ABSTRACT
Various raw materials are used to produce vinegars that contain functional compounds associated with disease prevention. We evaluated changes in functional compounds during tomato vinegar production and superoxide dismutase-like activity of tomato vinegar. Tomato vinegar contained abundant anti-hypertensive compounds, e.g., γ-aminobutyric acid and potassium derived from tomatoes and acetic acid and pyroglutamic acid produced during fermentation. It had stronger superoxide dismutase-like activity than commercial vinegars because of tomato-derived superoxide dismutase-like compounds, including phenolic acids, flavonoids, and glutathione. These data indicate that tomato vinegar is a candidate dietary supplement with potential preventive effects against cardiovascular diseases due to its anti-hypertensive and superoxide dismutase-like compounds.
Introduction
Vinegar is a common seasoning that has long been used as a traditional medicine to regulate blood pressure and blood glucose, promote calcium absorption and fatigue recovery, and stimulate appetite.[Citation1–Citation4] Acetic acid is an essential and major constituent of vinegar; it has beneficial effects against hypertension,[Citation5] hyperglycemia,[Citation6] and dyslipidemia.[Citation7] In Japan, acetic acid is an active constituent of foods categorized as government-approved Food for Specified Health Uses for people with higher than normal blood pressure (https://hfnet.nih.go.jp/). In addition to acetic acid, vinegar contains various functional compounds derived from its raw material. In a previous study, we reported that apple vinegar made with high quantities of raw material contains abundant apple phenolic compounds with potent superoxide dismutase (SOD)-like activity.[Citation8] Budak et al.[Citation9] reported that red wine vinegar contains grape polyphenols that have high SOD-like activity. Horiuchi et al.[Citation10] developed onion vinegar that was abundant in onion-derived potassium, which has anti-hypertensive effects. These results demonstrate that the health benefits of vinegar may be related to functional compounds derived from raw material as well as products obtained by fermentation.
Tomatoes have the highest production value of any fruits in the world.[Citation11] Tomatoes and tomato products are rich in chemo-preventive and health-promoting compounds, including organic acids, amino acids, minerals, phenolic compounds, carotenoids, and vitamins.[Citation12–Citation14] γ-Aminobutyric acid (GABA) is a well-known non-standard amino acid with a blood pressure lowering (BPL) effect[Citation15] and GABA-rich tomato show a significant BPL effect in spontaneously hypertensive rats.[Citation16] Potassium, which is abundant in tomato fruits, also has a BPL effect via natriuretic activity.[Citation17] In addition, phenolic compounds and vitamins exert pharmacological effects via anti-oxidant action. Anti-oxidant compounds trap radical oxidants, such as SOD, which is an important enzyme involved in cellular defense against reactive oxygen species (ROS).[Citation18] Phenolic compounds, including flavonoids, are potent SOD-like compounds in tomatoes and their products.[Citation19] Vitamin C is a major SOD-like compound in freshly consumed tomatoes.[Citation14]
In 2008, tomato vinegar was developed by Mizkan Co., Ltd. (Aichi, Japan). Its production involved two steps: alcohol fermentation of tomato juice to tomato wine and acetic acid fermentation of tomato wine to tomato vinegar. The tomato vinegar was expected to be a functional food containing acetic acid and tomato-derived functional compounds. Despite recent studies of tomato vinegar,[Citation20,Citation21] their functional compounds and SOD-like activity have not been examined. We quantified the functional compounds in raw tomato juice and during the tomato vinegar fermentation processes. Additionally, we examined SOD-like activity in tomato vinegar and analyzed compounds with SOD-like activity using a liquid chromatography mass spectrometry (LC-MS) and database collation. The results of these analyses provide a basis for determining the health benefits of tomato vinegar. The present study analyzed changes in functional compounds during fermentations, SOD-like activity, and SOD-like compounds to estimate the functionality of the tomato vinegar.
Materials and methods
Chemicals
Formic acid, high-performance liquid chromatography (HPLC)-grade acetonitrile, methanol, p-toluenesulfonic acid, ethylenediamine tetraacetate (EDTA)’ after ‘methanol’ disodium hydrogen phosphate (Na2HPO4), sodium dihydrogen phosphate (NaH2PO4), citric acid, malic acid, pyroglutamic acid, tyrosine, phenylalanine, glutathione, wogonoside, naringin, chlorogenic acid, and rutin were purchased from Kanto Chemical Co., Inc. (Tokyo, Japan). Ninhydrin reagent, uridylic acid, adenylic acid, guanylic acid, and caffeic acid were purchased from Wako Pure Chemical Industries (Osaka, Japan). Trifluoroacetic acid (TFA) was purchased from Watanabe Chemical Industries, Ltd. (Hiroshima, Japan). Apple and rice vinegars were purchased from a local supermarket.
