ABSTRACT
Acid- and rennet-induced gelation properties of milk with modified casein micelles, produced by partial renneting at 4oC for 15 min, followed by inactivation of enzymes by heat at 60oC/3 min (referred as low heat treatment milk) and 85oC/30 min (high heat treatment milk), were investigated to provide a mechanistic understanding of gel formation from partially renneted milk. Acidification of low heat treatment milk gave firmer gel quality, this was reflected by its high elastic modulus (G′) and hardness. In addition, the high heating condition for enzyme inactivation of high heat treatment milk alone increased the elastic modulus of both the control and renneted milk samples. Gel development of the two milk types (low heat treatment and high heat treatment milks) was different. In contrast with acid gelation, rennet-induced gelation of partially pre-rennet treated milk had no impact on the elastic modulus of low heat treatment milk and the rennet gels were very weak. Similarly, the addition of rennet to pre-rennet treated high heat treatment milk did not produce “true gels,” most likely due to the effect of the heat treatment on impairing the rennet coagulation. The findings in this study confirmed that pre-rennet treated milk had positive effects on the end-product acid gels of low heat treatment and high heat treatment milk.
Introduction
Gelation is the formation of a three-dimensional protein matrix of aggregated protein. There are several factors that can cause the gelation of milk, especially the addition of acid and rennet. Acid and rennet-induced gels are different, although in both cases the stability of casein micelles is lost, leading to aggregation.[Citation1,Citation2] Gelation induced by acid, rennet or a combination of acid and rennet, is very practical in dairy products, and it is used for making yogurt, natural or/and fresh cheese.
Glucono-δ-lactone (GDL) often used to study the mechanism and effects of acid gelation. GDL degrades to gluconic acid in which the degradation rate can be regulated to match the rate of development of acid in fermented foods. The application of acid causes a decrease in milk pH. The change in pH then modifies the nature of the milk environment, causing a release of colloidal calcium phosphate (CCP) from the micelles[Citation1] and the collapse of the hairy layer of casein micelles.[Citation3] This collapse reduces the protective ability, leading to instability of casein micelles[Citation4] and, in some cases, affects the casein micelle size.[Citation5,Citation6] The loss of steric and electrostatic stability by acidification results in the formation of a three-dimensional gel network. The modified casein micelles then start to get closer to each other, forming a thread-like gel. Finally, the casein micelles develop a full gel structure at their isoelectric point ~ pH 4.6 to 4.8.[Citation7]
In rennet gelation, the enzyme added primarily targets the κ-casein of casein micelles. The surface location of the κ-casein component makes it readily accessible for enzyme hydrolysis. The enzyme cleaves the κ-casein into para-κ-casein that is still intact on the micelle, and caseinomacropeptide (CMP), known as hairy layers in the micelle surface, which are released into the serum milk.[Citation2] CMP contains the hydrophilic parts of κ-casein, which is primarily responsible for the steric stabilization of the casein micelles. The absence of CMP also reduces the particle charge of the micelles and eventually diminishes the micelles’ stability.[Citation8,Citation9] The full enzyme reaction then completely destroys the steric stabilization of the modified casein micelles. The final net result is that aggregation takes place.
There are several reports in the literature that describe the gelation properties of milks with various heat treatments, solids concentrations, and composition. Some articles examined the acid-gelation that occurs in yogurt making or acid-cheese production, using skim milk,[Citation10] concentrated skim milk,[Citation11] heated milk[Citation12,Citation13] including heated milk in alkaline pH conditions,[Citation14,Citation15] and heated milk with various temperature and holding times.[Citation16] Acidification has also been observed in milk with pH alteration,[Citation17] in pressure-treated milk,[Citation18] high-pressure homogenized milk,[Citation19] and sonicated milk.[Citation20] Others have reported on the behavior of rennet gelation (as in the manufacture of cheese) of cold milk,[Citation21] unheated skim milk,[Citation22–Citation27] and milk protein concentrate (MPC85),[Citation28] as well as heated skim milk.[Citation29] Most publications related combined acid-rennet gels have only focused on either unheated or heated milk.[Citation30–Citation32] There is a less understanding of the effects of the combination of acid (either by the addition of GDL or with mesophilic cultures) and rennet gelation. The gelation properties of such milk have showed different gel properties or final elastic modulus values.
Partial renneting is applied for specific purpose, such as controlling the enzyme reaction, so that the desired protein hydrolysis level can be targeted. Partial renneting can be employed by lowering the enzyme concentration, reducing the renneting time, incubating at low temperature or by using all of the applied treatments.[Citation6] There is relatively little information available on the gelation of milk containing casein micelles that have been pre-treated with rennet. In this study, the partial renneting action was expected to modify casein micelle’s behavior, with the changes in these fundamental properties then affecting their gelation properties. The aim of this study was to provide more information on the characteristics of partially rennet pre-treated milk following acid- and rennet-induced gelation. Gel development, gelation time, elastic modulus, gel hardness, and syneresis were the gelation properties measured.
Materials and methods
Materials
There were two types of pasteurised milk samples used in this study.[Citation6] The treatments represented are the following:
Milk with 15 min at 4oC rennet treatment followed by enzyme inactivation using heat (60oC) for 3 min (low heat treatment milk [LHT])
Milk with 15 min rennet treatment followed by enzyme inactivation by heating the milk to 85oC for 30 min (high heat treatment milk [HHT])
Rennet (Chymosin EC 3.4.23.4; Chy-Max Plus; 200 international milk coagulating units (IMCU) mL−1) was kindly provided by CHR Hansen Pty Ltd. Melbourne, Australia. The rennet concentrations used were 0.00, 0.01, 0.02, and 0.03 IMCU mL−1, with CMP release at level of 0, 10, 20, and 15%, respectively.[Citation6] Control or milk with 0.00 IMCU mL−1 rennet, was milk with the addition of water to represent the same amount of enzyme added to renneted milk. Renneted milk was used for milk with partial renneting treatment. The experimental procedure from this point on are common to all samples, which have been pre-treated with rennet and exposed to either low or high heat.
Rheological measurement
For acid-induced gel, milk samples were acidified using 2% (w/w) GDL, following the procedure of others.[Citation33] For rennet-induced gelation, a preliminary experiment on the series of levels of rennet concentrations (0.03 to 0.18 IMCU mL−1) added was conducted. It was found that 0.09 IMCU mL−1 was suitable for showing the best gel development, in which the G′ values reached their plateau within 360 min.
