ABSTRACT
Pu-erh tea has gained more and more popularity and attracted much attention for its various biological effects. The objective of this study was to determine the active phenolic compounds and the biological effects of 15 differently aged Pu-erh teas. The results showed that 43 active phenolics, containing 7 flavan-3-ols, 11 organic acids, and esters, 3 proanthocyanidin dimers, 2 benzotropolones, and 20 flavonoid glycosides were identified based on high-performance liquid chromatography coupled to electrospray ionisation and quadrupole time-of-flight tandem mass spectrometry (HPLC-ESI-QTOF-MS/MS). In particular, 2’-O-galloylhyperin and quercetin-3-O-p-coumaroyl-rhamnosyl-arabinosyl-hexoside were identified from constituents of tea for the first time. The 15 Pu-erh teas exhibited strong DPPH and ABTS scavenging activity in a concentration-dependent manner with IC50 0.99–2.12 and 0.97–2.67 mmol Trolox equivalent antioxidant capacity/g, respectively. All of the Pu-erh teas tested significantly inhibited the proliferation of SMMC-7721 cells, in a dosage-dependent manner. Of the 15 Pu-erh teas examined, the cake tea P6 had the strongest effect on SMMC-7721 cells with IC50 = 65.88 ± 3.53 µg/mL. The others Pu-erh teas had an IC50 = 96 – 509 µg/mL. Our results also indicated that not all the older Pu-erh teas display stronger anti-activities and anti-cancer than the younger ones and the youngest Pu-erh tea did not possess the highest level of active components. This study provides useful information for consumers to deeply understand the chemical constituents and bioactivity of Pu-erh tea preserved for a long time.
Introduction
Pu-erh tea is a unique fermented tea, which is originally produced in Yunnan Province, southwest of China using the leaves of the cultivated variety large-leaf tea of Camellia sinensis. It has been confirmed that Pu-erh tea exhibits multiple beneficial health effects, such as antioxidant,[Citation1,Citation2] anticancer,[Citation3] antimutagenic,[Citation4] antimicrobial,[Citation4] anti-atherogenic, antiobesity,[Citation5,Citation6] as well as hepatoprotective[Citation7] activities. The health benefits attributed to Pu-erh tea has increased its popularity.
In the production of Pu-erh tea, the sun-dried green tea leaves are autoclaved and dried to make Pu-erh raw tea and stored in natural conditions to make aged Pu-erh tea, or the leaves are artificially post-fermented with microorganisms for several months to 1 year to produce ripened Pu-erh tea. For fully fermented black tea, the oxidation of the tea polyphenols is performed mainly by polyphenol oxidases from the tea leaves. However, the polyphenol oxidases are inactivated by heat during the steam-compression process. Thus, the tea polyphenols in Pu-erh tea are oxidized mainly by the action of microorganisms.[Citation8] The microorganisms oxidize tea polyphenols more completely than polyphenol oxidase in black tea, leading to lower concentrations of tea polyphenols and tea catechins in Pu-erh tea.
It is generally believed that the longer the aged Pu-erh tea is kept, the better it will be in both quality and taste. Thus, the commercial value of older Pu-erh tea is much higher than that of the younger ones. However, there is still an argument whether aged Pu-erh teas with longer storage times have stronger bioactivities. Some researchers showed that the chemical constituents and beneficial health effects were significantly different in the Pu-erh teas with different storage years.[Citation9] Others have reported that younger Pu-erh teas had stronger free radical scavenging activities than older ones of the same type.[Citation10] Furthermore, another study demonstrated that the quality of tea infusion could reach levels as high as the long-term storage Pu-erh teas after the fresh tea leaves were artificially inoculated with the appropriate microorganisms for a few weeks.[Citation11] However, little research has been carried out on the bioactivities and biochemical components of the aged Pu-erh teas with 25 or much longer storage years due to their rarity and precious.
Modern pharmacological research suggests that the health benefits of tea consumption are mainly due to the tea polyphenols. Many types of polyphenols have been identified in tea, including catechins, flavonols, flavones, gallic acid (GA), quinic esters of gallic, caffeic and coumaric acid, theaflavins, thearubigins, and proanthocyanidins.[Citation12,Citation13] Identification and determination of the previously mentioned phenolic components has been performed using hyphenated analytical techniques, such as liquid chromatography-mass spectrometry (LC-MS).