Preparation of tomato vinegar
Tomato vinegar was provided by Mizkan Co., Ltd. (Handa, Japan). The product was made from raw tomato juice by alcohol fermentation and acetic acid fermentation. Tomato fruits (Lycopersicon esculentum “Roma VF”) was crushed with water (2.5 kg tomato/L). After filtration and heat sterilization, the raw clear tomato juice was used for alcohol fermentation with yeast. Saccharomyces cerevisiae (25 g; Oriental Yeast Co., Ltd., Tokyo, Japan) was used as the starter and pectinase was used to promote fermentation; both were added to 20 L of clear tomato juice (Brix 15%), and the mixture was incubated at 30°C for 96 h. After filtration, the alcohol-fermented product with 3.6% (v/v) alcohol was obtained. The interim tomato wine and previously prepared unsterilized tomato vinegar including Acetobacter aceti with 7% acid were mixed in the ratio of 7:3, and then acetic acid fermentation was performed until the alcohol concentration decreased to 0.1% (v/v) in deep fermentation at 32°C for 110 h. The acetic acid fermentation product was added water, and the acid concentration was adjusted to 4%. After filtration and heat sterilization, the final tomato vinegar product was obtained. The unsterilized tomato vinegar was prepared in a similar manner for preparing the tomato vinegar using a usual vinegar starter culture of Acetobacter aceti with 7% acid prepared by Mizkan Co., Ltd., and the final product of tomato vinegar in three lots was prepared using the same tomato vinegar starter. In this study, the raw clear tomato juice (tomato juice), the interim product with 2.5% (v/v) alcohol (tomato wine), and the final product with 4% acid (tomato vinegar) were used for subsequent analyses.
Analysis of functional compounds in the tomato vinegar, interim product, and raw material
The functional compounds in the tomato vinegar, interim product, and raw material were analyzed at Mizkan Co., Ltd. To compare functional compounds among samples, the test volumes of the interim product and raw material were corresponded with that of the tomato vinegar obtained in the production process. Each of the three samples was prepared in triplicate and each preparation was analyzed. The results are expressed as means ± standard error (SE).
Organic acids
Organic acids were analyzed using an HPLC system (Shimadzu Co., Kyoto, Japan) equipped with an electronic conductivity detector. Separation was performed at 50°C on an RSpak KC-811 column (8.0 × 300 mm i.d., Showa Denko K.K., Tokyo, Japan) at a flow rate of 0.6 mL/min using an isocratic method. The mobile phases and injection volume were 4 mM p-toluenesulfonic acid aqueous solution and 20 μL, respectively. Detection was conducted with a postcolumn pH buffered method. Mixtures of 4 mM p-toluenesulfonic acid and 100 μM EDTA were used as postcolumn reagents.
Sugars
Sugars were analyzed using an HPLC system (Jasco Co., Tokyo, Japan) equipped with a refractive index detector. Separation was performed at 25°C on a Shodex NH2P-50 Column (4.6 × 250 mm i.d., Showa Denko K.K.) at a flow rate of 1.0 mL/min using an isocratic method. The mobile phases and injection volume were acetonitrile/water (75:25, v/v) and 5.0 μL, respectively.
Minerals
An atomic absorption spectrometer AA-6800 (Shimadzu) was used for analyses of Ca, Na, K, Mg, and Fe following methods described previously.[Citation22]
Amino acids
Amino acids in each sample were first separated by ion exchange chromatography, secondary reacted with ninhydrin reagent, and finally detected by visible light absorption detector on a JEOL AminoTac JLC-500V analyzer (JEOL, Tokyo, Japan). Samples without pre-treatment were injected directly to the analyzer.
Identification of SOD-like compounds in tomato vinegar by LC-MS and database collation
Preparation of 10% and 70% MeOH fractions from tomato vinegar
Tomato vinegar (2 mL) was applied to a Sep-Pak Vac C18 2 g Cartridge (Waters Co., Milford, MA, USA) pretreated with 24 mL of methanol/0.1% TFA water (10:90 v/v) at room temperature. The first 12 mL eluted with methanol/0.1% TFA water (10:90 v/v) was collected as the 10% MeOH fraction, and the latter 12 mL eluted with methanol/0.1% TFA water (70:30 v/v) was collected as the 70% MeOH fraction. The eluent composition was determined in preliminary experiments. The 10 and 70% MeOH fractions were lyophilized after evaporation under a vacuum and 174.6 and 9.4 mg were obtained, respectively. The lyophilized fractions were used for the LC-MS analysis and SOD-like activity assay.
LC-MS analysis of the MeOH fractions and database collation
The lyophilized fractions were dissolved in 500 μL of 0.1% formic acid-water and used for the LC-MS analysis. The analysis was performed using the Quattro micro API (MS) with a Waters 2695 LC system (Waters Co.) at the CREFAS (Collaborated Research Center for Food Functions, Faculty of Agriculture, Shinshu University). Separation was performed at 40°C on a CHEMCOBOND 5-ODS-W reversed-phase column (4.6 × 150 mm; Chemco Plus Scientific Co., Ltd. Kyoto, Japan) with an injection volume of 10 μL. Elution was performed at 0.8 mL/min using 0.1% formic acid-water (solvent A) and 0.1% formic acid-acetonitrile (solvent B): 0–6 min, 0–5% solvent B; 6–15 min, isocratic 5% solvent B; 15–30 min, 5–20% solvent B; 30–40 min, isocratic 20% solvent B. The UV detection wavelength was 215 nm. Mass spectra were acquired in electrospray ionization mode using 3500 V capillary voltage, 20 V cone voltage, 350 L/h N2 gas flow (desolvation), 50 L/h N2 gas flow (cone), 100°C source temperature, and 350°C desolvation temperature. The mass spectrometer was operated in negative and positive modes. In the analysis, compounds in the tomato vinegar fractions were identified by comparing the observed data (retention time, precursor ion, and fragment ions) with a spectral database, i.e., the Kazusa OMICS (KOMICS) Database: http://webs2.kazusa.or.jp/komicmarket.[Citation23] Validation of the identified compounds was performed by spiking the fractions with standards and repeating the analysis. The MS data for non-commercially available glycosides were reconfirmed based on literature information combined with experimental data derived from LC-MS-based metabolomics experiments. In each case, the identification was verified.
