ABSTRACT
Antidiabetic and anti-inflammatory potential of sulphated polygalactans isolated from the red seaweeds Kappaphycus alvarezii and Gracilaria opuntia were acquired by employing different in vitro systems. The sulphated galactopyran motif derived from G. opuntia possessed significant antidiabetic properties as identified by α-amylase (IC50 0.04 mg/mL), α-glucosidase (IC50 0.09 mg/mL) and dipeptidyl peptidase-4 (DPP-4, IC50 0.09 mg/mL) inhibitory activities. Based on the detailed nuclear magnetic resonance spectroscopy experiments the sulphated galactopyran motif of G. opuntia was designated as →3)-4-O-sulfonato-(6-O-acetyl)-β-D-galactopyranosyl-(1→4)-3,6-anhydro-(2-O-sulfonato)-α-D-galactopyranosyl-(1→3)-4-O-sulfonato-(6-O-acetyl)-β-D-xylosyl-(1→3)-4-O-sulfonato-(6-O-acetyl)-β-D-galactopyranosyl-(1→4)-3,6-anhydro-(2-O-sulfonato)-α-D-galactopyranan, while the one from K. alvarezii was demonstrated to be →4)-4-O-sulfonato-(2-O-methyl)-β-D-galactopyranosyl-(1→4)-3,6-anhydro-(2-O-methyl)-α-D-galactopyranan. The sulphated galactans from G. opuntia showed greater anti-inflammatory inhibitory activities as determined by cyclooxygenase-1 (COX-1, IC50 0.01 mg/mL), cyclooxygenase-2 (COX-2, IC50 0.03 mg/mL), and 5-lipoxygenase inhibitory activities (5-LOX, IC50 0.24 mg/mL). This study revealed that the sulfated polygalactan enriched concentrate from G. opuntia can be used as potential therapeutic candidate to suppress the hyperglycemic response in diabetic conditions and inflammatory activity. They can be used to develop functional food ingredient in nutraceutical products.
Introduction
Diabetes mellitus is a metabolic disorder resulting from a defect in insulin secretion, insulin action, or both. Insulin deficiency in turn leads to chronic hyperglycaemia with disturbances of carbohydrate, fat, and protein metabolism. As the disease progresses tissue or vascular damage ensues leading to severe complications, such as retinopathy neuropathy and nephropathy. Thus, diabetes covers an extensive range of heterogeneous diseases.[Citation1] Two decades back sulphonyl ureas were the only orally possible drugs for regulation of hyperglycaemia, but the last decade witnessed the availability of a newer class of oral anti-diabetic drugs. Despite the accessibility of an enormous number of drugs to control hyperglycaemia, greater cost and side effects of these synthetic drugs are the major concerns. In this context naturally derived seaweed concentrate have prominent effect because they are free from adverse side effects.
One relevant remedy to treat type-2 diabetes is to reduce the post-prandial hyperglycemia by inhibiting the actions of carbohydrate hydrolyzing enzymes like α-amylase, α-glucosidase, and DPP-4 (dipeptidyl peptidase-4).[Citation2] Increase in the chronic subclinical inflammation is one of the vital pathogenic factors in insulin resistance syndrome and type-2 diabetes.[Citation3] It was known that the adipose tissue can synthesize and release the pro-inflammatory cytokines, tumor necrosis factor-alpha (TNF-α), interleukins (IL-1 and IL-6), and that the inflammatory markers are bound to a multiple metabolic pathways for insulin resistance, lipoprotein lipase action, and adipocyte function.[Citation4] Ongoing studies displayed that inflammation, and more exactly pro-inflammatory cytokines, play a typical role in the development of microvascular diabetic disorders.[Citation4]
Over the last decade, a plethora of information has emerged demonstrating a close link between type-2 diabetes and inflammatory response. The present study targeted its attention on the mechanistic insight of anti-inflammatory and antidiabetic potentials of seaweeds. The red and green seaweeds were found to be potential inhibitors of α-glucosidase.[Citation5] Bromophenols, 2-piperidione, benzene acetamide, n-hexadecanoic acid, and polysaccharide derivatives were found in red seaweeds like Rhodomela confervoides, Symphyocladia latiuscula, Polysiphonia urceolata, and were found to exhibit hypoglycemic potentials by inhibiting α-glucosidase.[Citation5] Bioactive properties of the seaweeds were reported to be due to the presence of sulphated polysaccharides, phenolics, and terpenoids.[Citation6,Citation7] However, substantial medical and pharmaceutical researches are required to expand therapeutic agents from these marine sources. In the last few years, diverse inspections have shown that low-grade inflammation is associated with the possibility of developing type-2 diabetes. Innovative proposal for treatment of type-2 diabetes on the gut hormone glucagon-like peptide-1 (GLP-1), a potent insulinotropic peptide, which is antidiabetic due to its consolidated action to stimulate insulin secretion, increase β-cell mass, inhibit glucagon secretion, reduce the rate of gastric emptying, and induces satiety. The peptide is inactivated by the enzyme dipeptidyl peptidase-4 (DPP-4), resulting in a half-life of active GLP-1 of only approximately 1–2 min. Inhibition of DPP-4 expanding the levels of endogenous active GLP-1 and prolongs its half-life. Thus, DPP-4 inhibition can be an effective approach to treat type-2 diabetes mellitus by potentiating insulin secretion.[Citation8]
The polysaccharides from seaweeds were reported to possess various bioactivities and were found to assume an essential part to combat different oxidative stress induced diseases.[Citation9] The bioactivities of polysaccharides depends on various structural parameters, including the degree of sulfation, the molecular weight, the sulfation position and type of sugar units and glycosidic linkaging and branching. Among different seaweeds found in the Gulf of Mannar region in the southeastern coast of the Indian subcontinent, Kappaphycus alvarezii and Gracilaria opuntia are abundantly available. These genera of seaweeds have been widely used in traditional medicine, and are considered to be economically important since they are used in the pharmaceutical, nutraceutical, cosmetic, and food industries.[Citation10–Citation12] Numerous studies have been carried out to determine the antioxidant activities in the red seaweed K. alvarezii extract.[Citation13–Citation16] The acetone extract of K. alvarezii possess significant antioxidative activity.[Citation17] K. alvarezii extracts displayed significant protection against DNA damage induced by H2O2, and enhanced antioxidant potential and protection against tissue lipid peroxidation and cell damage.[Citation18] The methanolic extract derived from K. alvarezii was screened for the antidiabetic (α-amylase) anti-inflammatory activity (hyaluronidase inhibition) and cytotoxicity against the stannous chloride in E. coli AB 1157.[Citation18] The red seaweed belonging to Gracilaria spp were found to be rich in sulphated polysaccharides, and were generally related with anti-inflammatory property.[Citation19]
In this background, the objectives of the present study were to screen these seaweeds belonging to K. alvarezii and G. opuntia for lead polysaccharide fractions containing different sulphated galactan motifs with anti-inflammatory and antidiabetic properties. The seaweed concentrate containing the sulphated galactans were described utilizing nuclear magnetic resonance spectroscopic assays and were correlated with their in vitro anti-inflammatory and antidiabetic properties.