In the present study, 15 aged Pu-erh teas with storage periods from 10 to 105 years were used to evaluate their phenolic constituents and bioactivities. The phenolic compounds of these aged Pu-erh teas were identified by the high-performance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry (HPLC-QTOF-MS) technique. Their antioxidant activities were determined by both diphenylpicryl-hydrazyl (DPPH) and 2,2′-azinobis(3-ethylbenzthiazoline-sulfonic acid) diammonium salt (ABTS) assays. And their effects on the proliferation of human hepatocellular carcinoma were investigated using the 3-(4,5-dimethylthiazolyl)-2,5-diphenyl-tetrazolium bromide (MTT) method.
Materials and methods
Tea samples
Fifteen Pu-erh tea samples with storage periods from 10 to 105 years were obtained from Kemingyuan Tea Shop, Xiamen, Fujian, China, as shown in Table S1. Cake tea P7 (ripened pu-erh tea) is manufactured according to artificially post-fermented process in Yunnan province, and then stored at room temperature; others are raw Pu-erh teas, which are stored at natural conditions for a long time (25–105 years) to ferment, that so-called aged Pu-erh teas. More detailed information on these Pu-erh tea samples is summarized in the supporting information (Fig. S1). The tea samples were all kept in desiccators at room temperature. All of the experiments were carried out in 2015.
Chemicals
The standard samples of GA, caffeine, theobromine, theophylline, (+)-catechin (C), (–)-epicatechin (EC), (–)-gallocatechin (GC), (–)-epigallocatechin (EGC), (-)-epicatechin gallate (ECG), (–)-epigallocatechin gallate (EGCG), and (–)-gallocatechin gallate (GCG) were purchased from Sigma (St. Louis, MO). DPPH was obtained from Aldrich (Milwaukee, WI). Acetonitrile, methanol, and formic acid were high-performance liquid chromatography (HPLC)-grade and obtained from Merck (Darmstadt, Germany). Deionized water was prepared using a Millipore Milli Q-Plus system (Millipore, Billerica, MA). Fetal bovine serum (FBS) was obtained from Hyclone. RPMI-1640 medium, penicillin, streptomycin and 0.25% trypsin were purchased from Gibco. Dimethylsulfoxide (DMSO) was obtained from Amresco and MTT was obtained from Sigma.
Preparation of tea infusion samples
Two grams of each tea sample and 50 mL of boiling water were added into a conical flask and heated in a water bath (95°C) for 15 min. The tea infusions were filtrated through 0.22 μm membrane filter after cooling down. Finally, the resulting tea infusions were lyophilized.
HPLC-QTOF-MS analyses
HPLC-QTOF-MS analysis was performed using a Agilent 1260 Series Rapid Resolution LC system equipped with a binary pump with degasser, high-performance autosampler and thermostated column compartment (Agilent Corporation, Milford, MA), coupled with a Agilent 6520 Series quadrupole time-of-flight mass spectrometer. Chromatographic separations were performed on a 2.1 mm × 150 mm 1.8 μm ZORBAX SB-C18 (Agilent, USA) chromatography column. The column was maintained at 35°C and the injection volume was 10 µL. The mobile phase consisted of solvent A (0.5% formic acid water solution) and solvent B (0.5% formic acid acetonitrile solution) at a flow rate of 0.30 mL/min: 0–8 min, 5–5% B; 20–38 min, 5–95% B; 38–45 min, 95–95% B; 47–55 min, 5–5% B. The ESI source was operated in negative and positive mode. Nitrogen (N2) was used as sheath, auxiliary, and collision gas. The drying temperature and drying gas flow were 350°C and 5 L/min, respectively. The nebulizer pressure, collision energy, fragmentor, and skimmer were 40 psi, 35, 175, and 65 V, respectively. The mass spectra were obtained at a mass-to-charge ratio (m/z) scan range from 100 to 3000. Auto MS/MS parameters were used for the analysis in a full-scan mode.
Determination of aged Pu-erh tea antioxidant activity
DPPH radical-scavenging activity of the tea infusions was determined by the method described by Gaulejac et al.[Citation14] with some modifications. Briefly, 0.1 mL of lyophilized tea powder water solution of various concentrations was added to 3 mL of the DPPH methanol solution (6 × 10−Citation5 mol/L). The samples were incubated at room temperature for 30 min. Subsequently, the absorbance of each sample was measured at 517 nm using a Shimadzu ultraviolet (UV)-1602PC spectrophotometer (Kyoto, Japan). The radical-scavenging ability was calculated by the formula: scavenging ability (%) = 100 × [(A0 – Ai /A0)]. Where Ai is absorbance of the tea sample; A0 is absorbance of the methanol blank control.