The coagulant was added to the sample (20 mL) in a beaker; the mixture was then stirred for 2 min, after which 660 µL of the sample was transferred to a rheometer. A water trap and cover arrangement was placed over the sample to prevent evaporation. A time sweep at 30oC for 360 min gelation and a temperature sweep from 30 to 5oC after the completion of gelation were run. The remainder of the sample was used to determine milk pH at regular intervals during the rheology measurement. The change in pH at 30oC over time was monitored using a standard pH meter. All samples were found to be at ~pH 4.5 after about 150 min.
The rheological properties were determined in a stress and strain control rheometer Discovery HR1 model (DHR-1; TA instruments, USA) using TRIOS software with a cone geometry (diameter 40 mm, a cone angle 2o, cone truncation 54 nm) at a frequency of 0.1 Hz, with a constant oscillation stress of 0.1 Pa, a strain of 0.5%, and a temperature of 30 ± 0.1oC, as described by others.[Citation34] In this study, gelation time was defined as the point when gels had a G′ = 1 Pa, representing a transition from liquid-like to solid-like behavior. Accordingly, the milk pH in which the G′ reached ≥1 Pa was determined as gelation pH. Rheological measurements of samples were carried out twice under the selected testing conditions (varied milk types and pre-rennet concentration). A representative measurement was chosen for each sample for the presentation of the development of G′ in the results section.
Gel hardness and syneresis test
Gel hardness of the bulk sample was measured using a TA-XT2 texture analyzer (Brookfield CT3 Texture Analyzer, Essex, UK), controlled by a personal computer (PC) equipped with Exponent (version 5.0.8.0) software. Measurements were made in triplicate at 25oC, following the procedure described by others.[Citation35] Samples were prepared in a container of diameter 40 mm and the sample height was 40 mm. The penetrometric test was performed 360 min after the coagulation agent was added. A flat base cylinder probe (diameter 12.7 mm) was used, with a test speed 1.00 mm s−1. Gel hardness was defined as the maximum force needed to break the curd.
The syneresis test was performed by using a centrifugation test, as described by others.[Citation36,Citation37] After the addition of coagulating agents, 40 g of the milk was held undisturbed in a water bath at 30oC for 360 min incubation. Then, the gels were centrifuged at 1000 × g for 15 min at 25oC. The liberated liquid of the curd was poured off, weighed, and expressed as a percentage of grams of liquid to the weight of the original sample. Tests were done in triplicate for each sample.
Statistical analysis
Experiments were conducted in triplicate (randomised complete block design), unless otherwise stated. Significant differences were analyzed by using analysis of variance (ANOVA) with Tukey’s honest significant difference (HSD) test using Minitab 16. All graphs were plotted by using SigmaPlot 12.5.
Results and discussion
Time sweep
A time sweep was initially performed throughout the study to monitor the build-up or the breakdown of gel structure through changes in the elastic modulus (G′) values (). In addition, enzyme inactivation in response to the application of LHT and HHT milk, respectively, had different effects on the elastic modulus of the resulting gels, as shown in . Generally, acidification of partially rennet treated milk with various concentrations gave different gelation behavior when compared to that of the control milk.
Table 1. Effect of glucono-δ-lactone (GDL) addition on the properties of acid-induced gels made from low heat (LHT) and high heat treatment (HHT) milk with rennet concentrations of 0, 0.01, 0.02, and 0.03 IMCU mL−1. Gels were formed at 30oC for 360 min.
Figure 1. Representative graphs of elastic modulus (G′) as a function of time (A and B) and milk pH (C and D) for acid-induced gels made from enzyme treated low heat (A and C) and high heat treatment (B, and D) milk with rennet concentrations of 0.00 (●), 0.01 (○), 0.02 (Δ), and 0.03 (□) IMCU mL−1. Gels were made at 30oC for 6 h.
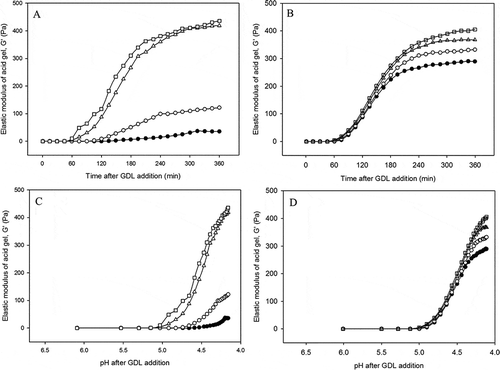
In LHT milk, the G′ of control milk gel was significantly lower than that of the rennet-altered milk (). When milk was acidified with GDL, the G′ of control milk was <1 Pa for the first hour; only renneted LHT samples started to gel. This indicates that the higher degree of κ-casein breakdown affects the gelation behavior from the time of acidulant addition. The onset of gelation of the control LHT milk started after 120 min of GDL incubation (). The gel development of control milk was very slow and the final G′ was around 30 Pa. In all renneted samples, the onset of gelation started earlier, while during gel ageing the G′ increased to their maximum G′ after 360 min gelation time. Moreover, the G′ value of gels rose with increases in the rennet concentration. It was clear that even with the lowest concentration of rennet (0.01 IMCU mL−1), the changes in G′ were very clear.
High thermal inactivation caused different gel behavior for HHT milk (). It can be clearly seen that all HHT samples started to gel within the first hour. After the onset of gelation, a sudden increase in elastic modulus developed, the increase being with the higher pre-rennet levels. The final G′ values of gels made from control milk was ~280 Pa. The elasticity of HHT milk increased to >300 Pa during gel ageing; however, the rate of increase became slower, especially after 240 min of GDL addition. Compared to control LHT milk, acid gelation of control HHT milk resulted in a 10 times higher final G′. As the difference between LHT and HHT milk was only the temperature used for enzyme inactivation, the elevated G′ of control HHT milk suggests that heat treatment alone produced gel with higher elasticity. The effect of heat treatment on acid gel texture and rheology is very well-known and widely applied in yogurt processing.[Citation10,Citation38,Citation39] In addition, gel with the lowest rennet level (0.01 IMCU mL−1) produced a higher elasticity (3×) when compared to that of its corresponding LHT milk at pH < 4.6. This result indicated a similar effect of heat treatment on renneted HHT samples, although the effect was lower when compared to that of control HHT milk. It was interesting to note that renneted samples showed the same gel development trends as the control gel, but produced higher G′ values. The G′ values range in this study was in agreement with another reported study, who found that final G′ of renneted samples ranged from 300 to 400 Pa.[Citation40] Although the onset of gelation was statistically similar, a higher rennet level tended to cause a shorter gelation time in the range of 50 to 65 min of GDL incubation. In addition, a similar pattern up to 180 min after GDL addition was also detected. This finding might indicate that the heat treatment had a greater effect than pre-partial renneting treatment, during the initial stages of gel development.