Materials and methods
Seaweed material
The red seaweeds were Kappaphycus alvarezii (Doty) Doty ex Silva (Family: Solieriaceae, Order: Gigartinales) and Gracilaria opuntia (Family: Hypneaceae, Order: Gracilariales). They were freshly collected from the Gulf of Mannar in Mandapam region situated between 8º48′ N, 78º9′ E and 9º14′ N, 79º14′E in the south east coast of India. The seaweed samples were washed in running water for 10 min to remove the epiphytes, dirt, and salt particle, before being transported to the laboratory and shade dried (35 ± 3°C) for 72 h. The shade dried seaweeds were powdered and utilized for further experiments.
Chemicals and reagents
All chemicals, solvents and reagents used in this study were of analytical grade, and were obtained from E-Merck (Darmstadt, Germany) or Sigma-Aldrich Chemical Co. Inc. (St. Louis, MO).
Isolation and preparation of sulphated polygalactan enriched seaweed concentrate
The dried seaweed powder (200 g) was extracted with hot water at 80–90°C for 3–4 h to yield aqueous extract,[Citation20] which was cooled and centrifuged (8500 rpm for 15 min, 4°C, Sorvell Biofuge Stratos, Thermo Scientific, USA) to remove the solid residues. The supernatant (1000 mL) obtained after centrifugation was concentrated to 1/10th of the original volume (100 mL) by utilizing a rotational vacuum concentrator (Martin Christ RVC 2-33 IR, Martin Christ, Germany), cooled, and precipitated with three volumes of ethanol (500 mL, 3:1, v/v) overnight at 4°C for the precipitation of the sulphated polygalactans. The lyophilization of the precipitated material in a laboratory freeze-drier (Martin Christ alpha 1-4 LDplus, Martin Christ, Germany) yielded sulphated polygalactan-enriched seaweed concentrate (144 g; yield based on powdered seaweed, 72%).
In vitro anti-inflammatory activity of sulphated polygalactan enriched seaweed concentrate
Cyclooxygenase (COX-1 and COX-2) inhibition assay
In vitro anti-inflammatory activities of the sulphated polygalactan enriched seaweed concentrate were assessed by cyclooxygenase (COX-1 and COX-2) inhibition assays by 2, 7-dichlorofluorescein method[Citation21] and the 5-lipoxygenase (5-LOX) inhibition assay.[Citation12] Leuco-2, 7-dichlorofluorescein diacetate (5 mg) was hydrolyzed at room temperature in NaOH (1 M, 50 μL) for 10 min, and HCl (1 M, 30 μL) was added to neutralize the excess of NaOH before the resulting 1-dichlorofluorescein (DCF) was diluted in Tris-HCl buffer (0.1 M, pH 8). COX enzymes (COX-1 and COX-2) were diluted in Tris-buffer (0.1 M, pH 8). The test samples were pre-incubated with the enzymes at room temperature for 5 min in the presence of hematin. Premixed phenol, 1-DCF, and arachidonic acid were added to the enzyme mixture to initiate the reaction, and to give a final reaction mixture of arachidonic acid (50 μM), phenol (500 μM), 1-DCF (20 μM), and hematin (1 μM) in the final volume of Tris-HCl buffer (0.1 M, 1 mL, pH 8). The absorbance at 502 nm (ΔAsample) was recorded spectrophotometrically within a 1 min. A blank reaction mixture was analyzed in the spectrophotometer reference cell against each test reaction to account for any non-enzymatic activity attributed to the test sample. This blank consisted of the reaction mixture without the addition of enzyme. The IC50 value (mg/mL), named effective concentration, which is the concentration of the sulphated polygalactan enriched concentrate of K. alvarezii and G. opuntia that inhibit 50% of the pro-inflammatory enzyme activity (COX-1 and 2), was calculated from the non-linear regression curve. To calculate IC50, a series of dose-response data have been used, whereas the simplest estimate of IC50 is to plot x-y and fit the data with a straight line. IC50 value was estimated using the fitted line, i.e., y = mx + c, and expressed as IC50 = (0.5 – c) /m.
Lipoxygenase (5-LOX) inhibition assay
The 5-LOX inhibition assay performed according to the modified method of Baylac and Racine.[Citation22] The test samples (50 μL in different concentrations 1000 and 5000 ppm, in DMSO and tween 20 mixture; 29:1, w/w) were added with the pre-warmed potassium phosphate buffer (0.1 M, 2.95 mL, pH 6.3) and linoleic acid solution (48 µL) before being placed in a cuvette (3 mL). Thereafter, ice-cold buffer (potassium phosphate; 12 μL) was mixed with 5-LOX enzyme (100 U). The absorbance was recorded at 234 nm.[Citation22] The IC50 value (mg/mL) was calculated from the non-linear regression curve as described in the earlier section.
In vitro antidiabetic activities of sulphated polygalactan enriched seaweed concentrate
Antidiabetic activities of the sulphated polygalactan enriched concentrate of K. alvarezii and G. opuntia were determined by inhibition of α-amylase, α-glucosidase, and dipeptidyl peptidase-4 enzymes.