The ABTS + radical scavenging activity was measured using the method described by Antolovich et al.[Citation15] with minor changes. First, the ABTS radical cation (ABTS·+) work solution was prepared by mixing ABTS·+ stock solution (7 mmol/L in water) with 2.5 mM potassium persulfate for 12 h at 4°C in the dark and then the work solution was diluted with ethanol to an absorbance of approximately 1.0 at 734 nm. Second, 0.1 mL of various concentrations of lyophilized tea powder water solution was mixed with 3.9 mL ABTS·+ work solution, and then the samples were incubated at room temperature for 6 min. Finally, the absorbance of each sample was measured at 734 nm using the Shimadzu UV-1602PC spectrophotometer (Kyoto, Japan) with ethanol as a blank control.
All of the experiments were repeated three times. The IC50 values (50% inhibition of the DPPH and ABTS radical formation, mg/mL) of the tea infusions and Trolox were calculated, respectively. The antioxidant activity of the tea sample was converted to the Trolox equivalent antioxidant capacity (TEAC) and calculated as follows: TEAC (mmol Trolox/g) = IC50 (Trolox) /IC50 (Pu-erh tea sample).
Determination of anticancer activity in the aged Pu-erh teas
The anticancer activity of the aged Pu-erh teas was determined using the MTT assay described by Van de Loosdrecht et al.[Citation16] Human hepatocarcinoma cancer cell line SMMC-7721 was provided by Xiamen Overseas Chinese Subtropical Plant Introduction Garden (Fujian, China). The SMMC-7721 cells were cultured at 37°C with 5% CO2 in a RPMI-1640 medium (pH = 7.2–7.4) supplemented with 10% FBS, 100 U/mL penicillin and 100 U/mL streptomycin. Exponentially growing cells were harvested and prepared into cell suspensions. Subsequently, 200 μL cell suspensions were inoculated into each well of the 96-well plates and the cells were incubated at 37°C for 24 h to achieve cell attachment. After that, the SMMC-7721 cells were treated with 180 μL/well tea infusion samples with different concentration for 24 h. Next, 20 μL MTT solution (5 mg/mL) was added to each well and the plates were incubated at 37°C for another 4 h. After the supernatants were removed, 150 μL DMSO was added to each well to dissolve the formazan crystals. Finally, the absorbance of each sample was read at 490 nm using a microplate reader. The cell survival percentage was calculated using the formula: Survival (%) = ODtea exposure /ODcontrol × 100. The IC50 values (50% inhibition of cell growth) were calculated. All of the experiments were repeated five times.
Total content determination of the phenolics, flavonoids, catechins, GA, and purine alkaloids
Total polyphenol content of the Pu-erh tea samples was determined by the Folin-Ciocalteu method described by Singleton et al. with minor modification.[Citation17] First, 1 mL of the tea infusion samples were mixed with 5 mL of 10% (v/v) Folin-Ciocalteu’s phenol reagent and 4 mL of 7.5% (w/v) Na2CO3 solution. Second, the mixtures were incubated for 1 h at room temperature and then their absorbance at 765 nm was measured. The total polyphenol content of each tea infusion sample was calculated according to a standard curve of GA solution and converted into milligrams of GA equivalents per gram of the Pu-erh tea of sample (mg/g). Each experiment was conducted in triplicate.
The total flavonoid content was analyzed using the Dowd method with minor modification.[Citation18] Briefly, 2 mL tea infusion sample, 8 mL of 1.5% (w/v) AlCl3, 4 mL HAc-NaAc buffer (pH = 5.5), and 11 mL of 50% (v/v) ethanol were added to 25-mL volumetric flask, respectively. After incubation for 30 min, the absorbance was measured at 415 nm using a UV-vis spectrophotometer. Rutin was used as a standard solution. Total flavonoid content was expressed as milligrams of rutin equivalents per gram of the Pu-erh teas. All experiments were carried out in triplicate.