Measurement of SOD-like activity
SOD-like antioxidant activity of tomato vinegar was measured on 96-well plates using the SOD Assay Kit-WST (Dojindo Laboratories, Kumamoto, Japan) according to a method reported previously[Citation8] and compared the results with those of rice (typical cereal vinegar) and apple vinegars (typical fruit vinegar). The WST method is an in vitro SOD-like activity assay suitable for food materials.[Citation8,Citation24] Briefly, a phosphate buffer solution (pH 7.0) was prepared by mixing 39 mL of 0.1 M NaH2PO4 solution and 61 mL of 0.1 M Na2HPO4. The vinegars were diluted in three dilution series with the phosphate buffer solution. The rice vinegar which is most popular vinegar in Japan and the apple vinegar which is abundant in phenolic compounds with SOD-like activity were employed as the comparison samples. The tomato vinegar fractions were prepared at three final concentrations using the buffer solution. The dilution ratio and test concentration were determined in preliminary experiments. The sample solution (20 μL) and WST working solution (200 μL) were mixed in a well. Enzyme solution (20 μL) was added to the mixture and the plates were incubated at 37°C for 20 min. After incubation, absorbance was measured at 520 nm using a Model 680 Microplate Reader (Bio-Rad Laboratories Ltd., Hercules, CA). The SOD-like activity (inhibition rate, %) for each sample solution was calculated using the following equation: SOD-like activity (inhibition rate, %) = {[(A blank – A blank control) – (A sample – A sample control)] /(A blank – A blank control)} × 100. In the blank, phosphate buffer was used in place of the sample solution. In the sample control, dilution buffer was used in place of the enzyme solution. In the blank control, phosphate and dilution buffers were used in place of the sample and enzyme solutions. All measurements were performed in triplicate for each sample concentration, and the results are expressed as means ± SE. The linear calibration curve for the dilution range using the liquid vinegar samples or the test concentrations for the tomato vinegar fractions (x) and the corresponding inhibition rate (y) was obtained, and the correlation coefficient was >0.98. The 50% inhibitory concentration (IC50) was calculated from the regression curve.
Statistical analysis
All analyses were performed in triplicate. Functional compounds in the tomato vinegar, raw materials, and tomato wine were quantitatively compared by one-way analysis of variance (ANOVA) followed by Tukey’s tests and p < 0.05 was considered statistically significant. Student’s t-tests were performed to compare SOD-like activity between tomato vinegar, rice vinegar, and apple vinegar and the tomato vinegar fractions. The significance levels were p < 0.05 and p < 0.01 for the t-tests.
Results and discussion
We investigated the functionality of tomato vinegar by evaluating changes in functional compounds during fermentation and by analyzing SOD-like activity and SOD-like compounds.
Functional compounds in tomato vinegar and quantitative changes during fermentation
The upper columns of show the total amounts of organic acids, amino acids, sugars, and minerals in tomato vinegar (final product), tomato wine (interim product), and tomato juice (raw material). The most abundant component in tomato vinegar was organic acids, followed by amino acids, sugars, and minerals. During tomato vinegar production, the total organic acid and total sugar contents significantly increased (p < 0.01) and decreased (p < 0.01), respectively, consistent with the conversion of sugar to ethanol by yeast during alcohol fermentation and conversion of ethanol to acetic acid by acetic acid bacteria during acetic acid fermentation. The total amino acid contents did not change significantly during fermentations, and most amino acids in the raw tomato juice were observed in the tomato vinegar. These results are consistent with a previous report that vegetable and fruit vinegars have similar amino acid compositions and contents to those of the raw materials.[Citation25] The total mineral contents were significantly lower (p < 0.05) in tomato wine than in the raw materials, suggesting that microbes metabolized minerals in the alcohol fermentation process.[Citation26] However, total minerals in the tomato vinegar were maintained at the same high level as that in the tomato wine.
Table 1. Summary of investigated organic acid, amino acid, sugar, and mineral concentrations during the production process of tomato vinegar (mg/100 mL).
The individual compounds in organic acids, amino acids, sugars, and minerals during the fermentation processes are summarized in . The tomato vinegar contained the following organic acids (in decreasing order): acetic acid > citric acid > malic acid > succinic acid > lactic acid (ANOVA; F(3,8) = 1412.0; p = 3.1 × 10−11). The acetic acid concentration increased significantly from 7.31 to 2824 mg/100 mL (386-fold, p < 0.01) during the alcohol and acetic acid fermentations. Succinic acid, which is produced as a metabolic byproduct of yeast during alcohol fermentation,[Citation27] also significantly increased 4.4-fold (from 8.51 to 37.7 mg/100 mL, p < 0.01) during alcohol fermentation. The concentrations of citric, malic, and lactic acids did not change significantly during the fermentation process.