Inhibition of α-amylase activity
In vitro anti-diabetic studies by inhibition of α-amylase were measured according to the method of Hamdan and Afifi[Citation23] with suitable modification. The test samples (500 μL) and standard drug (100–1000 μg/mL) were added to phosphate buffer (500 μL, 0.20 mM, pH 6.9) containing α-amylase (0.5 mg/mL) solution and were incubated at 25°C for 10 min. Thereafter, starch solution (500 μL, 1% w/v in 0.02 M sodium phosphate buffer pH 6.9) was added to the contents, and the reaction mixtures were incubated at 25°C for 10 min. The reaction was quenched with 3, 5 dinitrosalicylic acid reagent (1.0 mL) by heating in a boiling water bath for 5 min before being cooled at room temperature. The reaction mixture was then diluted with distilled water (10 mL), and the absorbance was measured at 540 nm. The plot of inhibition of α-amylase activity was recorded and the IC50 value (mg/mL) was calculated.
Inhibition of α-glucosidase activity
The α-glucosidase inhibition assay was performed according to the modified method of Dong et al.[Citation24] In brief, a solution of starch substrate (2% w/v maltose or sucrose, 1 mL in 0.2 M Tris buffer pH 8.0) and various concentrations of seaweed extracts were incubated for 5 min at 37°C. The reaction was initiated by adding α-glucosidase enzyme (1 mL, 1 U/mL) to the reaction mixture, followed by incubation for 10 min at 37°C. The reaction was stopped with 3, 5 dinitrosalicylic acid reagent (1 mL) by heating for 2 min in a boiling water bath before being cooled at room temperature. The reaction mixture was then diluted with distilled water (9 mL), and the absorbance was measured at 540 nm. The IC50 value (mg/mL), which is the concentration of the extracts/fractions that inhibit 50% of the α-glucosidase activity, was calculated from the non-linear regression curve as detailed earlier.
Inhibition of dipeptidyl peptidase-4
Diprotein-A (Ile-Pro-Ile) was used as reference inhibitor standard for the method (Hamdan & Afifi).[Citation23] The standard was diluted to various concentrations using Tris-HCl buffer (50 mM, pH 7.5) and the final volume was made to 35 µL. The absorbance of the reaction mixture was taken at 405 nm. DPP-4 enzyme (15 µL, 0.05 U/mL) was added to reaction mixture. One unit enzyme activity was defined as the amount of enzyme catalyzing the release of 1 µM of 4-nitro aniline (pNA) from the substrate (gly-pro-p-nitroanilide)/min under the standard assay conditions. After adding the enzyme, the reaction mixture was pre-incubated for 10 min at 37°C to enhance the binding capacity of the inhibitor. This was followed by the addition of substrate (gly-pro-p-nitroanilide [Gly-Pro-pNA, 50 µL, 0.2 M in Tris-HCl buffer) as a substrate. The reaction mixture was incubated for 30 min at 37°C, and the reaction was terminated by the addition of glacial acetic acid (25 µL of 25%). The absorbance of the reaction mixture was measured at 405 nm. The IC50 value (mg/mL), which is the concentration of the sulphated polygalactan enriched concentrate of K. alvarezii and G. opuntia that inhibit 50 % of the DPP-4, was calculated from the non-linear regression curve as described in the earlier section.
Spectroscopic methods
Fourier transform infrared (FTIR) spectra of KBr pellets were recorded utilizing a Perkin–Elmer Series 400 FTIR spectrophotometer scanning between 4000 and 400 cm−1. The solid samples of dried sulphated polygalactan fraction (10 mg) were mixed with KBr (100 mg) and compressed to prepare as a salt disc. The frequencies of different components present in each sample were analyzed. Proton nuclear magnetic resonance (1H-NMR, Proton Nuclear Magnetic Resonance Spectroscopy) spectra were recorded on a Bruker AVANCE DRX 600 MHz (AV 600) spectrometer (Bruker, Karlsruhe, Germany) in deuterium oxide (D2O) as aprotic solvent at ambient temperature (27°C) with tetramethylsilane (TMS) as the internal standard (δ 0 ppm) equipped with 5 mm probes. The samples were deuterium exchanged by successive freeze-drying steps in D2O (99.9%) before being dissolved in D2O (20–25 mg/mL).
Statistical analysis
Data were expressed as mean of triplicate ± standard deviation. Statistical evaluation was carried out by SPSS software (SPSS Inc., Chicago, USA, ver. 13.0). Descriptive statistics were calculated for all the studied traits. The Pearson correlation coefficient (r) was calculated (p < 0.05) to assess the strength of the linear relationship between two variables. The selected variables for principal component analysis (PCA) were the different bioactivities, as exhibited by different type of polysaccharides extracted from red seaweeds.
Results and discussion
Isolation and purification of the polysaccharides from seaweeds
The precipitation of sulphated polygalactan from the crude extracts of K. alvarezii with ethanol brought about significantly greater recovery (75%) than that in G. opuntia (55%). The ethanol precipitated material was found to be rigid and crystalline. The elementary variables in extraction were the base used to generate the seaweed material, and the temperature at which the reaction materialized, which affected the gelling properties and structure of sulphated galactan. The sulphated galactan was found to be a linear poly-galactose chain, where the galactose units integrate together and bear differing extent of sulphate entity ( and ). A few of the D-galactoses involved a 6-sulphate ester group while some 3, 6-anhydro-D-galactoses consisting of a 2-sulphate ester group. In the presence of potassium ions, sulphated polygalactan gels dissolved by heating and subsequent cooling, resulting in the evolution of a three-dimensional network by cationic interaction with sulphate group. The interesting aspects of mixture of sulphated polygalactan gel are its thermal reversibility, whereas it can gel and melt frequently, only defeating a little gel strength at each cycle. As potassium concentration rose, the gel stability was found to be increased until an optimum level is reached according to that potassium ions have a size and shape which comprise them fit into the mixture of sulphated polygalactan helix. This structure is sustained through the positively charged ions and the negatively charged sulphate groups in the sulphated galactan moiety. The sulphated polygalactan has 34% 3, 6 anhydro-D-galactose groups as segment of its repeating structure and 25% ester sulfate groups, which promptly dispersed when heated. Thermo-reversible gels engendered by polygalactan by the arrangement of the disjointed chains into double or triple helices. Hydrogen bonding, persuaded by aggregation of the ordered domains to form a firm, three-dimensional stable gels. The gel strength of polysaccharides derived from the seaweeds K. alvarezii and G. opuntia was found to be greater, when heated in an alkaline solution of potassium hydroxide for about 2 h. The hydroxide part of the reagent penetrates the seaweed, diminished the amount of sulphate in the sulphated galactan and increasing the 3, 6-anhydro galactose unit, thereby developing the gel strength of the sulphated galactan. The contemporary inspection showed that greater yield of sulphated galactan can be achieved with potassium hydroxide (KOH) treatment.