The content of free amino acid (glutamate as a standard solution) and total soluble saccharides (glucose as a standard solution) were determined according to the methods described by Liang et al.[Citation19] Total contents of GA, purine alkaloids and catechins were separately measured on the Agilent series 1100 (Agilent Technologies) liquid chromatograph. A Welch Ultimate XB-C18 column (250 mm × 4.6 mm i.d., 5 μm) with a precolumn was used in this study. The separation was achieved by a gradient elution of 0.1% formic acid solution (solvent A) and acetonitrile (solvent B) at a flow rate of 1 mL/min: 0–20 min, 5–17% B; 20–40 min, 17–17% B. The equilibration time was 20 min and the injection volume was 20 μL. The detection wavelength was set at 278 nm. The column was re-equilibrated with the initial conditions for 15 min before the next run.
Statistical analysis
Correlations (r) of data were evaluated through Pearson’s correlation analysis using SPSS 19.0 software, and a p-value of less than 0.05 was considered to be statistically significant.
Results and discussion
Polyphenolic identification by HPLC-QTOF-MS/MS analysis
Some studies demonstrated that the potential health benefits of the Pu-erh teas could be mainly attributed to their polyphenolic compounds.[Citation20,Citation21] Accordingly, the polyphenolic components in the 15 aged Pu-erh teas were identified by the HPLC-QTOF-MS/MS technique in this study. Combined with the relevant literature, the components of the tea polyphenols were putatively annotated by comparison of their accurate mass and MS/MS fragmentation patterns with those of the authentic standards.[Citation22–Citation26] Chromatogram profiles of the aged Pu-erh teas recorded as negative modes are shown in Fig. S2. The retention times, MS and MSCitation2 spectral data and the identification results of 43 compounds in the aged Pu-erh teas are listed in .
Table 1. Retention times, MS spectral characteristics, and identify of phenolic compound present in 15 Pu-erh teas.
Flavan-3-ols
Peaks 7, 14, 15, 25, 27, 30, and 31 were identified as GC, C, EGC, EGCG, EC, ECG, and CG by comparing the retention time, [M–H]– ions and MSCitation2 fragment ions with those of authentic standard compounds and literature data. The parent ion [M–H]– at m/z 289.0714, generated MSCitation2 fragments at m/z 203.0678 and 137.0241, corresponding to the loss of one C4H6O2 and C8H8O3 moieties, respectively. The parent ion [M–H]– at m/z 305.0669, generated MSCitation2 fragments at m/z 219.0645, 167.0348, and 165.0212, corresponding to the loss of one C4H6O2, C7H6O3, and C7H8O3 moieties, respectively. The parent ion [M–H]– at m/z 441.0857 produced MSCitation2 fragments 289.0694 and 169.0129 corresponding to (epi) catechin and GA moieties, respectively. The parent ion [M–H]– at m/z 457.0769 produced MSCitation2 fragments 305.0606 and 169.0136 corresponding to (epi) gallocatechin and GA moieties, respectively.
Proanthocyanidin dimers
Peak 16, 17, and 21 have the same [M–H]– at m/z 577.1337 and MSCitation2 ion at m/z 407.0780 and 289.0716 corresponding to the cleavage of GA (170 Da) and epi(catechin); therefore, these compounds were tentatively identified as (epi)catechin(epi)catechin. These proanthocyanidin dimers were formed through quinone-methide (QM), retro-Diels-Alder (RDA), and heterocyclic ring fission (HRF) pathways.[Citation10]
Organic acids and their esters
Peak 1 was identified as ethyl 3,4-dihydroxybenzoate because it had [M–H]– at m/z 181.068 and MSCitation2 fragments at m/z 135.9104 corresponding to the loss of a OC2H5 moiety. Peak 2 was identified as quinic acid because it has [M–H]– at m/z 191.0559 and MSCitation2 fragments at m/z 127.0389. Peak 5 was identified as GA, which had a [M–H]– at m/z 169.0147 and MSCitation2 ions at m/z 125.0239 with the loss of one CO2 molecule. Peak 6 was tentatively identified as 3-O-galloylquinic acid, which produced a [M–H]– at m/z 343.0676 and prominent MSCitation2 ions at m/z 191.0557 (quinic acid residue). Peak 11, 13, and 28 have the same [M–H]– at m/z 337.0919 and fragmented ions at m/z 163.0035 corresponding to the deprotonated ion of cinnamoyl; therefore, peaks 11, 13 and 28 were 3-p-coumaroylquinic acid, 4-p-coumaroylquinic acid, and 5-p-coumaroylquinic acid, respectively, according to the elute order described by Clifford et al.[Citation27] Peak 12 was determined to be caffeic acid, which had a [M–H]– at m/z 179.0580. Peak 24 was determined to be 3-caffeoylquinic acid, which had a [M–H]– at m/z 353.0872 and MSCitation2 fragment at m/z 191.0553 corresponding to the loss of a caffeoyl unit. Peak 43 was identified as ellagic acid, it had a [M–H]– at m/z 300.9992 that is almost identical to peak 49 (quercetin) with [M–H]– at m/z 301.0349. Peak 53 was tentatively identified as trigalloylglucose because it had a [M–H]– at m/z 635.0416 and MSCitation2 fragments at m/z 112.9858.