Following either the differences in G′ or gelation time of acid gels made from LHT and HHT milk, it can clearly be seen that their gel development behavior was not the same. In LHT milk, presumably, the degree of κ-casein breakdown affected gel development from the time the milk started to gel, which was indicated by the significant difference in gelation time for all samples. The mild discrepancy of HHT milk gelation time indicated that the heat treatment can have an effect on the initial onset of gelation, while the partial renneting treatment affected the subsequent gel development during gelation ageing.
Representative graphs of elastic modulus values as a function of milk pH are shown in and . For all renneted LHT and HHT samples, the G′ increased rapidly with the decrease in pH after the onset of gelation. In LHT samples, control milk showed gelation only at a low pH of 4.61. Moreover, the onset of gelation of milk with the lowest amount of enzyme addition occurred at pH 4.78. In milk with higher rennet concentration, gelation started at almost the same pH (~5.05; ). Gel development was very fast after the onset of gelation, especially for gels with the highest rennet concentration. In HHT milk, control and milk with lower concentration enzyme pre-treatment started to gel at pH around 5.0. Gel development started to increase sharply at pH below 5.0. For renneted HHT milk, there were positive correlations between the extent of κ-casein hydrolysis and G′ values at any particular pH (). As the shape of gel growth for the control and renneted HHT milk up to pH 4.5 were qualitatively similar, these results indicated that the extent of κ-casein hydrolysis would have a greater impact on gel development at pH < 4.5. The higher onset of gelation pH of acid gel made from severe heated milk is well-established.[Citation16,Citation41]
Two important mechanisms (internal and external structural) occur in casein micelles in response to the lowering of pH by GDL addition in this study. First, the change in the internal structure is also followed by changes in casein micelle size, as the micellar calcium is progressively dissolved into the serum milk. Second, there is also a modification of the external layer of CMs caused by the collapse of the hairy layer. Hence, the casein micelles become less stable and their inter-particle contact occurs as the milk gets closer to their isoelectric pH of ~4.6.[Citation3,Citation4,Citation42]
In the control LHT milk, the lower G′ of the acid gel indicates the weakness of the gel. The gel weakness may be in response to the surface layer of control milk being dominated by the presence of CMP. Although the CMPs are no longer “hairy,” they are still hydrophilic, providing a hydrated layer and preventing close contact between the micelles.[Citation43,Citation44] Therefore, even though the hairy layers collapsed at lower pH, their presence in the surface layer still affects the interface between the aggregating micelles. The formation of weak acid gels (<30 Pa) made from control or unmodified milk, has also been reported by the others.[Citation39,Citation40]
For LHT renneted samples, partial renneting prior to acidification resulted in the release of CMP to the solution, leaving the surface with small gaps. These gaps provide “reactive spots” on the surface of casein micelles. The decrease in hairy thickness as the result of the collapsing hairy layer facilitates the interaction of the reactive spots between the casein micelles. Higher κ-casein hydrolysis levels produced more reactive gaps, which enabled more extensive contact amongst the casein micelles, leading to higher G′ with the increase in rennet level.[Citation45,Citation46] The large increase in G′ gel formed from enzyme pre-treated milk at pHs higher than 4.6 can be explained in this way.
It is also noted that the application of severe thermal enzyme inactivation prior to coagulant addition resulted in a number of changes in their acid gelation properties. It is well-known that heat causes whey protein to denature. The denatured whey protein then makes complex associations with intact κ-casein on the surface of the micelle (surface complexes) and κ-casein in the serum milk (serum complexes) via the formation of disulphide bonds.[Citation47–Citation49] The binding of whey protein onto the casein micelles could create a “new version of intact κ-casein” on the surface of the micelles. For acid-induced gelation, this means that the onset of gelation pH would be higher (i.e., at pH ~5.1), due to the higher isoelectric point of whey proteins that coat the casein micelles.[Citation50–Citation52] As the result, earlier onset of gelation of HHT milk can be expected when compared to LHT milk, as was shown in this study.
In addition, the surface and serum κ-casein/ whey protein complexes can also participate in the acid gel matrix.[Citation41,Citation50,Citation53] They can bind to the inter particles and also act as a bridge between the aggregating micelles,[Citation47] and hence increase the number of bonds and strength between particles.[Citation50,Citation54,Citation55] This would then lead to the formation of stronger gels, leading to the increased elastic modulus of gels made from renneted HHT milk. This phenomenon not only happened in gels made from the control HHT milk, but also appeared in the gel of rennet-treated HHT milk, because similar severe heat was applied to all HHT milk. Furthermore, it was also observed that heating did not affect the pH profile during acidification. Therefore, the reduced gelation time was associated with milk gelling at higher pH.
The similarity in the onset of the gelation time or the narrow range of onset of gelation pH for all control and renneted HHT samples, indicated that, at the start, all milk shared the same pattern of gel development. The initial aggregation could be driven by the heat treatment, probably because all the samples were coated with the complex formations. Therefore, the surface layer would be mainly affected by the denatured whey protein rather than the nature of native or altered casein micelles.
Temperature sweep
Temperature sweeps were performed after 360 min of gelation to examine changes in the rheological behavior, especially G′ value. To understand the combination effect of time and temperature, the G′ values were monitored until the temperature was reduced to 5oC. Generally, the G′ of LHT and HHT samples increased to some extent at lower temperatures, dependent on the concentration of the enzyme added ( and ).
Figure 2. Representative graphs of elastic modulus (G′) as a function of temperature for acid-induced gels made from enzyme treated low heat (A) and high heat treatment (B) milk with rennet concentrations of 0.00 (●), 0.01 (○), 0.02 (Δ) and 0.03 (□) IMCU mL−1. Gels were made at 30oC for 6 h and cooled down to 5oC.