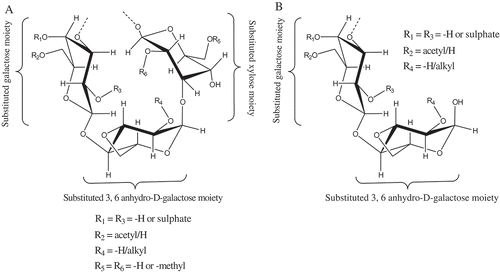
Figure 1. Structural representation of galactopyranan motifs of the sulphated polygalactans from A: G. opuntia and B: K. alvarezii. The sulphated galactopyran motif of G. opuntia was designated as →3)-4-O-sulfonato-(6-O-acetyl)-β-D-galactopyranosyl-(1→4)-3,6-anhydro-(2-O-sulfonato)-α-D-galactopyranosyl-(1→3)-4-O-sulfonato-(6-O-acetyl)-β-D-xylosyl-(1→3)-4-O-sulfonato-(6-O-acetyl)-β-D-galactopyranosyl-(1→4)-3,6-anhydro-(2-O-sulfonato)-α-D-galactopyranan, while the one from K. alvarezii was →4)-4-O-sulfonato-(2-O-methyl)-β-D-galactopyranosyl-(1→4)-3,6-anhydro-(2-O-methyl)-α-D-galactopyranan.
FTIR Spectral analysis of polysaccharides
The conspicuous attributes of the FTIR spectra of sulphated polygalactan from the red seaweeds were because of the sulfate ester and carbohydrate groups in the infrared spectra. The strong absorption bands at 1210–1260 cm–1 of the FTIR spectra demonstrated the region of S = O groups (attributed to the glycosidic linkage). The band at ~800 cm–1 was specific to 3, 6-anhydrogalactose-2-sulfate.[Citation25] The IR signals close to 3200–3400 cm–1 were credited to the region of –OH gatherings present in the sulphated polygalactans from the seaweeds. The S=O of the sulfate esters were showing up at 1250–1300 cm–1, while the C-O-C of sugar and C-O-S sulfate ester were displayed at around 950–1100 cm−1. The broad bands at 3200–3400 cm−1 were found to be a result of the sulphated polygalactan units. The C-H stretching vibrations were assigned to be present at around 2800–2900 cm−1. The absorbance peaks around 1050–1100 cm−1 displayed the pyranose ring structure. Intense absorbance at around 1700 cm−1 and bands of about 1420 cm−1 (C=O symmetric stretching vibrations), were due to carbonyl (acetyl) groups in the polysaccharide back bone chain. Three characteristic bands in the fingerprint anomeric region (950–700 cm−1), connected to the ester sulphate bonds were attributed to a special feature of the sulphated polygalactan type of polysaccharides.[Citation26] The absorption bands at ~1400 cm−1 in FT-IR spectra deduced the normality of β-glycosidic linkages. Moreover, the IR absorption bands unique to the presence of anomeric locale (800–900 cm−1), joined with the ester sulphate bonds are the special feature of the sulphated polygalactan type of polysaccharides.[Citation26]
NMR Structural analysis of polysaccharides
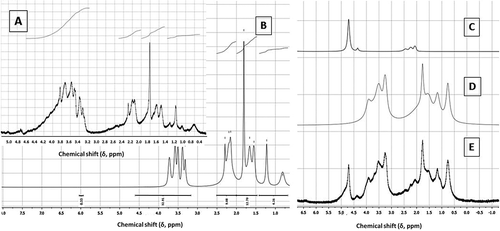
NMR spectroscopy has been carried out for the structural prediction of any regular and complex polysaccharides. Intense signals acquired from the 1H-NMR spectroscopy exhibited the region of hydrogen atoms at anomeric carbon (δ 5.4; and ). The 1H-NMR spectra of polysaccharides comprise of some well-resolved signals, counting those of anomeric protons (δ 4.4–5.5; and ). By virtue of polysaccharides, the resonances due to the anomeric protons were grouped between δ 3–4. These results were in accordance to those reported in the literature.[Citation27] The and exhibited the 1H-NMR range of blend of sulphated polygalactans (κ-, ι-) separated from K. alvarezii and G. opuntia. The peaks at δ 5 signified the vicinity of ι- and κ-monomers of sulphated polygalactans.[Citation28] The ring protons associated with sulphate functionalities demonstrated 1H-NMR signals at δ 5. The 1H-NMR showed proton signal at δ 5.34, which was relegated to be a direct result of the anomeric proton of the 3, 6-anhydrogalactose-2-sulfate.[Citation27] The anomeric proton signals at δ 4.5 and 5.0 were normal for κ-sulphated polygalactan. The 1H-NMR signals in the scope of δ 3.6–4.9 were portrayed to whatever is left of the methylene and methine hydrogens of the sulphated polygalactan moiety. The 1H-NMR spectrum of polysaccharides obtained from K. alvarezii got very much comprehended deshielded signals at δ 4.5–5, which demonstrated the region of κ- and ι-monomer of sulphated polygalactans. There absence of peaks at δ 5.26 indicated the absence of μ-monomers (precursor of κ-sulfated polygalactan).[Citation28] G. opuntia contains anomeric protons in the region between δ 3–4, which demonstrated that the sulphated polygalactan isolated from G. opuntia contains oligomeric building subunits, such as xylose and anhydro galactose besides κ- and ι-type of monomeric units. Weak proton signals at δ 2–2.5 for K. alvarezii were relegated to be because of the acetyl protons. These outcomes have been supported by earlier studies.[Citation29] Recognizable –O-alkyl signals (ideally –OCH3) in the 1H-NMR spectrum of galactan derivative from G. opuntia and K. alvarezii at δ 3.4 obviously revealed the region of more conspicuous number of alkoxy substitutions in the sulphated polygactans from the red seaweeds. The 13C-NMR spectrum of polygalactans showed signals with specific multiplicities, which suggested the positional differences of 1→3 and 1→4 linked residues in the sulphation patterns. The anomeric region of 13C-NMR exhibited the characteristic signals, and were relegated taking into account the data reported in the literature.