Flavonols
Peak 49 had a [M–H]– ion at m/z 301.0349 and MSCitation2 ion at m/z 151.0033, 121.0297, 107.0141 was tentatively identified as quercetin. Peak 52 with [M–H]– ion at m/z 285.0388 was tentatively identified as kaempferol. Peak 46 with [M–H]– ion at m/z 317.0309 and MSCitation2 ion at m/z 151.0025, 137.0235, and 109.0296 was tentatively identified as myricetin. Peak 20 with a [M–H]– ion at m/z 319.0416, and MSCitation2 ion at m/z 203.0337, 175.0404, 137.0263, 109.0304, has a molecular weight 2 amu higher than compound myricetin was tentatively identified as dihydromyricetin.
O-Glycosylated flavonols
A total of six myricetin, quercetin, or kaempferol glycosides conjugated to a range of sugars, including glucose, galactose, and rhamnose, as mono-, di-, and trisaccharides were identified, which were universally present in tea. Peak 34 had a [M–H]– at m/z 479.0816, which produced MSCitation2 ions at m/z 316.0220 and 317.0247 (myricetin) with the loss of m/z 162 corresponding to the cleavage of hexose sugar; thus, peak 34 was determined as myricetin 3-O-galactoside.
Peak 41 had a [M–H]– at m/z 755.1969 and MSCitation2 fragments at m/z 609.1176 and 301.0321 corresponding to the loss of 146 Da and 454 Da fragments. The loss of 146 Da was in keeping with the cleavage of a rhamnosyl unit, 301.0321 corresponding to quercetin. Generally, the more sugar moieties attached to the flavonoid aglycone, the earlier the retention time in reversed phase HPLC. Therefore, peak 41 was identified as quercetin-3-O-dirhamnosylglucoside.
Peak 42 was identified as rutin with [M–H]– at m/z 609.1448 and MSCitation2 fragment at m/z 301.0333 by losing a sugar molecule of glucose and rhamnose from aglycone quercetin. Peak 44 and 47 have the same [M–H]– at m/z 593.1435 and MSCitation2 fragment at m/z 285.0354 by losing a sugar molecule of hexose-rhamnose from aglycone kaempferol. Galactosides elute earlier than the corresponding glucosides; therefore, peak 44 and 47 were identified as kaempferol-3-O-rhamnosyl-galactoside and kaempferol-3-O-rhamnosyl-glucoside, respectively. Peak 45 was tentatively identified as kaempferol-rhamnose-hexose-rhamnose with a [M–H]– at m/z 739.2004 and MS2 fragment at m/z 593.1507 corresponding to the loss of a 146 Da fragment (equates with the cleavage of a rhamnosyl unit) and 285.0440 (kaempferol).
C-Glycosylated flavones
Eight C-glycosylated apigenins were identified in 15 aged Pu-erh teas. Peak 29 had a [M–H]– ion at m/z 593.1544 and MSCitation2 ions at m/z 473.1105 (M–H–Hexosyl)–, 383.0786, 353.0680 (M–H–dihexosyl)–, showing the existence of two glycosyls; thus, peak 29 was tentatively identified as 6,8-C-diglucosylapigenin. Peak 26 had a [M–H]– ion at m/z 595.1636 and predominant MSCitation2 ions at m/z 475.1165, 385.0935, and 355.0826 (M–H–dihexosyl)–, which has a molecular weight 2 amu higher than compound 29; therefore, it was identified as an unknown C-glycosylated flavones.