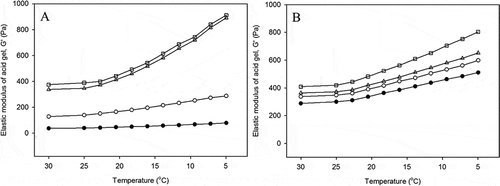
LHT milk with lowest the concentration of added rennet had qualitatively similar behavior to the control milk, in which the G′ increased around 50% upon cooling to 5oC (). Moreover, the G′ of milk with the addition of a higher amount of rennet increased sharply following the decrease in temperature, reaching G′ values of >800 Pa. Similarly, the decrease in temperature resulted in an increase of G′ of HHT milk (). The elastic modulus of control milk was lower relative to all renneted HHT milk, at any particular temperature. Lowering the temperature to 25oC resulted in an increase in all G′ values, with the G′ values almost doubling at 5oC, especially for gels made from LHT and HHT milk with higher pre-rennet levels. The increase of G′ can be related to gels that were more solid-like at low temperature. Similar behavior has been previously reported relating to the study of acid casein gels.[Citation56,Citation57] They reported that cooling gels from the incubation temperatures of 30 or 40oC caused an increase in G′, with the elastic modulus almost doubling with a drop in the temperature from 40 to 5oC. In this study, the increase in G′ was slow until the temperature dropped to 25oC, while the gels became rapidly firmer below 20oC. The high G′ could possibly be the result of extensive swelling of casein particles at low temperature, combined with an increase in the contact area between particles.[Citation57] A large contact area means the strengthening some types interactions that are affected by cooling conditions (e.g., hydrogen bonding), which is reflected in the higher G′ with decreasing temperature.[Citation52,Citation57]
Acid gel hardness and syneresis
Rheology measurement of the LHT and HHT samples was followed by characterization of bulk gel hardness, which was measured by using a texture analyzer ( and ). The hardness of the gel was defined as the peak force of the first compression of the product. From both graphs, it was apparent that the increase in enzyme concentration improved the hardness of the gels. Thus, increasing the rennet level not only shortened the gelation time, but also produced a firmer curd. The hardness of gels made from control LHT () and HHT () milk was around 10 g, which was in the range of gel hardness reported in another study.[Citation58] The hardness values of the gels made from renneted LHT and HHT milk ranged from 20 to 35 g and 10 to 20 g, respectively. In LHT milk, the highest gel hardness was obtained with the higher pre-rennet concentration, which was up to 3 times higher than that of control LHT milk. Texture analysis supported that the extensive contacts between casein micelles due to the partial removal of the hairy layer not only increased the G′ values, but also produced higher bulk gel hardness. The increased hardness of the renneted LHT product when compared with the control LHT milk also means that the gel structure is stronger in response to partial enzyme treatment. Similarly, the application of severe heat of HHT milk also affected the gel hardness, although the effect was less than that on the hardness of LHT gels. The hardness of renneted samples increased relative to the pre-rennet level, with increased rennet levels of 0.02 and 0.03 IMCU mL−1 producing ~50% firmer gel when compared to the control HHT gel.
Figure 3. Gel hardness (A and B) and syneresis (C and D) of acid-induced bulk gel made from enzyme treated low heat (A and C) and high heat treatment (B and D) milk. Gels were formed at 30oC for 6 h.
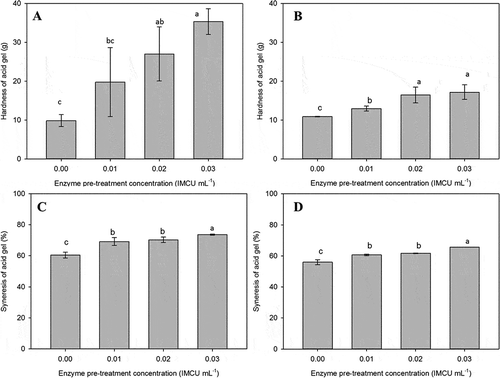
Syneresis is also known as serum holding capacity and is defined as the amount of whey expelled from the curd after centrifugation of milk gels. Under the experimental conditions, the acid gels of LHT milk did not show any apparent syneresis if the gels were allowed to form undisturbed. The centrifugation test showed that control and rennet-altered LHT milk had syneresis values above 60% (). Higher κ-casein breakdown increased the syneresis level by around 10%, which was significantly different from the control milk. This indicated that more serum was expelled from the curd after partial rennet treatment, in which the values ranged between 60 to 75%. For HHT milk, control milk had a syneresis value around 55%, which was less than the syneresis of gels made from control LHT milk. Similar to the trend in acid gels of LHT milk, the renneted HHT milk gave an increase in syneresis value with higher pre-rennet application (). In general, the syneresis level of HHT milk was lower than for corresponding LHT milk, indicating that the HHT milk had a better water holding capacity compared to LHT milk.
Other reports noted that heat treatment reduced the separation of whey from the gel.[Citation37,Citation59–Citation61] They proposed that a higher syneresis level of acid gel of enzyme treated milk was due to the alteration in their interaction in the gel network. Another study has proposed that syneresis of acid-rennet gels is higher than from pure-acid gels (in this study: control acid gels) because the former samples had higher tan δ values. In a rheological study by Vanvliet et al.,[Citation62] it was reported that a higher tan δ is related to greater tendency to exhibit syneresis.[Citation62] In this study, the tan δ of renneted gel (0.25–0.29) was also higher than for the control milk gel (0.23). As the tan δ is due to the balance between viscous and elastic components, the increase in tan δ values suggests gels having a more viscous character and may lead to the expulsion of more liquid from the gels. The increase in gel strength and syneresis following higher rennet dosage on milk concentrates has also been reported by others.[Citation61] The syneresis caused by the mechanical (in this case centrifugal force) may not necessarily mean that there will be a higher level of syneresis in the product at rest.
In addition to a higher tan δ of gels made of HHT milk, a microstructure study of acid gels made from heated milk reported that the application of severe heat to milk prior to acidification produced gels with lower porosity. The low porosity would then cause lower syneresis, as it can enhance the water holding capacity of the protein network.[Citation39] In contrast, gels made from unheated milk gave a similar microstructure to milk heated at <75oC, which appeared to have an irregular protein clustered network. Therefore, it can be noted that the differences in enzyme inactivation temperature affects the gel microstructure.
Syneresis is also an important parameter in the processing of milk. As syneresis is not expected to occur in yogurt production,[Citation58] this finding on HHT milk supports the use of pre-heated milk for yogurt production. However, for cheese- or curd-making, syneresis is desirable.[Citation60] As syneresis level of renneted LHT samples was higher than that of control milk, it might be possible for rennet-altered treatment before acidification to also be used as a pre-treatment to promote syneresis in cheese production.
Rennet-induced gelation of LHT and HHT enzyme pre-treated milks
Time sweep
In this study, the rennet gelation property of partially rennet treated milk was investigated. The gelation pH for all gels made with rennet was the natural pH of milk (6.62), as has been reported for other studies.[Citation63] In acid-induced gelation, it was noted that acid coagulant had a significant effect on gels made from LHT and HHT milk. Pre-rennet and thermal denaturation of whey protein resulted in an increase in elastic modulus and onset of gelation pH, and a decrease in the gelation time of acid gel. However, the opposite effects were obtained for rennet gel.