[Citation27] Despite the normal sulphated polygalactan repeating units, the samples were found to contain some minor constituents that were as often as possible experienced in carragenophytes. A small amount of 3-linked 6-O-methyl-d-galactose residues was found in the κ-sulphated polygalactan from K. alvarezii as additionally supported by past studies.[Citation30] Pyruvic acid is a typical segment of numerous complex sulphated polygalactans. It outlined a cyclic acetal at positions C-4 and C-6 of the 3-linked galactose residues. The pyruvic acid ketals might likewise be experienced in the 1H-NMR spectra of the polysaccharides in the present study as evident by the methyl proton resonances at δ 1.45. These outcomes have been supported by earlier studies.[Citation29] The anomeric region of 13C-NMR exhibited the characteristic signals, and were assigned based on the literature data.[Citation27] The 13C-NMR signals at δ 58.91 and 65.25 were suggested as the –CH2-groups on the C-6 of the 3-linked galactopyranosyl-4-sulfate moiety. In addition the frail proton signals at δ 29.18 and 23.34 and carbon signals at δ 174–178 were related to the methyl and carboxyl carbons of the pyruvated galactopyranosyl residues. The 13C-NMR chemical shift values in the present study were in accordance with the chemicals shifts reported for the basic sulfated polygalactan structure.[Citation27] Based on the detailed NMR experiments the sulphated galactopyran motif of G. opuntia was designated as →3)-4-O-sulfonato-(6-O-acetyl)-β-D-galactopyranosyl-(1→4)-3,6-anhydro-(2-O-sulfonato)-α-D-galactopyranosyl-(1→3)-4-O-sulfonato-(6-O-acetyl)-β-D-xylosyl-(1→3)-4-O-sulfonato-(6-O-acetyl)-β-D-galactopyranosyl-(1→4)-3,6-anhydro-(2-O-sulfonato)-α-D-galactopyranan, while the one from K. alvarezii was demonstrated to be →4)-4-O-sulfonato-(2-O-methyl)-β-D-galactopyranosyl-(1→4)-3,6-anhydro-(2-O-methyl)-α-D-galactopyranan ( and ).
Figure 2. 1H-NMR spectral representation of sulphated polygalactan from G. opuntia and K. alvarezii. A: The originally acquired 1H-NMR spectrum of the sulphated galactopyran of G. opuntia. B: The large number of transitions in 1H-NMR spectra in the sulphated galactopyran of G. opuntia was made streamlined by the peak analysis algorithm, global spectral deconvolution (GSD), which applies a deconvolution of the entire range consequently making us to work out the signature peaks. C–D: Deconvoluted compound peaks obtained from the originally acquired 1H-NMR spectrum of the galactopyranan derived from K. alvarezii. E: The originally acquired 1H-NMR spectrum of the sulphated galactopyran of K. alvarezii.
In vitro antidiabetic activities of sulphated polygalactan enriched seaweed concentrate
Inhibition of α-amylase and α-glucosidase activities
Competitive inhibitor of α-glucosidase is acarbose and miglitol, which reduces consumption of starch and disaccharides.[Citation31] Hence, one of the therapeutic pathways for contracting postprandial blood glucose levels in patient with diabetes mellitus is to impede carbohydrate absorption after food intake. The α-amylase established a family of endo-amylases catalyzing the initial hydrolysis of starch into shorter oligosaccharides through the cleavage of α-D-(1-4) glycosidic bonds. Inhibition of these enzymes (α-amylase and α-glucosidase) reduced the high postprandial blood glucose peaks in diabetes.[Citation32] Acarbose and miglitol are competitive inhibitor of α-glucosidase resulting in lower absorption of starch and disaccharides. Vicinity of 13C-NMR signals at δ 170 of the EtOAc-MeOH extracts derived from the seaweeds are a decent sign about the acyl carbonyl carbon that were accounted for to repress α-glucosidase protein.[Citation33]
The results from the present study demonstrated that there were no difference in α-glucosidase inhibitory activity (IC50 0.09 mg/mL) of the sulphated polygalactans purified from two seaweed species considered in the present study (). However, the sulphated galactans of G. opuntia exhibited greater α-amylase inhibitory activity (IC50 0.04 mg/mL) than that of K. alvarezii (IC50 0.15 mg/mL) and the positive control (acarbose, IC50 0.2 mg/mL). The antidiabetic effect of the sulphated polysaccharides from seaweeds might attribute to their inhibitory effects against α-amylase that retard the digestion of carbohydrate to delay the postprandial rise in blood glucose. Likewise, there was no significant difference in the α-glucosidase inhibitory activity of the sulphated galactans derived from these two red seaweed species (IC50 ~0.09 mg/mL). Seaweeds were reported to possess α-amylase and α-glucosidase inhibitory activities,[Citation34] which substantiate the results obtained in the present study that these seaweed species are good source for antidiabetic agents.
Table 1. Antidiabetic and anti-inflammatory inhibitory activities (IC50) of the sulphated polygalactans derived from K. alvarezii and G. opuntia.