The amino acid contents in the vinegar varied widely and were as follows (from highest to lowest concentration): pyroglutamic acid (pyro-Glu) > Glu > GABA > Asp > Ala > Asn > Gln > Phe > His > Ser > Cys > Thr > Arg > Lys > Val > Tyr > Ile > Leu > Gly > Pro > Met > Trp (ANOVA; F(21,44) = 133.1; p = 4.9 × 10−33). The major amino acids were pyro-Glu, Glu, GABA, and Asp (558, 519, 398, and 291 mg/100 mL, respectively). The same four amino acids were also major components of the raw tomato juice and remained in the tomato vinegar at high levels. During the two-step fermentation, the pyro-Glu, Gln, Ile, Met, Phe, and Trp contents changed significantly (p < 0.05). In particular, pyro-Glu and Gln increased by 51.8% and decreased by 51.6% during alcohol fermentation, respectively. Pyro-Glu is chemically produced from Gln in the alcohol fermentation process.[Citation28]
There was no significant variability in the sugar components in the tomato vinegar (ANOVA; F(2,6) = 0.76; p = 0.51). However, except for sucrose, the sugar contents changed significantly during the fermentation processes (p < 0.01). Glucose decreased significantly by 96.9% during alcohol fermentation and fructose, which was observed in the raw material, was not detected in the vinegar or wine. These sugars are converted to ethanol during alcohol fermentation, providing a carbon source for acetic acid bacteria during alcohol oxidation.
The minerals in the vinegar varied widely and were observed as follows (in decreasing order): K > Mg > Na > Ca > Fe (ANOVA; F(3,8) = 226.4; p = 4.5 × 10−8); K constituted 90.0% of all minerals. Although K decreased significantly by 27.9% during alcohol fermentation (p < 0.05), it was the most abundant mineral in the vinegar (497 mg/100 mL). The lowering of K contents during the vinegar production could be caused by removal of yeast cell containing high levels of internal K+ in filtration processes. The levels of Mg, Na, and Fe were the same before and after the fermentations.
Collectively, the tomato vinegar contained abundant tomato-derived GABA and potassium, in addition to acetic acid and pyro-Glu produced during the fermentations. Acetic acid intake reduces blood pressure via vasorelaxation in vivo.[Citation5] GABA has a BPL effect due to the inhibition of norepinephrine release from sympathetic nerves via presynaptic GABA B receptors.[Citation15] Additionally, pyro-Glu decreases blood pressure by reducing vascular resistance in rat carotid arteries.[Citation29] Potassium also has a BPL effect due to its natriuretic activity.[Citation17,Citation30] Hypertension is a multifactorial disorder characterized by persistent increases in blood pressure and involves abnormalities in cardiovascular function.[Citation31] Based on our results, we inferred that tomato vinegar is likely to have an anti-hypertensive effect owing to the functional compounds derived from raw tomato juice and their increased concentrations during fermentation.
SOD-Like activity of tomato vinegar
SOD-like activity is a well-known indicator of food functionality in tomato fruits.[Citation32] It is expected that tomato vinegar also have SOD-like anti-oxidant activity. Therefore, we measured the activity using the WST-1 method. The activities of the vinegar samples (tomato, apple, and rice vinegars) are expressed as the dilution that caused 50% inhibition ().[Citation8] The IC50 dilutions for tomato, apple, and rice vinegars were 19.6, 4.7, and 1.5 times, respectively. Tomato vinegar demonstrated more potent SOD-like activity (p < 0.01) than commercial apple and rice vinegars. To characterize SOD-like compounds responsible for the SOD-like activity of tomato vinegar, we measured the SOD-like activities of 10 and 70% MeOH fractions prepared from tomato vinegar by ODS solid phase extraction.
Figure 1. SOD-like activities of tomato, apple, and rice vinegars (A), 70% MeOH and 10% MeOH fractions, and lyophilized tomato vinegar (B). Significance (assessed using Student’s t-tests): **p < 0.01.
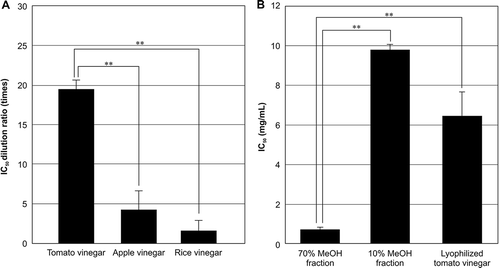
The lyophilized fractions were assayed, and lyophilized tomato vinegar was used as a control. The activities of the lyophilized samples are expressed as the concentrations that caused 50% inhibition (IC50; ). The IC50 values for the 10% and 70% MeOH fractions and lyophilized tomato vinegar were 9.8, 0.72, and 6.5 mg/mL, respectively. The SOD-like activity of the 70% MeOH fraction was significantly higher (p < 0.01) than that of the 10% MeOH fraction. The activity increased by 9.0 times (p < 0.01) compared with that of lyophilized tomato vinegar. However, the 10% MeOH fraction had moderate SOD-like activity. These results demonstrated that major SOD-like compounds in tomato vinegar were concentrated in the 70% MeOH fraction. Therefore, we investigated the SOD-like compounds contained in both fractions using a database search based on LC-MS.
