seed oil was susceptible for oxidation, so more research should be done in the future to improve its OSI. Physical refining, such as deodorization or short path distillation, might be a way to improve the oil stability by reducing the free FAs. Furthermore, antioxidants addition may be necessary to stabilize the oil before commercial use. In general, this study provides some basic information of the cold-pressed T. kirilowii seed oil for its further application in the food industry.
FUNDING
This study was supported by the National Natural Science Foundation of China (No. 31171704 and No. 31471668).
Additional information
Funding
REFERENCES
- Yang, J.; Zhou, C.; Yuan, G.; Li, D. Effects of Geographical Origin on the Conjugated Linolenic Acid of Trichosanthes kirilowii Maxim Seed Oil. Journal of the American Oil Chemists’ Society 2012, 89, 401–407.
- Dat, N.T.; Jin, X.; Hong, Y.S.; Lee, J.J. An Isoaurone and Other Constituents from Trichosanthes kirilowii Seeds Inhibit Hypoxia-Inducible Factor-1 and Nuclear Factor-kB. Journal of Natural Products 2010, 73, 1167–1169.
- Wu, S.; Xu, T.; Akoh, C.C. Effect of Roasting on the Volatile Constituents of Trichosanthes kirilowii Seeds. Journal of Food and Drug Analysis 2014, 22, 310–317.
- Huang, Y.; He, P.; Bader, K.P.; Radunz, A.; Schmid, G.H. Seeds of Trichosanthes kirilowii, An Energy-Rich Diet. Zeitschrift für Naturforschung 2000, 55, 189–194.
- Joh, Y.G.; Kim, S.J.; Christie, W.W. The Structure of the Triacylglycerols, Containing Punicic Acid, in the Seed Oil of Trichosanthes kirilowii. Journal of the American Oil Chemists’ Society 1995, 72, 1037–1042.
- Lee, K.W.; Lee, H.J.; Cho, H.Y.; Kim, Y.J. Role of the Conjugated Linoleic Acid in the Prevention of Cancer. Critical Reviews in Food Science and Nutrition 2005, 45, 135–144.
- Park, Y.; Pariza, M.W. Mechanisms of Body Fat Modulation by Conjugated Linoleic Acid (CLA). Food Research International 2007, 40, 311–323.
- Salas-Salvado, J.; Marquez-Sandoval, F.; Bullo, M. Conjugated Linoleic Acid Intake in Humans: A Systematic Review Focusing on Its Effect on Body Composition, Glucose, and Lipid Metabolism. Critical Reviews in Food Science and Nutrition 2006, 46, 479–488.
- Pande, G.; Akoh, C.C. Antioxidant Capacity and Lipid Characterization of Six Georgia-Grown Pomegranate Cultivars. Journal of Agricultural and Food Chemistry 2009, 57, 9427–9436.
- Jing, P.; Ye, T.; Shi, H.; Sheng, Y.; Slavin, M.; Gao, B.; Liu, L.; Yu, L.L. Antioxidant Properties and Phytochemical Composition of China-Grown Pomegranate Seeds. Food Chemistry 2012, 132, 1457–1464.
- Koski, A.; Psomiadou, E.; Tsimidou, M.; Hopia, A.; Kefalas, P.; Whl, K.; Heinonen, M. Oxidative Stability and Minor Constituents of Virgin Olive Oil and Cold-Pressed Rapeseed Oil. European Food Research and Technology 2002, 214, 294–298.
- Teh, S.S.; Birch, J. Physicochemical and Quality Characteristics of Cold-Pressed Hemp, Flax, and Canola Seed Oils. Journal of Food Composition and Analysis 2013, 30, 26–31.
- Tynek, M.; Pawłowicz, R.; Gromadzka, J.; Tylingo, R.; Wardencki, W.; Karlovits, G. Virgin Rapeseed Oils Obtained from Different Rape Varieties by Cold Pressed Method–Their Characteristics, Properties, and Differences. European Journal of Lipid Science and Technology 2012, 114, 357–366.
- Sun, X.; Li, Q.; Lv, P.; Li, W. Effect of Different Esterification Methods on the Type and Content of Octadecatrienoic Acid in Trichosanthes kirilowii Maxim Seed Oil. Modern Food Science and Technology 2013, 29, 647–650.
- Dhibi, M.; Flamini, G.; Issaoui, M.; Hammami, M. Volatile Compounds and Oxidative Stability of Pinus halepensis Mill. Seed Oil Under Heating Conditions. International Journal of Food Science and Technology 2012, 47, 1158–1164.
- Zhang, Z.; Wang, L.; Li, D.; Li, S.; Özkan, N. Characteristics of Flaxseed Oil from Two Different Flax Plants. International Journal of Food Properties 2011, 14, 1286–1296.
- Kataoka, H.; Lord, H.L.; Pawliszyn, J. Applications of Solid-Phase Microextraction in Food Analysis. Journal of Chromatography A 2000, 880, 35–62.
- Lee, J.; Pangloli, P. Volatile Compounds and Storage Stability of Potato Chips Fried in Mid-Oleic Sunflower Oil. International Journal of Food Properties 2013, 16, 563–573.
- Pradhan, R.C.; Mishra, S.; Naik, S.N.; Bhatnagar, N.; Vijay, V.K. Oil Expression from Jatropha Seeds Using a Screw Press Expeller. Biosystems Engineering 2011, 109, 158–166.
- Martínez, M.L.; Mattea, M.A.; Maestri, D.M. Pressing and Supercritical Carbon Dioxide Extraction of Walnut Oil. Journal of Food Engineering 2008, 88, 399–404.
- Wang, W.; Wang, L.; Jiang, J. Fatty Acid Profile of Trichosanthes kirilowii Maxim. Seed Oil. Chemical Papers 2009, 63, 489–492.
- Małecka, M. Antioxidant Properties of the Unsaponifiable Matter Isolated from Tomato Seeds, Oat Grains, and Wheat Germ Oil. Food Chemistry 2002, 79, 327–330.
- Tengku Rozaina, T.M.; Birch, E.J. Physicochemical Characterisation and Oxidative Stability of Refined Hoki Oil, Unrefined Hoki Oil and Unrefined Tuna Oil. International Journal of Food Science and Technology 2013, 48, 2331–2339.
- Tan, C.; Man, Y.C. Differential Scanning Calorimetric Analysis of Edible Oils: Comparison of Thermal Properties and Chemical Composition. Journal of the American Oil Chemists’ Society 2000, 77, 143–155.
- Schieberle, P.; Grosch, W. Potent Odorants of Rye Bread Crust-Differences from the Crumb and from Wheat Bread Crust. Zeitschrift für Lebensmittel-Untersuchung und Forschung 1994, 198, 292–296.
- Morales, M.; Rios, J.; Aparicio, R. Changes in the Volatile Composition of Virgin Olive Oil During Oxidation: Flavors and Off-Flavors. Journal of Agricultural and Food Chemistry 1997, 45, 2666–2673.