Inhibition of dipeptidyl-peptidase-4 enzyme activity
Dipeptidyl peptidase-4 (DPP-4) is involved in the inactivation of glucagon like peptide-1 (GLP-1), a potent insulinotropic peptide. Thus, DPP-4 inhibition can be an efficient approach to treat type-2 diabetes mellitus by potentiating insulin secretion.[Citation8] The present study described the biological effects of sulphated polygalactans isolated from two different red seaweeds. Significant differences were observed in the polysaccharide fractions, when compared with control. DPP-4 inhibitory activity of the sulphated galactans of G. opuntia was found to be significantly greater (IC50 0.09 mg/mL) than that derived from K. alvarezii (IC50 0.12 mg/mL) and the standard diprotin A (IC50 1.54 mg/mL; p < 0.05). The synthetic DPP-4 inhibitors, such as vildagliptin, sitagliptin, saxagliptin, etc, were reported to have several side effects like headache, dizziness, hypoglycemic disorders, nausea, weight gain, and swelling of the legs and ankles due to excess fluid retention.[Citation35] Similarly, other synthetic hypoglycemic agents (acarbose and voglibose) that inhibit α-amylase and α-glucosidase were found to cause hepatic and gastrointestinal disorders.[Citation36] Polyphenols and sulphated polysaccharides present in seaweeds have been proven for antiviral, antitumoral, anti-inflammatory, and anticoagulant activity.[Citation37] The bioactive compounds from seaweeds were reported to be effective for the treatment of major chronic diseases like diabetes through the inhibition of starch digesting enzymes and the regulation of glucose induced oxidative stress.[Citation38] The bioactivity of the seaweed extracts are due to the interaction of functional groups in the sulphated polygalactan with DPP-4 by H-bonding and hydrophilic interactions. The solvent fractions of G. opuntia were found to possess greater number of electronegative functional groups, which can form H-bond with DPP-4 resulting in greater antidiabetic activity. The seaweeds considered in the present study can be used as potential alternative therapy for treatment of diabetes.
In vitro anti-inflammatory activity of sulphated polygalactan enriched seaweed concentrate
The sulphated polygalactan from G. opuntia exhibited significantly greater (p < 0.05) COX-1 and COX-2 inhibition activity (IC50 values of 0.01 and 0.03 mg/mL, respectively). G. opuntia polygalactan also exhibited greater 5-LOX inhibitory activity (0.24 mg/mL) than that of K. alvarezii (IC50 0.34 mg/mL; ). It is to be noted that sulphated polygalactan isolated in the present study showed greater anti-inflammatory activity in comparison with the positive control aspirin. Polysaccharides were reported to be one of the major bioactive components with selective activity against inflammation in the aqueous extract of seaweed D. obtusata.[Citation39,Citation40] Red seaweeds were found to be the most important source of many biologically active metabolites in contrast to other algal classes.[Citation39] The sulphated galactans got from G. opuntia were found to have more prominent number of electronegative functional groups, which are characteristic of sulphated polygalactans, in the downfield space of the NMR spectra. These electronegative functional groups in the substituted polysaccharides derived from the seaweeds prevent abstraction of hydrogen from arachidanoic acid in cyclooxygenases by ion pairing, and in this manner prevent synthesis of the pro-inflammatory prostaglandins.
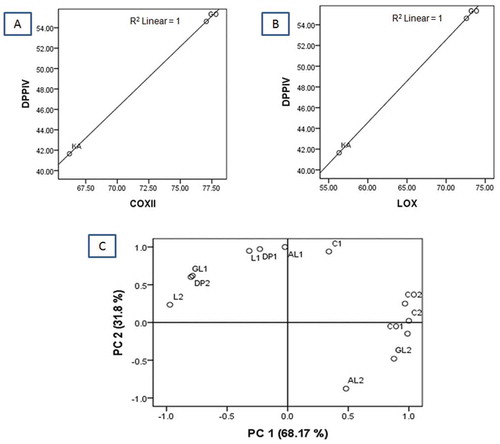
A positive correlation was noticed between the DPP-4 inhibitory activities of the sulphated polygalactan enriched concentrate derived from G. opuntia (GO) and K. alvarezii (KA) and their anti-inflammatory activities. A significant positive correlation between anti-inflammatory and antidiabetic activity of both the sulphated polygalactans isolated from G. opuntia (GO) and K. alvarezii (KA; DPP-4/COX-2: R2 = 1, DPPIV/LOX: R2 = 1) were noted ( and ). A positive correlation was observed between DPP-4 and α-amylase/α-glucosidase activity of sulphated polygalactan (DPP-4/α-amylase: R2 = 1, DPP-4/α-glucosidase: R2 = 1). The relationships between anti-inflammatory and antidiabetic activities of the sulphated polygalactan enriched concentrate of K. alvarezii and G. opuntia were statistically analyzed using PCA (). The loading of first and second principle components (PC1 and PC2) were accounted for 68.17 and 31.8% of the variance, respectively. The component, PC1 was mainly influenced by inhibitory activities of sulphated polygalactan derived from G. opuntia and K. alvarezii (GO, KA) toward the pro-inflammatory enzymes, COX-1 (denoted as C1; GO and C2; KA) and COX-2 (denoted as CO1; GO and CO2; KA), along with α-amylase (AL2; KA) and α-glucosidase (GL2; KA). On the other hand, 5-LOX (denoted as L1; GO and L2; KA), DPPIV (DP1; GO and DP2; KA), α-amylase (AL1; GO), and α-glucosidase (GL1; GO) inhibiotory properties of the sulphated polygalactans of the title seaweeds were mainly contributed to PC2 ( and ). The similarity in the greater loading of DPP-4, COX-2, 5-LOX·inhibitory activities, and a significant positive correlation between DPP-4 inhibition activity (DP1-DP2) with anti-COX and LOX properties of seaweed derived sulphated polygalactans apparently demonstrated that these bioactivities were in close relation. The significant correlation of antidiabetic activities with anti-inflammatory properties of the sulphated polygalactans isolated from the seaweeds G. opuntia and K. alvarezii also indicated that these polysaccharides derived from seaweeds were responsible for potential anti-inflammatory and antidiabetic properties.