Identification of SOD-like compounds in the 10 and 70% MeOH fractions of tomato vinegar
Compounds in the 10% and 70% MeOH fractions were identified by collating observed MS data (i.e., the observed peaks) with reference data from the KOMICS tomato derived-compounds database.[Citation23] presents all compounds detected in the 10 and 70% MeOH fractions. shows the LC chromatograms of the fractions. In the 10% MeOH fraction, the major compounds were amino acids and organic acids with moderate SOD-like activity.[Citation43–Citation45] The 70% MeOH fraction contained phenolic acids, flavonoids and its glycosides, and glutathione. As phenolic acids, caffeic acid (peak N in ) and chlorogenic acid (peak P) were identified. Additionally, phenolic acid glycosides, such as caffeic acid-hexose (peak I), p-coumaric acid-hexose (peak J), and sinapic acid-hexose (peak K), were detected. Flavonoids and their glycosides, i.e., naringin (peak O), naringenin chalcone hexose (peak Q), quercetin-hexose-deoxyhexose-pentose (peak R), rutin (peak S), and wogonoside (peak M), were also identified. Glutathione was detected (peak L). Phenolic acids, flavonoids, and their glycosides with at least one aromatic ring bearing one or more hydroxyl groups possess strong SOD-like activity against ROS.[Citation46,Citation47] Glutathione is an important low-molecular-weight sulfhydryl containing anti-oxidant compounds in vivo.[Citation48] Vitamins and carotenoids, including vitamin C, lycopene, and β-carotene, were not detected. Thermally unstable Vitamin C might be lost in heat sterilization of the raw tomato juice and final product,[Citation49] and carotenoids (lycopene and β-carotene) with high lipophilicity might be precipitated and removed as residue by filtration in the vinegar production processes.
Table 2. Mass spectrometry ion data for compounds detected in the tomato vinegar fractions by liquid chromatography electrospray ionization mass spectrometry.
Figure 2. Chromatograms from liquid chromatography analyses of tomato vinegar: 10% MeOH (A) and 70% MeOH fraction (B). The detection wavelength was 215 nm.
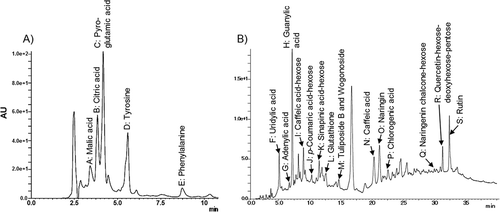
Based on the LC-MS analyses and the database collation, 19 distinct compounds were identified in the MeOH fractions. The 10 and 70% MeOH fraction contained SOD-like compounds with moderate and potent activity, respectively. The results supported higher SOD-like activity for the 70% MeOH fraction than the 10% MeOH fraction (). Tomato fruits contain abundant non-enzymatic SOD-like compounds, such as phenolic acids and flavonoids.[Citation12–Citation14] We have observed that the SOD-like activity of vinegar is greatly affected by the raw materials used for its production.[Citation8] Based on our results, we inferred that the tomato-derived compounds confer potent SOD-like activity in tomato vinegar.
The excessive generation and accumulation of ROS causes the activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase in cardiovascular tissue.[Citation50] NADPH oxidase activation impairs vasodilatory nitric oxide (NO) production and bioavailability in vascular smooth muscle cells,[Citation51] which causes vascular dysfunction.[Citation52,Citation53] Therefore, ROS scavenging can prevent vascular dysfunction. SOD converts superoxide anions, including ROS, into hydrogen peroxide.[Citation54] Accordingly, tomato vinegar, which contained tomato-derived SOD-like compounds, might have preventative effects on vascular dysfunction.
Conclusion
The quantification of organic acids, amino acids, sugars, and minerals as well as the LC-MS database collation analysis revealed existence of BPL and SOD-like compounds in tomato vinegar. Tomato vinegar contained abundant tomato-derived functional compounds with anti-hypertensive effects, such as GABA and potassium, in addition to acetic acid and pyro-Glu, which were produced during fermentation. Moreover, it had potent SOD-like activity, which was attributed to various tomato-derived compounds, including phenolic acids, flavonoids and its glycosides, and glutathione. Hypertension and vascular dysfunction are major risk factors of cardiovascular diseases, including stroke, heart failure, and atherosclerosis.[Citation55] These observed properties of tomato vinegar suggest that tomato vinegar is a useful functional food with potential preventive effects against cardiovascular diseases via its anti-hypertensive and SOD-like compounds.
References
- Parrondo, J.; Herrero, M.; García, L.A.; Díaz, M. A Note—Production of Vinegar from Whey. Journal of the Institute of Brewing 2003, 109, 356–358.