The experiments with rennet-induced gel demonstrated that the pre-rennet treatment to modify the casein micelle had a negative effect on elastic modulus values of LHT milk ( and ). The G′ of control and renneted LHT milk were >1 Pa in the first hour. Then, the gel development was very fast; the G′ values increased two-fold after 120 min of rennet addition. From 120 to 360 min, the increase in G′ was slow. Quantitatively, the highest G′ value was observed for casein gels of control LHT milk. The final G′ value for rennet-induced gel made from control milk was ~65 Pa, which was in agreement with other reports.[Citation64] Then, the trend of gel development was similar between control milk and rennet-altered milk after rennet application, up to 360 min. However, the increase of pre-rennet concentration tended to produce gels with lower elastic modulus and longer gelation time. The small differences in the average values of G′ between pre-rennet samples, indicates that the addition of rennet prior to rennet gelation did not improve the gel elasticity. This could arise from initial differences in the level of κ-casein hydrolysis as a result of pre-rennet treatment. For renneted LHT milk, the higher amount of CMP released suggests that the extent of κ-casein hydrolysis increased with increased rennet concentration. When the κ-casein was hydrolyzed, the number of intact κ-casein was reduced. As the primary target of rennet is the κ-casein, the reduced amount of substrate (i.e., κ-casein) in rennet-altered milk was the limiting factor in the reaction, leading to a looser gel network. Zoon and co-workers reported several important parameters that are related to the rheological properties of rennet-induced gel, including the effects of temperature,[Citation65] calcium and phosphate,[Citation66] along with pH and NaCl.[Citation67] However, none of these effects applied to the experimental milk in this study, because the difference between the control and enzyme treated milk was only in their surface layer, for example, the number of intact κ-casein. Therefore, the alteration in their gelation properties was little affected by the environment conditions among the samples.
Table 2. Effect of rennet addition on the gelation properties of rennet-induced gels made from low heat (LHT) and high heat treatment (HHT) milk with rennet concentrations of 0, 0.01, 0.02, and 0.03 IMCU mL−1. Gels were formed at 30oC for 360 min.
Figure 4. Representative graphs of elastic modulus (G′) as a function of time for rennet-induced gels made from enzyme treated low heat (A) and high heat treatment (B) milk with rennet concentrations of 0.00 (●), 0.01 (○), 0.02 (Δ), and 0.03 (□) IMCU mL−1. Gels were made at 30oC and cooled down to 5oC.
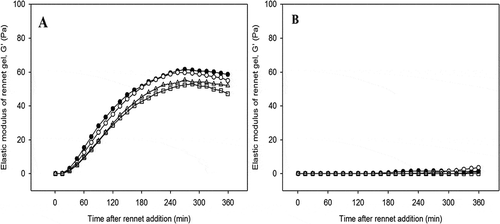
In contrast, the addition of 0.09 IMCU mL−1 rennet was not sufficient to initiate the gel formation of HHT milk (). Therefore, no gel was formed in HHT samples with this rennet concentration, although some gels showed ≥1 Pa. It was observed that the pre-rennet treatment alone, such as in LHT milk and in combination with severe heat treatment as in HHT milk, did not have a positive effect on the rennet-induced gelation. Compared to LHT milk with mild thermal enzyme inactivation, severe heat to HHT milk markedly reduced the elastic modulus of the gels. This was in broad agreement with Anema and others,[Citation68] who reported that the G′ of unheated milk was ~70 Pa, while heating the milk decreased the G′ to 10 Pa.
The poor renneting of samples because of the whey protein and κ-casein complexes has also been referred to in the literature.[Citation69,Citation70] In HHT milk, the protruding hair was reported to be covered by denatured whey protein, forming “filamentous appendages” in the micelle surface.[Citation16] Many studies have suggested that denatured whey protein and κ-casein at the casein micelle surface delay the primary phase, as the chymosin-sensitive-bond of the casein micelles becomes covered by the complexes.[Citation71] Therefore, the modified casein micelles became “more stable”[Citation29] and the prolonged rennet incubation for up to 360 min in the current study did not initiate aggregation of the samples, resulting in no gel being formed. Moreover, as the accessibility to the reactive sites was very limited, the formation of fewer interactions would result in weaker bonds, hence very weak gels.
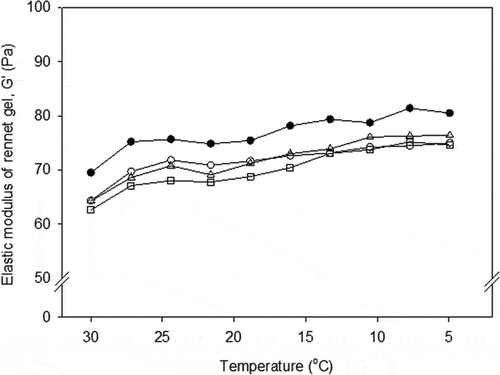
Temperature sweep
Lowering the temperature to 5oC had a similar effect on the G′ of the control and renneted samples made from LHT milk (). Subsequent cooling of the rennet gels resulted in a slight increase in G′ values. The final G′ ranged from 50 to 70 Pa. The higher final G′ values at 30oC were followed by the greater G′ of control milk when compared to renneted LHT milk at any particular temperature. Cold storage of gels made from LHT milk could double the G′ of acid gels, but a similar affect was not apparent for rennet gels. For HHT milk, cooling the rennet gels was not presented, as “true” gels were not formed.
Rennet gel hardness and syneresis
The bulk gels were analyzed by a texture analyzer for their hardness after 360 min of rennet addition (). Similar to the rheological parameters, acid and rennet-induced gels of enzyme-pre-treated LHT milk had opposite effects on gel strength (G′ and firmness). The concentration of enzyme during pretreatment had no significant effect on the rennet gel hardness. Gel hardness value of the control gel was around 13 g, while the value of renneted samples ranged between 8 to 12 g. Gels made from enzyme pre-treated LHT milk did not show any statistically significant differences between each other. However, they tended to form weaker gels (up to 30% reduction in firmness) when compared to gels made from control LHT milk.
Figure 6. Gel hardness (A and B) and syneresis (C) of rennet-induced bulk gels made from enzyme treated low heat (A and C) and high heat treatment (B). Gels were formed at 30oC for 6 h.