Figure 3. Correlation plot between inhibition of antidiabetic (dipeptidyl peptidase, DPP-4) and anti-inflammatory (cyclooxygenase-2, COX-2 and 5-lipoxygenase, 5-LOX) activities (A-B). A: DPP-4 versus COX-2; and B: DPP-4 versus 5-LOX activities; C: PCA loading plot diagrams showing the correlation between antidiabetic and anti-inflammatory activities of the sulphated polygalactan fractions from G. opuntia and K. alvarezii.
Conclusions
The sulphated polygalactans isolated from the red seaweeds K. alvarezii and G. opuntia possess a number of bioactivities against different disease targets, namely, inflammation and type-2 diabetes. The sulphated polygalactan enriched concentrate obtained from G. opuntia showed greater anti-inflammatory activities than that from K. alvarezii as determined by in vitro cycloxygenase/lipoxygenase inhibitory activities. The activities showed significant positive correlation with the antidiabetic activities as determined by in vitro α-amylase, α-glucosidase, and dipeptidyl peptidase-4 inhibitory properties. This study demonstrated the candidacy of red seaweeds particularly, G. opuntia as potential source of bioactive sulphated polygalactans for use as functional food supplements to deter inflammation and type-2 diabetes.
Acknowledgments
The authors thank the Director, Central Marine Fisheries Research Institute, for his guidance and support. Thanks are due to the Head, Marine Biotechnology Division for facilitating the research works. Fasina Makkar thanks Department of Science and Technology for fellowship.
Funding
This work is supported by the funding under the Science and Engineering Research Board (SERB) Scheme (Grant number SR/S1/OC-96/2012 SERB) from Department of Science and Technology, Ministry of Science and Technology, New Delhi, India.
Additional information
Funding
References
- Bearse, M.A.; Han Jr., T.; Schneck, M.E. Local Multifocal Oscillatory Potential Abnormalities in Diabetes and Early Diabetic Retinopathy. Investigative Ophthalmology and Visual Sciences 2004, 45, 3259–3265.
- Baishakhi, D.; Analava, M. Chemo-Profiling of Eucalyptus and Study of Its Hypoglycemic Potential. World Journal of Diabetes 2013, 4(5), 170–176.
- Festa, A.D.; Agostino, R.; Howard, G.; Mykkanen, L.; Tracy, R.P.; Haffner, S.M. Chronic Subclinical Inflammation as Part of the Insulin Resistance Syndrome: The Insulin Resistance Atherosclerosis Study. Circulation 2000, 102, 42–47.
- Crook, M. Type 2 Diabetes Mellitus: A Disease of the Innate Immune System an Update. Diabetic Medicine 2004, 21, 203–207.
- Seung-Hong, L; You-Jin, J. Anti-Diabetic Effects of Brown Algae Derived Phlorotannins, Marine Polyphenols Through Diverse Mechanisms. Fitoterapia 2013, 86, 129–136.
- Chakraborty, K.; Paulraj, R. Sesquiterpenoids with Free Radical Scavenging Properties from Marine Macroalga Ulva fasciata Delile. Food Chemistry 2010, 122, 31–41.
- Chakraborty, K.; Joseph, D.; Praveen, N.K. Antioxidant Activities and Phenolic Contents of Three Red Seaweeds (Division: Rhodophyta) Harvested from the Gulf of Mannar of Peninsular India. Journal of Food Science and Technology 2015, 52(4), 1924–1935.
- Mentlein, R. Dipeptidyl-Peptidase IV (CD26): Role in the Inactivation of Regulatory Peptides. Regulatory Peptides 1999, 24, 85–89.
- Leonard, F.; Collnot, E.M.; Lehr, C.M. A Three-Dimensional Co-Culture of Enterocytes, Monocytes and Dendritic Cells to Model Inflamed Intestinal Mucosa in vitro. Molecular Pharmaceutics 2010, 7, 2103–2119.
- Blouin, N.A.; Brodie, J.A.; Grossman, A.C.; Xu, P.; Brawley, S.H. Porphyra: A Marine Crop Shaped by Stress. Trends in Plant Science 2011, 16, 29–37.
- Gressler, V.; Yokoya, N.S.; Fujii, M.T.; Colepicolo, P.; Mancini, J.; Torres, R.P.; Pinto, E. Lipid, Fatty Acid, Protein, Amino Acid and Ash Contents in Four Brazilian Red Algae Species. Food Chemistry 2010, 120, 585–590.
- Holdt, S.L.; Kraan, S. Bioactive Compounds in Seaweed: Functional Food Applications and Legislation. Journal of Applied Phycology 2011, 23, 543–597.
- Chew, Y.L.; Lim, Y.Y.; Omar, M.; Khoo, K.S. Antioxidant Activity of Three Edible Seaweeds from Two Areas in South East Asia. LWT–Food Sciences and Technology 2008, 41, 1067–1072.
- Matanjun, P.; Suhaila, M.; Noordin, M.M.; Kharidah, M.; Ming, C.H. Antioxidant Activities and Phenolic Content of Eight Species of Seaweeds from North Borneo. Journal of Applied Phycology 2008, 20, 367–373.
- Ganesan, P.; Kumar, C.S.; Bhaskar, N. Antioxidant Properties of Methanol Extracts and Its Solvent Fractions Obtained from Selected Indian Red Seaweeds. Bioresource Technology 2008, 99, 2717–2723.
- Kumar, K.S.; Ganesan, K.; Subba Rao, P.V. Antioxidant Potential of Solvent Extracts of Kappaphycus alvarezii (Doty) Doty an Edible Seaweed. Food Chemistry 2008, 107, 289–295.
- Farah Diyana, A.; Abdullah, A.; Shahrul Hisham, Z.A.; Chan, K.M. Antioxidant Activity of Red Algae Kappaphycus alvarezii and Kappaphycus striatum. International Food Research Journal 2015, 22(5), 1977–1984.
- Nagarani, N.; Kumaraguru, A.K. Evaluation of Anti-Inflammatory, Antidiabetic, Cytotoxic Activity of K. alvarezii. International Journal of Pharma and Biosciences 2013, 4(1), 921–929.