Peaks 32 and 33 had the same [M–H]– at m/z 563.1396 and MSCitation2 ions at m/z 473.1096 (M–H–pentosyl)–, 443.0984 (M–H–hexosyl)–, 383.0756 and 353.0644, which were assigned as 6-C-arabinosyl-8-C-glucosyl, apigenin, and 6-C-glucosyl-8-C-arabinosyl apigenin, respectively based on the different ratio of ions m/z 443:473. Peak 40 was tentatively identified as apigenin 6,8-C-depentoside because it had a [M–H]– ion at m/z 533.1318 and MSCitation2 fragments at 473.1049 (due to loss of 60 amu from one pentosyl), 443.0953, 413.0793 (due to loss of two pentosyls of 60 amu), 383.0763, and 353.0643.
Peak 36 and 39 have the same [M–H]– ion at m/z 431.0989 and main MSCitation2 fragment ions at m/z 311.0553 (M–H–hexosyl)–, 283.0599, which were assigned as vitexin and isovitexin. Peak 37 had a [M–H]– ion at m/z 615.0925 and main MSCitation2 fragment ions 463.0774 and 301.0267; thus, peak 37 was tentatively identified as 2’-O-galloylhyperin, which was not previously reported in tea.
Acylated glycosylated flavonols
Two acylated flavonol glycosides contained one p-coumaroyl group were identified in the tested Pu-erh teas. Peak 50 had a [M–H]– at m/z 887.2237 and MSCitation2 fragment at m/z 741.1873 corresponding to the loss of a 146 Da. This 146 amu loss was in keeping with the cleavage of a p-coumaroyl group. The ion 741.1873 produced 595.1124 and 301.0348 ions corresponding to the loss of a 146 Da (a rhamnose moiety) and 294 Da (a sugar molecule of arabinosyl and hexosyl) fragments. The fragment ion at m/z 301 corresponded to the aglycone quercetin. Therefore, peak 50 was tentatively identified as quercetin-3-O-p-coumaroyl-rhamnosyl-arabinosyl-hexoside, which is a new compound identified in teas.
Peak 51 was speculated as quercetin-3-O-p-coumaroyl-dirhamnosylhexoside with [M–H]– at m/z 901.2346 and MSCitation2 fragment at m/z 755.1764 corresponding to the loss of a 146 Da fragment that was in keeping with the cleavage of a p-coumaroyl group. The fragment ion at m/z 755.1764 was similar to the deprotonated ion of compound xx, which produced fragment ions at m/z 609.1458 and 301.0336.
Substance of benzotropolones
Peak 3 had a [M–H]– ion at m/z 221.0256 and a predominant MSCitation2 ion fragment at m/z 113.0205, corresponding to the loss of a pyrogallol unit; thus, peak 3 was tentatively identified as purpurogallin. Purpurogallin is well known to play a key role in affecting the availability of micronutrients in the soil, the bioavailability of metals in the body and the oxidation of theaflavins and their physiological activity.[Citation28] Peak 8 had a [M–H]– ion at m/z 399.1055 and main MSCitation2 ion fragments at m/z 275.1064 and 110.0260, corresponds with (C14H12O6–H)– ion and C6H6O2; therefore, peak 8 was tentatively identified as (epi) theaflagallin, which was formed from the oxidation of (epi) gallocatechin and pyrogallol.
In total, 43 phenolic compounds including 7 catechins, 3 proanthocyanidin dimers, 11 organic acids, and their esters, 4 flavonols, 6 O-glycosylated flavonols, 8 C-glycosylated flavones, 2 acylated glycosylated flavonols, and 2 benzotropolones were identified in 15 Pu-erh teas. Many of the previously stated phenolic compounds have been reported to exhibit a broad range of biological activities, especially the catechins, which are strong antioxidants in vitro and in vivo. For example, Qian et al.,[Citation10] reported that 10 major free radical scavengers were found in Pu-erh tea, and nine of them were identified as GA, GC, EGC, C, EC, EGCG, ECG, rutin, and quercetin-3-glucoside by HPLC-DAD-MS coupled online with ABTS-based analysis.