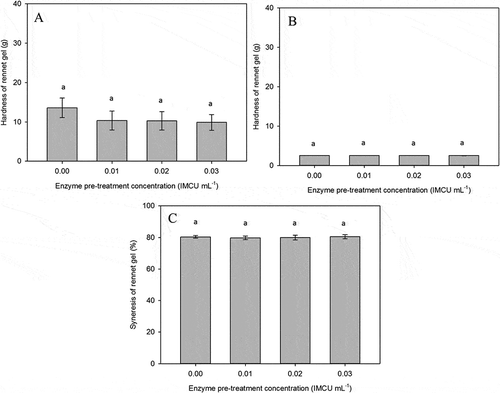
The syneresis test results on rennet-induced gel of enzyme pre-treated LHT milk are presented in . Following comparable results of gel hardness, rennet gel made from the control and renneted samples had similar syneresis levels, around 80%. The similarity in syneresis levels was also supported by the comparable value of tan δ (0.31–0.34). This implied that the pre-rennet treatment did not affect the amount of whey expelled from the rennet curd. The mild variation in elastic modulus values might be responsible for the similarity of their syneresis levels. Again, this behavior differed in a comparison with acidification of the LHT samples, in which the enzyme pre-treatment had an impact on syneresis level. In the experimental conditions of this study, it was also found that syneresis of LHT rennet gels was greater than that for LHT acid gels; a similar result was reported in an earlier study.[Citation72] The higher syneresis level was also supported by the larger value of tan δ of rennet gels (0.31–0.34) when compared to that of acid gels (0.25–0.29; results not shown).
The lack of gel formation after the addition of rennet to HHT milk prevented the measurement of hardness and syneresis of the gel.
Conclusions
In the dairy industry, heat treatment of milk is used to increase the performance of the product. It can be concluded from this study that thermal denatured whey protein contributed to the difference in gel development of pre-rennet treated LHT and HHT milks, especially for control and milk with very low rennet concentration. The initial gel development of HHT milk was more affected by the whey protein-casein complexes. In most cases, rennet-induced gels did not improve gelation properties. For example, rennet addition to renneted LHT milk had no significant effect on rennet gels. A tendency for an increase in rennet gelation time and a decrease in gel firmness were observed with increased pre-rennet concentration. In addition, the application of severe heat impaired the rennet gelation and the “true gel” did not form. The significant finding from this study relates to the impact of partial renneting by the addition of a small amount of rennet with only 15 min incubation. It was noted that acid-induced gelation could improve the gelation properties, in which the milk samples containing hydrolyzed κ-casein can be used to produce stronger gels. Therefore, partial rennet treated milk may provide a product with unique functional properties which could be useful for the development of new or improved dairy products.
Acknowledgment
The authors would like to thank Dr John M. Schiller (Honorary Senior Fellow, School of Agriculture and Food Sciences, The University of Queensland), for his assistance in the proofreading of this manuscript.
Funding
The authors would like to thank Dairy Innovation Australia Ltd. (DIAL) for the financial support provided to undertake this research, DIKTI for the financial support granted to Hotnida Sinaga during her study.
Additional information
Funding
References
- Dalgleish, D.G.; Law, A.J.R. pH-Induced Dissociation of Bovine Casein Micelles. 2. Mineral Solubilization and Its Relation to Casein Release. Journal of Dairy Research 1989, 56(5), 727–735.
- Mistry, V.V. Chymosin in Cheese Making. In Food Biochemistry and Food Processing; Simpson, B.K.; Ed.; Wiley-Blackwell: Ames, IA, 2012; 223–231.
- De Kruif, C.G. Skim Milk Acidification. Journal of Colloid and Interface Science 1997, 185(1), 19–25.
- McMahon, D.J.; Du, H.; McManus, W.R.; Larsen, K.M. Microstructural Changes in Casein Supramolecules During Acidification of Skim Milk. Journal of Dairy Science 2009, 92(12), 5854–5867.
- Sinaga, H. Effect of manipulation of casein micelles on the functional properties of milk, in School of Land and Food Sciences. Ph.D. Thesis, University of Queensland: Brisbane, Australia, May 2015, p. 232.
- Sinaga, H.; Bansal, N.; Bhandari, B. Partial Renneting of Pasteurised Bovine Milk: Casein Micelle Size, Heat and Storage Stability. Food Research International 2016, 84, 52–60.
- Ye, R.; Harte, F. Casein Maps: Effect of Ethanol, pH, Temperature, and Cacl(2) on the Particle Size of Reconstituted Casein Micelles. Journal of Dairy Science 2013, 96(2), 799–805.
- Augustin, M.A.; Udabage, P. Influence of Processing on Functionality of Milk and Dairy Proteins. In Advances in Food and Nutrition Research; Steve, L.T.; Ed.; Academic Press: Cambridge, MA, 2007; 1–38.
- Walstra, P.; Bloomfield, V.A.; Wei, G.J.; Jenness, R. Effect of Chymosin Action on the Hydrodynamic Diameter of Casein Micelles. Biochimica et Biophysica Acta 1981, 669(2), 258–259.
- Anema, S.G. Effect of Temperature and Rate of Acidification on the Rheological Properties of Acid Skim Milk Gels. Journal of Food Processing and Preservation 2008b, 32(6), 1016–1033.
- Bienvenue, A.; Jimenez-Flores, R.; Singh, H. Rheological Properties of Concentrated Skim Milk: Influence of Heat Treatment and Genetic Variants on the Changes in Viscosity During Storage. Journal of Agricultural and Food Chemistry 2003, 51(22), 6488–6494.
- Purwanti, N.; Smiddy, M.; van der Goot, A.J.; de Vries, R.; Alting, A.; Boom, R. Modulation of Rheological Properties by Heat-Induced Aggregation of Whey Protein Solution. Food Hydrocolloids 2011, 25(6), 1482–1489.
- Anema, S.G.; Lee, S.K.; Lowe, E.K.; Klostermeyer, H. Rheological Properties of Acid Gels Prepared from Heated pH-Adjusted Skim Milk. Journal of Agricultural and Food Chemistry 2004, 52(2), 337–343.
- Guyomarc’h, F.; MAhieux, O.; Renan, M.; Chatriot, M.; Gamerre, V.; Famelart, M.H. Changes in the Acid Gelation of Skim Milk as Affected by Heat-Treatment and Alkaline pH Conditions. Lait 2007, 87(2), 119–137.
- Menard, O.; Camier, B.; Guyomarc’h, F. Effect of Heat Treatment at Alkaline pH on the Rennet Coagulation Properties of Skim Milk. Lait 2005, 85(6), 515–526.
- Lucey, J.A.; Teo, C.T.; Munro, P.A.; Singh, H. Rheological Properties at Small (Dynamic) and Large (Yield) Deformations of Acid Gels Made from Heated Milk. Journal of Dairy Research 1997, 64(04), 591–600.
- Sinaga, H.; Bansal, N.; Bhandari, B. Effects of Milk pH Alteration on Casein Micelle Size and Gelation Properties of Milk. International Journal of Food Properties 2016, 20, 179–197.
- Anema, S.G. Effect of pH at Pressure Treatment on the Acid Gelation of Skim Milk. Innovative Food Science & Emerging Technologies 2010, 11(2), 265–273.