- Liljeberg, H.; Björck, I. Delayed Gastric Emptying Rate May Explain Improved Glycaemia in Healthy Subjects to a Starchy Meal with Added Vinegar. European Journal of Clinical Nutrition 1998, 52, 368–371.
- Kondo, S.; Tayama, K.; Tsukamoto, Y.; Ikeda, K.; Yamori, Y. Antihypertensive Effects of Acetic Acid and Vinegar on Spontaneously Hypertensive Rats. Bioscience, Biotechnology, and Biochemistry 2001, 65, 2690–2694.
- Fushimi, T.; Tayama, K.; Fukaya, M.; Kitakoshi, K.; Nakai, N.; Tsukamoto, Y.; Sato Y. Acetic Acid Feeding Enhances Glycogen Repletion in Liver and Skeletal Muscle of Rats. Journal of Nutrition 2001, 131, 1973–1977.
- Carmichael, F.J.; Saldivia, V.; Varghese, G.A.; Israel, Y.; Orrego, H. Ethanol-Induced Increase in Portal Blood Flow: Role of Acetate And A1- and A2-Adenosine Receptors. American Journal of Physiology 1988, 255, 417–423.
- Sakakibara, S.; Yamauchi, T.; Oshima, Y.; Tsukamoto, Y.; Kadowaki, T. Acetic Acid Activates Hepatic AMPK and Reduces Hyperglycemia in Diabetic KK-A(Y) Mice. Biochemical and Biophysical Research Communications 2006, 344, 597–604.
- Fushimi, T.; Suruga, K.; Oshima, Y.; Fukiharu, M.; Tsukamoto, Y.; Goda, T. Dietary Acetic Acid Reduces Serum Cholesterol and Triacylglycerols in Rats Fed a Cholesterol-Rich Diet. British Journal of Nutrition 2006, 95, 916–924.
- Nakamura, K.; Ogasawara, Y.; Endou, K.; Fujimori, S.; Koyama, M.; Akano, H. Phenolic Compounds Responsible for the Superoxide Dismutase-Like Activity in High-Brix Apple Vinegar. Journal of Agricultural and Food Chemistry 2010, 58, 10124–10132.
- Budak, N.H.; Aykin, E.; Seydim, A.C.; Greene, A.K.; Guzel-Seydim, Z.B. Functional Properties of Vinegar. Journal of Food Science 2014, 79, 757–764.
- Horiuchi, J.; Kanno, T.; Kobayashi M. New Vinegar Production from Onions. Journal of Bioscience and Bioengineering 1999, 88, 107–109.
- Food and Agriculture Organization of the United Nations, & World Health Organization. 2014. http://www.fao.org/ (access on January 24th, 2016).
- Alarcon-Flores, M.I.; Romero-Gonzalez, R.; Vidal, J.L.M.; Frenich A.G. Multiclass Determination of Phenolic Compounds in Different Varieties of Tomato and Lettuce by Ultra High Performance Liquid Chromatography Coupled to Tandem Mass Spectrometry. International Journal of Food Properties 2016, 19, 494–507.
- Siddiqui, M.W.; Chakraborty, I.; Homa, F.; Dhua, R.S. Bioactive Compounds and Antioxidant Capacity in Dark Green, Old Gold Crimson, Ripening Inhibitor, and Normal Tomatoes. International Journal of Food Properties 2016, 19, 688–699.
- Boggio, S.B.; Palatnik, J.F.; Heldt, H.W.; Valle, E.M. Changes in Amino Acid Composition and Nitrogen Metabolizing Enzymes in Ripening Fruits of Lycopersicon Esculentum Mill. Plant Science 2000, 159, 125–133.
- Hayakawa, K.; Kimura, M.; Kasaha, K.; Matsumoto, K.; Sansawa, H.; Yamori, Y. Effect of a γ-Aminobutyric Acid-Enriched Dairy Product on the Blood Pressure of Spontaneously Hypertensive and Normotensive Wistar–Kyoto Rats. British Journal of Nutrition 2004, 92, 411–417.
- Yoshimura, M.; Toyoshi, T.; Sano, A.; Izumi, T.; Fujii, T.; Konishi, C.; Inai, S.; Matsukura, C.; Fukuda, N.; Ezura, H.; Obata, A. Antihypertensive Effect of a Gamma-Aminobutyric Acid Rich Tomato Cultivar “DG03-9” in Spontaneously Hypertensive Rats. Journal of Agricultural and Food Chemistry 2010, 58, 615–619.
- Meneely, G.R.; Battarbee, H.D. High Sodium-Low Potassium Environment and Hypertension. American Journal of Cardiology 1976, 38, 768–785.
- Sies, H. Strategies of Antioxidant Defense. European Journal of Biochemistry 1993, 215, 213–219.
- Shahidi, F.; Wanasundara, P.K. Phenolic Antioxidants. Critical Reviews in Food Science and Nutrition 1992, 32, 67.
- Lee, J.H.; Cho, H.D.; Jeong, J.H.; Lee, M.K.; Jeong, Y.K.; Shim, K.H.; Seo, K.I. New Vinegar Produced by Tomato Suppresses Adipocyte Differentiation and Fat Accumulation in 3T3-L1 Cells and Obese Rat Model. Food Chemistry 2013, 141, 3241–3249.