- Mendonça, P.; Freitas, J.C. Topical Antiedematous Activity of the Organic Extract of Liagora farinosa Algae (Rhodophyta, Nemaliales). Ciencia e Cultura 2000, 52, 175–178.
- Praveen, N.K.; Chakraborty, K. Antioxidant and Anti-Inflammatory Potential of the Aqueous Extract and Polysaccharide Fraction from Brown Marine Macroalgae Padina sp from Gulf of Mannar of Peninsular India. Journal of Coastal Life Medicine 2013, 1(1), 38–48.
- Larsen, T.A.; Gujer, W. Separate Management of Anthropogenic Nutrient Solutions (Human Urine). Water Science and Technology 1996, 34(3–4), 87–94.
- Baylac, S.; Racine, R. Inhibition of 5-Lipoxygenase by Essential Oils and Other Natural Fragrant Extracts. International Journal of Aromatherapy 2003, 13, 138–142.
- Hamdan, I.I.; Afifi, F.U. Studies on the in vitro and in vivo Hypoglycemic Activities of Some Medicinal Plants Used in Treatment of Diabetes in Jordanian Traditional Medicine. Journal of Ethnopharmacology 2004, 93, 117–121.
- Dong, H.Q.; Li, M.; Zhu, F.; Liu, F.L; Huang, J.B. Inhibitory Potential of Trilobatin from Lithocarpus Polystachyus Rehd Against α-Glucosidase and α-Amylase Linked to Type 2 Diabetes. Food Chemistry 2012, 130, 261–266.
- Villanueva, R.D.; Montano, M.N.E.; Romero, J.B. Iota-Carrageenan from a Newly Formed, Rare Variety of Eucheumoid Seaweed. Journal of Applied Phycology 2009, 21, 27–30.
- Gomez-Ordonez, E.; Rupere, P. FT-IR-ATR Spectroscopy as a Tool for Polysaccharide Identification in Edible Brown and Red Seaweeds. Food Hydrocolloids 2011, 25, 1514–1520.
- Cases, M.R.; Cerezo, A.S.; Stortz, C.A. Separation and Quantitation of Enantiomeric Galactoses and Their Mono-O-Methylethers as Their Diastereomeric Acetylated 1-Deoxy-1-(2-Hydroxypropylamino) Alditols. Carbohydrate Research 1995, 269(2), 333–341.
- Vandevelde, F.; Knutsen, S.H.; Usov, A.I.; Rollema, H.S; Cerezo, A.S. 1H and 13C High Resolution NMR Spectroscopy of Carrageenans: Application in Research and Industry. Trends in Food Science and Technology International 2002, 13, 73–92.
- Chiovitti, A.; Bacic, A.; Craik, D.J.; Kraft, G.T.; Liao, M.L.; Falshaw, R. A Pyruvated Carrageenann from Australian Specimens of the Red Alga Sarconema filiforme. Carbohydrate Research 1998, 310, 77–83.
- Bellion, C.; Brigand, G.; Prome, J.C.; Welti, D.; Bociek, S. Identification Characterization des Précurseurs Biologiques des Carraghénanes par Spectroscopie. Carbohydrate Research 1983, 119, 31–48.
- Davis, S.N.; Granner, D.K. Insulin, Oral Agents and the Pharmacology of the Endocrine Pancreas. In Goodman and Gilman’s The Pharmacological Basis of Therapeutics; Hardman, J.G.; Limbird, L.E.; Gilman, A.G.; Eds.; McGraw-Hill Co Inc.: New York, NY, 2011; 1679–1714 p.
- Conforty, F.; Loizzo, M.R.; Statty, G.A.; Menichini, F. Comparative Radical Scavenging and Antidiabetic Activities of Methanolic Extract and Fractions from Achillea ligustica. Biological and Pharmaceutical Bulletin 2005, 28, 1791–1794.
- Matsui, T.; Ueda, T.; Sugita, K.; Terahara, N.; Matsumoto, K. α-Glucosidase Inhibitory Action of Natural Acylated Anthocyanins. α-Glucosidase Inhibiton by Isolated Acylated Anthocyanins. Journal of Agricultural Food Chemistry 2001, 49, 1952–1956.
- Apostolidis, E.; Karayannakidis, P.D.; Kwon, Y.I.; Lee, C.M; Seeram, N.P. Seasonal Variation of Phenolic Antioxidant-Mediated α-Glucosidase Inhibition of Ascophyllum nodosum. Plant Foods for Human Nutrition 2011, 66(4), 313–319.
- Idris, I.; Donnelly, R. Dipeptidyl Peptidase-IV Inhibitors: A Major New Class of Oral Antidiabetic Drug. Diabetes Obesity Metabolism 2007, 2, 153–165.
- Murai, A.; Iwamura, K.; Takada, M.; Ogawa, K.; Usui, T.; Okumura, J.I. Control of Postprandial Hyperglycaemia by Galactosyl Maltobionolactone and Its Novel Anti-Amylase Effect in Mice. Life Science 2002, 71, 1405–1415.
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.A. Comparative Study of the Anti-Inflammatory, Anticoagulant, Antiangiogenic, and Antiadhesive Activities of Nine Different Fucoidans from Brown Seaweeds. Glycobiology 2007, 17(5), 541–552.
- Lee, S.H.; Han, J.S.; Heo, S.J.; Hwang, J.Y.; Jeon, Y.J. Protective Effects of Dieckol Isolated from Ecklonia cava Against High Glucose-Induced Oxidative Stress in Human Umbilical Vein Endothelial Cells. Toxicology in Vitro 2010, 24(2), 375–381.
- Frias, J.P.; Sobral, P.; Ferreira, A.M. Organic Pollutants in Microplastics from Two Beaches of the Portuguese Coast. Marine Pollution Bulletin 2010, 60(11), 1988–1992.
- Silva, E.D.; Scheuer, P.J. Manoalide, an Antibiotic Sesterterpenoid from the Marine Sponge Luffariella variabilis (Poleajaeff). Tetrahedron Letters 1980, 21(17), 1611–1614.