- Hernandez, A.; Harte, F.M. Manufacture of Acid Gels From Skim Milk Using High-Pressure Homogenization. Journal of Dairy Science 2008, 91(10), 3761–3767.
- Madadlou, A.; Emam-Djomeh, Z.; Mousavi, M.E.; Mohamadifar, M.; Ehsani, M. Acid-Induced Gelation Behavior of Sonicated Casein Solutions. Ultrasonics Sonochemistry 2010, 17(1), 153–158.
- Bansal, N.; Fox, P.F.; McSweeney, P.L.H. Aggregation of Rennet-Altered Casein Micelles at Low Temperatures. Journal of Agricultural and Food Chemistry 2007, 55(8), 3120–3126.
- Mellema, M.; Walstra, P.; van Opheusden, J.H.; van Vliet, T. Effects of Structural Rearrangements on the Rheology of Rennet-Induced Casein Particle Gels. Advances in Colloid and Interface Science 2002, 98(1), 25–50.
- Hidalgo, M.E.; Pires, M.S.; Risso, P.H. A Study on Bovine Kappa-Casein Aggregation After the Enzymatic Action of Chymosin. Colloids and Surfaces B-Biointerfaces 2010, 76(2), 556–563.
- Sandra, S.; Ho, M.; Alexander, M.; Corredig, M. Effect of Soluble Calcium on the Renneting Properties of Casein Micelles as Measured by Rheology and Diffusing Wave Spectroscopy. Journal of Dairy Science 2012, 95(1), 75–82.
- Klandar, A.H.; Lagaude, A.; Chevalier-Lucia, D. Assessment of the Rennet Coagulation of Skim Milk: A Comparison of Methods. International Dairy Journal 2007, 17(10), 1151–1160.
- Madadlou, A.; Khosroshahi, A.; Mousavi, M.E. Rheology, Microstructure, and Functionality of Low-Fat Iranian White Cheese Made with Different Concentrations of Rennet. Journal of Dairy Science 2005, 88(9), 3052–3062.
- Titapiccolo, G.I.; Corredig, M.; Alexander, M. Modification to the Renneting Functionality of Casein Micelles Caused by Nonionic Surfactants. Journal of Dairy Science 2010, 93(2), 506–514.
- Hunter, R.J.; Hemar, Y.; Pinder, D.N.; Anema, S.G. Effect of Storage Time and Temperature of Milk Protein Concentrate (MPC85) on the Renneting Properties of Skim Milk Fortified with MPC85. Food Chemistry 2011, 125(3), 944–952.
- Anema, S.G.; Lee, S.K.; Klostermeyer, H. Rennet-Induced Aggregation of Heated pH-Adjusted Skim Milk. Journal of Agricultural and Food Chemistry 2011, 59(15), 8413–8422.
- Tranchant, C.C.; Dalgleish, D.G.; Hill, A.R. Different Coagulation Behaviour of Bacteriologically Acidified and Renneted Milk: The Importance of Fine-Tuning Acid Production and Rennet Action. International Dairy Journal 2001, 11(4–7), 483–494.
- Salvatore, E.; Pirisi, A.; Corredig, M. Gelation Properties of Casein Micelles During Combined Renneting and Bacterial Fermentation: Effect of Concentration by Ultrafiltration. International Dairy Journal 2011, 21(11), 848–856.
- Alexander, M.; Dalgleish, D.G. Application of Transmission Diffusing Wave Spectroscopy to the Study of Gelation of Milk by Acidification and Rennet. Colloids and Surfaces B-Biointerfaces 2004, 38(1–2), 83–90.
- Anema, S.G. Heat and/or High-Pressure Treatment of Skim Milk: Changes to the Casein Micelle Size, Whey Proteins and the Acid Gelation Properties of the Milk. International Journal of Dairy Technology 2008a, 61(3), 245–252.
- Hidalgo, M.E.; Riquelme, B.D.; Alvarez, E.M.; Wagner, J.R.; Risso, P.H. Acid-Induced Aggregation and Gelation of Bovine Sodium Caseinate-Carboxymethylcellulose Mixtures. In Food Industrial Processes—Methods and Equipment; Valdez, B.; Ed.; InTech, open access book, 2012.
- Nian, Y.; Chen, B.Y.; Aikman, P.; Grandison, A.; Lewis, M. Naturally Occurring Variations in Milk pH and Ionic Calcium and Their Effects on Some Properties and Processing Characteristics of Milk. International Journal of Dairy Technology 2012, 65(4), 490–497.
- Daviau, C.; Famelart, M.H.; Pierre, A.; Goudedranche, H.; Maubois, J.L. Rennet Coagulation of Skim Milk and Curd Drainage: Effect of pH, Casein Concentration, Ionic Strength and Heat Treatment. Lait 2000, 80(4), 397–415.
- Gastaldi, E.; Trial, N.; Guillaume, C.; Bourret, E.; Gontard, N.; Cuq, J.L. Effect of Controlled Kappa-Casein Hydrolysis on Rheological Properties of Acid Milk Gels. Journal of Dairy Science 2003, 86(3), 704–711.
- Lankes, H.; Ozer, H.B.; Robinson, R.K. The Effect of Elevated Milk Solids and Incubation Temperature on the Physical Properties of Natural Yoghurt. Milchwissenschaft-Milk Science International 1998, 53(9), 510–513.
- Lucey, J.A.; Tamehana, M.; Singh, H.; Munro, P.A. Effect of Heat Treatment on the Physical Properties of Milk Gels Made with Both Rennet and Acid. International Dairy Journal 2001, 11(4–7), 559–565.
- Lucey, J.A.; Tamehana, M.; Singh, H.; Munro, P.A. Effect of Interactions Between Denatured Whey Proteins and Casein Micelles on the Formation and Rheological Properties of Acid Skim Milk Gels. Journal of Dairy Research 1998b, 65(4), 555–567.
- Donato, L.; Guyomarc’H, F. Formation and Properties of the Whey Protein/Kappa-Casein Complexes in Heated Skim Milk—A Review. Dairy Science & Technology 2009, 89(1), 3–29.
- Roefs, S.; P.F.M.; Walstra, P.; Dalgleish, D.G.; Horne, D.S. Preliminary Note on the Change in Casein Micelles Caused by Acidification. Netherlands Milk and Dairy Journal 1985, 39(2), 119–122.
- Dalgleish, D.G. The Casein Micelle and Its Reactivity. Lait 2007, 87(4–5), 385–387.
- De Kruif, C.G. Casein Micelle Interactions. International Dairy Journal 1999, 9(3–6), 183–188.
- Li, J.; Dalgleish, D. Controlled Proteolysis and the Properties of Milk Gels. Journal of Agricultural and Food Chemistry 2006, 54(13), 4687–4695.