- Seo, K.I.; Lee, J.; Choi, R.Y.; Lee, H.I.; Lee, J.H.; Jeong, Y.K.; Kim, M.J.; Lee, M.K. Anti-Obesity and Anti-Insulin Resistance Effects of Tomato Vinegar Beverage in Diet-Induced Obese Mice. Food and Function 2014, 5, 1579–1586.
- Yasui, A.; Mukiseibun (Mineral). In Sin Shokuhin Bunseki-Ho Methods of Food Analysis; Japanese Society for Food Science and Food Technology and Editorial Committee for Shin Shokuhin Bunseki-Ho, Korin: Tokyo, Japan, 1996; 156–199.
- Iijima, Y.; Nakamura, Y.; Ogata, Y.; Tanaka, K.; Sakurai, N.; Suda, K.; Suzuki, T.; Suzuki, H.; Okazaki, K.; Kitayama, M.; Kanaya, S.; Aoki, K.; Shibata, D. Metabolite Annotations Based on the Integration of Mass Spectral Information. The Plant Journal 2008, 54, 949–962.
- Matsuura, R.; Moriyama, H.; Takeda, N.; Yamamoto, K.; Morita, Y.; Shimamura, T.; Ukeda, H. Determination of Antioxidant Activity and Characterization of Antioxidant Phenolics in the Plum Vinegar Extract of Cherry Blossom (Prunus Lannesiana). Journal of Agricultural and Food Chemistry 2008, 56, 544–549.
- Callejón, R.M.; Troncoso, A.M.; Morales, M.L. Determination of Amino Acids in Grape-Derived Products: A Review. Talanta 2010, 81, 1143–1152.
- Lokeshwari, M.; Swamy, C.N. Vermicomposting of Municipal and Agricultural Solid Waste with Sewage Sludge. Journal of Environmental Research and Development 2008, 3. 51–61.
- Rossi, C.; Hauber, J.; Singer, T.P. Mitochondrial and Cytoplasmic Enzymes for the Reduction of Fumarate to Succinate in Yeast. Nature 1964, 10, 167–170.
- Gilbert, J.B.; Price, V.E.; Greenstein, J.P. Effect of Anions on the Non-Enzymatic Desamidation of Glutamine. The Journal of Biological Chemistry 1949, 180, 209–218.
- Semkina, G.A.; Matsievskii, D.D.; Mirzoyan, N.R. Effect of Pyrrolidone-Pyroglutamic Acid Composition on Blood Flow in Rat Middle Cerebral Artery. Bulletin of Experimental Biology and Medicine 2006, 141, 51–52.
- Brunner, H.R.; Baer, L.; Sealey, J.E.; Ledingham, J.G.; Laragh, J.H. The Influence of Potassium Administration and of Potassium Deprivation on Plasma Renin in Normal and Hypertensive Subjects. Journal of Clinical Investigation 1970, 49, 2128–2138.
- World Health Organization. A global brief on hypertension-silent killer, global public health crisis. WHO reference number: WHO/DCO/WHD/2013.2, 2013.
- Abushita, A.A.; Daood, H.G.; Biacs, P.A. Change in Carotenoids and Antioxidant Vitamins in Tomato as a Function of Varietal and Technological Factors. Journal of Agricultural and Food Chemistry 2000, 48, 2075–2081.
- Carrari, F.; Baxter, C.; Usadel, B.; Urbanczyk-Wochniak, E.; Zanor, M-I.; Nunes-Nesi, A.; Nikiforova, V.; Centero, D.; Ratzka, A.; Pauly, M.; Sweetlove, L-J.; Fernie, A.R. Integrated Analysis of Metabolite and Transcript Levels Reveals the Metabolic Shifts That Underlie Tomato Fruit Development and Highlight Regulatory Aspects of Metabolic Network Behavior. Plant Physiology 2006, 142, 1380–1396.
- Winter, M.; Herrmann, K. Esters and Glucosides of Hydroxycinnamic Acids in Vegetables. Journal of Agricultural and Food Chemistry 1986, 34, 616–620.
- Buta, J.G.; Spaulding, D.W. Endogenous Levels of Phenolics in Tomato Fruit During Growth and Maturation. Journal of Plant Growth Regulation 1997, 16, 43–46.
- Schmidtlein, H.; Herrmann, K. Uber die phenolsauren des gemuses. II. Hydroxyzimtsauren und hydroxybenzoesauren der frucht- und samengemusearten. Z Lebensm Unters Forsch [About the phenolic acid of Gemuses. II. Hydroxycinnamic acid and monohydroxybenzoic acid of fruit and Samengemusearten]. 1975, 159, 213–218.
- Shoji, K.; Ubukata, M.; Momonoi, K.; Tsuji, T.; Morimatsu, T. Anther-Specific Production of Antimicrobial Tuliposide B in Tulips. Journal of the Japanese Society for Horticultural Science 2005, 74, 469–475.
- Seo, O.N.; Kim, G.S.; Kim, Y. H.; Park, S.; Jeong, S.W.; Lee, S.J.; Jin, J.S.; Shin, S.C. Determination of Polyphenol Components of Korean Scutellaria Baicalensis Georgi Using Liquid Chromatography Tandem Mass Spectrometry: Contribution to Overall Antioxidant Activity. Journal of Functional Foods 2013, 5, 1741–1750.