Proanthocyanidin dimers are known to be good free radical scavengers because it contains many more hydroxyl groups than a monomer. Because hydroxyl groups in dimers are conjugated with a double bond, dimers may act as a hydrogen donor, thereby stabilizing the electron distribution in the molecule and enhancing antioxidative properties.[Citation29] Flavonoids in tea exist in aglycone-free and glycoside forms. The major flavonoids in tea are the conjugates of quercetin and kaempferol with conjugating moiety varying from mono- to tetra-glycosides.[Citation30–Citation33] GA, quinic esters of gallic, coumaric, and caffeic acids are the common phenolic acids in teas.[Citation32] Flavonoids and phenolic acids have been demonstrated to possess many biological activities, such as antioxidant capacity, antiproliferative, apoptotic activity, antiviral, and antimicrobial.[Citation34–Citation37]
DPPH and ABTS radical scavenging activity
Free radicals can damage diverse cellular macromolecules, including proteins, carbohydrates, lipids, and nucleic acids due to their high reactivity, further leading to many diseases (such as diabetes mellitus, cancer, atherosclerosis, neurodegenerative diseases, and arthritis).[Citation38] Previous studies have reported that the Pu-erh tea extracts can effectively eliminate the free radicals, preventing living organisms from oxidative damage.[Citation2,Citation39] Therefore, the radical-scavenging activities of 15 aged Pu-erh teas with the storage periods from 10 to 105 years were separately evaluated by the DPPH and ABTS assays in present study.
The IC50 values of DPPH and ABTS assay are shown in . The results showed that the brick tea B4 (75-year storage) exhibited simultaneously the lowest DPPH and ABTS radical-scavenging activities. In the DPPH assay, the bowl tea T1 (75-year storage) was observed to possess the highest DPPH radical-scavenging activity, followed by the bowl tea T2 (71-year storage). In the ABTS assay, the cake tea P3 (75-year storage) appeared to have the highest ABTS radical-scavenging activity. Furthermore, the DPPH and ABTS radical-scavenging activities of the 15 aged Pu-erh teas occurred in a concentration-dependent manner (Fig. S3). The DPPH and ABTS radical-scavenging rates of the 14 aged pu-erh tea extracts with concentrations from 0.6 to 2.5 mg/mL could all reach at least 70% except the extracts of the brick tea B4.
Table 2. IC50 values of DPPH and ABTS assay of 15 Pu-erh teas.
Our results showed that the radical-scavenging activities of different aged Pu-erh teas varied, and not all the older Pu-erh teas exhibit stronger biological ability than the younger ones. This result is not in accordance with an earlier study performed by Qian et al., in which the ABTS radical-scavenging activities of the aged Pu-erh teas decreased with the increase of the Pu-erh tea storage years.[Citation10] Phenolic compounds have been reported to be excellent free radical scavengers due to their strong hydrogen-donating ability. The inconsistency between these results might be attributed to the difference in phenolic constituents of the aged Pu-erh tea samples used by these two independent studies.
Anticancer activity of aged Pu-erh teas
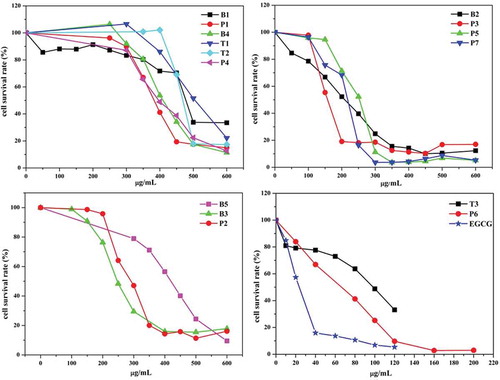
MTT is one of the most common in vitro assays to estimate the cytotoxic and/or cytostatic, or apoptosis-inducing effects of anticancer agents prior to preclinical and clinical testing. In the present study, effects of the aged Pu-erh tea samples on the proliferation of human hepatoma SMMC-7721 cells were examined in vitro using MTT assay with the standard substances of EGCG as a positive control. As shown in , growth and proliferation of SMMC-7721 cells were inhibited in a dose-dependent manner by the treatments of both EGCG and the Pu-erh tea samples for 24 h. The IC50 values of 15 aged Pu-erh teas and EGCG were summarized in . The results showed that the cake tea P6 (25-year storage) exhibited the strongest inhibitory activity on the proliferation of the SMMC-7721 cells with an IC50 value of 65.88 ± 3.53 µg/mL. Simultaneously, the cell viability of the SMMC-772 cells was less than 10% when the cells were treated with 120 µg/mL tea extracts of the cake tea P6 for 24 h. The IC50 value of the bowl tea T3 (27-year storage) was 96.94 ± 2.15 µg/mL, while those of the other 13 Pu-erh teas were from 154 to 509 µg/mL. Although the anticancer activities of the 15 aged Pu-erh tea samples were all weaker than that of the positive control, pure EGCG, of which the IC50 value was 24.24 ± 0.92 µg/mL, the Pu-erh teas used in this study all exhibited potent anticancer activities, revealing their beneficial health effects.