- Vanhooydonk, A.C.M.; Hagedoorn, H.G.; Boerrigter, I.J. pH-Induced Physicochemical Changes of Casein Micelles in Milk and Their Effect on Renneting 1. Effect of Acidification on Physicochemical Properties. Netherlands Milk and Dairy Journal 1986, 40(2–3), 281–296.
- Vasbinder, A.J.; de Kruif, C.G. Casein-Whey Protein Interactions in Heated Milk: The Influence of pH. International Dairy Journal 2003a, 13(8), 669–677.
- Giroux, H.J.; Houde, J.; Britten, M. Preparation of Nanoparticles from Denatured Whey Protein by pH-Cycling Treatment. Food Hydrocolloids 2010, 24(4), 341–346.
- Hae, D.J.; Swaisgood, H.E. Disulfide Bond Formation Between Thermally Denaturated Beta-Lactoglobulin and Kappa-Casein in Casein Micelles. Journal of Dairy Science 1990, 73(4), 900–904.
- Guyomarc’h, F.; Queguiner, C.; Law, A.J.; Horne, D.S.; Dalgleish, D.G. Role of the Soluble and Micelle-Bound Heat-Induced Protein Aggregates on Network Formation in Acid Skim Milk Gels. Journal of Agricultural and Food Chemistry 2003b, 51(26), 7743–7750.
- Morand, M.; Guyomarch, F.; LEgland, D.; Famelart, M.H. Changing the Isoelectric Point of the Heat-Induced Whey Protein Complexes Affects the Acid Gelation of Skim Milk. International Dairy Journal 2012, 23(1), 9–17.
- Lucey, J.A.; Munro, P.A.; Singh, H. Rheological Properties and Microstructure of Acid Milk Gels as Affected by Fat Content and Heat Treatment. Journal of Food Science 1998a, 63(4), 660–664.
- Guyomarc’h, F.; Law, A.J.R.; Dalgleish, D.G. Formation of Soluble and Micelle-Bound Protein Aggregates in Heated Milk. Journal of Agricultural and Food Chemistry 2003a, 51(16), 4652–4660.
- Schorsch, C.; Wilkins, D.K.; JOnes, M.G.; Norton. I.T. Gelation of Casein-Whey Mixtures: Effects of Heating Whey Proteins Alone or in the Presence of Casein Micelles. Journal of Dairy Research 2001, 68(3), 471–481.
- Van Vliet, T.; Lakemond, C.M.M.; Visschers, R.W. Rheology and Structure of Milk Protein Gels. Current Opinion in Colloid & Interface Science 2004, 9(5), 298–304.
- Zhong, Q.X.; Daubert, C.R.; Velev, O.D. Physicochemical Variables Affecting the Rheology and Microstructure of Rennet Casein Gels. Journal of Agricultural and Food Chemistry 2007, 55(7), 2688–2697.
- Roefs, S.; Vanvliet, T. Structure of Acid Casein Gels. 2. Dynamic Measurements and Type of Interaction Forces. Colloids and Surfaces 1990, 50, 161–175.
- Domagala, J. Instrumental Texture, Syneresis, and Microstructure of Yoghurt Prepared from Ultrafiltrated Goat Milk: Effect of Degree of Concentration. International Journal of Food Properties 2012, 15(3), 558–568.
- Cayot, P.; Fairise, J.F.; Colas, B.; Lorient, D.; Brule, G. Improvement of Rheological Properties of Firm Acid Gels by Skim Milk Heating Is Conserved After Stirring. Journal of Dairy Research 2003, 70, 423–431.
- Walstra, P.; Vandijk, H.J.M.; Guerts, T.J. The Syneresis of Curd 1. General Considerations and Literature Review. Netherlands Milk and Dairy Journal 1985, 39(4), 209–246.
- Smith, M.; McMahon, D.J. Aseptic Rennet Coagulation of Ultra-High Temperature Processed Milk Concentrates. Journal of Dairy Science 1996, 79(9), 1513–1520.
- Vanvliet, T.; van Dijk, J.M.; Zoon, P.; Walstra, P. Relation Between Syneresis and Rheological Properties of Particle Gels. Colloid and Polymer Science 1991, 269(6), 620–627.
- Lucey, J.A.; Tamehana, M.; Singh, H.; Munro, P.A. Rheological Properties of Milk Gels Formed by a Combination of Rennet and Glucono-Delta-Lactone. Journal of Dairy Research 2000, 67(3), 415–427.
- Horne, D.S.; Banks, J.M. Rennet-Induces Coagulation of Milk in Cheese: Chemistry, Physics and Microbiology; Fox, P.F.; McSweeney, P.L.H.; Cogan, T.M.; Guinee, T.P.; Eds.; Elsevier Academic Press: Amsterdam, 2004; 48–70.
- Zoon, P.; Vanvliet, T.; Walstra, P. Rheological Properties of Rennet-Induced Skim Milk Gels. 2. The Effect of Temperature. Netherlands Milk and Dairy Journal 1988a, 42(3), 271–294.
- Zoon, P.; Vanvliet, T.; Walstra, P. Rheological Properties of Rennet-Induced Skim Milk Gels. 3. The Effect of Calcium and Phosphate. Netherlands Milk and Dairy Journal 1988b, 42(3), 295–312.
- Zoon, P.; Vanvliet, T.; Walstra, P. Rheological Properties of Rennet-Induced Skim Milk Gels 4. The Effect of pH and NaCl. Netherlands Milk and Dairy Journal 1989, 43(1), 17–34.
- Anema, S.G.; Lee, S.K.; Klostermeyer, H. Effect of pH at Heat Treatment on the Hydrolysis of Kappa-Casein and the Gelation of Skim Milk by Chymosin. LWT–Food Science and Technology 2007, 40(1), 99–106.
- Kethireddipalli, P.; Hill, A.R.; Dalgleish, D.G. Protein Interactions in Heat-Treated Milk and Effect on Rennet Coagulation. International Dairy Journal 2010, 20(12), 838–843.
- Dinkov, K.; Dushkova, M. Effect of Heat Treatment of Milk on the Rennet Coagulation and Rheological Properties of Milk Gels. Milchwissenschaft-Milk Science International 2007, 62(2), 133–135.
- Leaver, J.; Law, A.J.R.; Horne, D.S.; Banks, J.M. Influence of Heating Regime and pH on the Primary Phase of Renneting of Whole Milk. International Dairy Journal 1995, 5(2), 129–140.
- Lucey, J.A. The Relationship Between Rheological Parameters and Whey Separation in Milk Gels. Food Hydrocolloids 2001, 15(4–6), 603–608.