- Le Gall, G.; Colquhoun, I.J.; Davis, A.L.; Collins, G.J.; Verhoeyen, M.E. Metabolite Profiling of Tomato (Lycopersicon Esculentum) Using 1HNMR Spectroscopy as a Tool to Detect Potential Unintended Effects Following a Genetic Modification. Journal of Agricultural and Food Chemistry 2003, 51, 2447–2456.
- Bovy, A.; de Vos, C.H.R.; Kemper, M.; Schijlen, E.; Almenar Pertejo, M.; Muir, S.; Collins, G.; Robinson, S.; Verhoeyen, M.; Hughes, S.; Santos-Buelga, C.; van Tunena, A. High-Flavonol Tomatoes Resulting from the Heterologous Expression of the Maize Transcription Factor Genes LC and C1. Plant Cell 2002, 14, 2509–2526.
- Bino, R.J.; de Vos, C.H.R.; Lieberman, M.; Hall, R.D.; Bovy, A.; Jonker, H.H.; Tikunov, Y.; Lommen, A.; Moco, S.; Levin, I. The Light-Hyperresponsive High Pigment-2dg Mutation of Tomato: Alterations in the Fruit Metabolome. New Phytologist 2005, 166, 427–438.
- Minoggio, M.; Bramati, L.; Simonetti, P.; Gardana, C.; Iemoli, L.; Santangelo, E.; Mauri, P.L.; Spigno, P.; Soressi, G.P.; Pietta, P.G. Polyphenol Pattern and Antioxidant Activity of Different Tomato Lines and Cultivars. Annals of Nutrition and Metabolism 2003, 47, 64–69.
- Hras, A.R.; Hadolin, M.; Knez, Z.; Bauman, D. Comparison of Antioxidative and Synergistic Effects of Rosemary Extract with α-Tocopherol, Ascorbyl Palmitate and Citric Acid in Sunflower Oil. Food Chemistry 2000, 71, 229–233.
- Hanachi, P.; Golkho, S.H. Using HPLC to Determination the Composition and Antioxidant Activity of Berberis Vulgaris. European Journal of Scientific Research 2009, 29, 47–54.
- Gülçin, I. Comparison of in Vitro Antioxidant and Antiradical Activities of l-Tyrosine and l-DOPA. Amino Acids 2007, 32, 431–438.
- Yu, L.; Haley, S.; Perret, J.; Harris, M.; Wilson, J.; Qian, M. Free Radical Scavenging Properties of Wheat Extracts. Journal of Agricultural and Food Chemistry 2002, 50, 1619–1624.
- Chen, H.; Zhou, Y.; Shao, Y.; Chen, F. Free Phenolic Acids in Shanxi Aged Vinegar: Changes During Aging and Synergistic Antioxidant Activities. International Journal of Food Properties 2016, 19, 1183–1193.
- Afanas’ev, I.B.; Dorozhko, A.I.; Brodskii, A.V.; Kostyuk, V.A.; Potapovitch, A.I. Chelating and Free Radical Scavenging Mechanisms of Inhibitory Action of Rutin and Quercetin in Lipid Peroxidation. Biochemical Pharmacology 1989, 38, 1763–1769.
- Gahler, S.; Otto, K.; Bohm, V. Alterations of Vitamin C, Total Phenolics, and Antioxidant Capacity as Affected by Processing Tomatoes to Different Products. Journal of Agricultural and Food Chemistry 2003, 51, 7962–7968.
- Martins, J.B.; Chaudhary, A.K.; Jiang, S.; Kwofie, M.; Mackie, P.; Miller, F.J. Role of NADPH Oxidase and Xanthine Oxidase in Mediating Inducible Vt/Vf and Triggered Activity in a Canine Model of Myocardial Ischemia. International Journal of Molecular Sciences 2014, 15, 20079–20100.
- Rodríguez, A.; Gómez-Ambrosi, J.; Catalán, V.; Fortuño, A.; Frühbeck, G. Leptin Inhibits the Proliferation of Vascular Smooth Muscle Cells Induced by Angiotensin II Through Nitric Oxide-Dependent Mechanisms.Mediators of Inflammation 2010, 2010, 105489.
- Cameron, E.N.; Cotter, A.M. Effects of Antioxidants on Nerve and Vascular Dysfunction in Experimental Diabetes. Diabetes Research and Clinical Practice 1999, 45, 137–146.
- Shi, J.; Yu, J.; Pohorly, J.E.; Kakuda, Y. Polyphenolics in Grape Seeds—Biochemistry and Functionality. Journal of Medicinal Food 2003, 6, 291–299.
- Karasu, C. Time Course of Changes in Endothelium-Dependent and –Independent Relaxation of Chronically Diabetic Aorta: Role of Reactive Oxygen Species. European Journal of Pharmacology 2000, 392, 163–173.
- Khalil, R.A. Hypertension and Vascular Dysfunction. In Interdisciplinary Concepts in Cardiovascular Health. Springer International Publishing: Cham, Switzerland, 2013; 1–37.