Table 3. IC50 values of MTT assay of 15 Pu-erh teas.
Comparison of the contents of major active compounds of various aged Pu-erh teas
Previous reports showed that levels of active compounds in Pu-erh tea are affected by raw materials with varied cultivation environments, different manufactures, storage conditions, and so on, leading to the differences of partly beneficial health effects. The contents of total polyphenols, total flavonoids, total catechins, free amino acid, total soluble saccharides, purine alkaloids, and GA in the 15 aged Pu-erh teas with long-tern storage were determined ().
Table 4. The contents of the total polyphenols, total flavonoids, total catechins, free amino acid, total soluble saccharides, purine alkaloids, and gallic acid.
The brick tea B5 (45-year storage) was found to have the highest total polyphenol content (64.33 ± 3.98 mg/g), followed by the cake tea P3 (75-year storage, 63.91 ± 3.22 mg/g), compared with the total content of polyphenol of 17.34–58.92 mg/g in other 13 tea tested samples. The contents of total catechin in the 15 aged Pu-erh teas were 0.27–1.94 mg/g, which are significantly lower than that of 38.69 ± 16.51 mg/g in aged pu-erh teas (6-years storage) reported by Zhang et al.[Citation40] The highest total catechins content (1.94 mg/g) was found in the bow l tea T1, whereas the lowest level (0.27 mg/g) was observed in brick tea B4 with the similar storage times (75-years storage). Low content of total catechins in present study is observed because tea catechins could be oxidized into orthoquinones, which could be condensed to further form theaflavins, thearubigins or others compounds during their long-term preservation process.[Citation40]
The bowl tea T3 (27-year storage) had the highest GA content with 10.12 mg/g, which was 78 times higher than that of brick tea B4 (75-year storage, 0.13 mg/g). The GA contents in other 13 Pu-erh teas were 0.14–5.81 mg/g. All the GA levels of 15 aged Pu-erh teas are lower than the one (18.27–68.25 mg/g) of 6 aged Pu-erh teas reported by Qian et al [Citation10]. The total flavonoid, free amino acid, purine alkaloid and soluble saccharide content in the 15 Pu-erh tea samples were 8.99–14.26, 3.42–4.15, 18.69–30.29, and 6.16–29.04 mg/g, respectively.
15 aged Pu-erh teas were divided in to four storage stages (10–30, 40–60, 70–90, >100 years), and the average contents of important chemical components were summarized in . The average contents of total polyphenols, total flavonoids, GA, and total catechins in aged Pu-erh tea at different storage stages showed no significant difference (p > 0.05). The results indicated that the youngest Pu-erh tea did not possess the highest level of active components.
Table 5. Average contents of the main active compounds of Pu-erh tea at four different storage stages.
The coefficient of correlation (r) between the IC50 values of radical-scavenging and anticancer activities and the contents of the active components in the aged Pu-erh teas were analyzed (). The results indicated that the total polyphenol and catechin content were significantly correlated with the DPPH radical scavenging activities of all of the samples (p < 0.05). While the total polyphenol and GA content were significantly correlated with the ABTS radical scavenging activities of all of the samples (p < 0.01), only the GA content was correlated with the anti-cancer abilities across all samples (p < 0.05).
Table 6. Correlation coefficients between antioxidant and anticancer activities and the main active compound contents.
Conclusion
Generally, the older the Pu-erh tea, the higher commercial value it possesses. Our results indicated that the anti-oxidant and anti-cancer activities of the 15 aged Pu-erh teas with different storage years displayed minor differences. Not all the older Pu-erh teas exhibit stronger biological ability than the younger ones and the oldest Pu-erh tea did not have the lowest content of active components. The value of Pu-erh tea is affect by the taste, odor, beneficial component amounts, and others. Thus, to assess the value of Pu-erh tea preserved at different times based on our research results is not enough. Forty-three active components were identified in the 15 Pu-erh tea samples by the HPLC-ESI-QTOF-MS/MS method, including 7 flavan-3-ols, 11 organic acids and esters, 3 proanthocyanidin dimers, 2 benzotropolones, and 20 flavonoid glycosides. Importantly, our results demonstrated that: (1) both total polyphenol and catechin content were significantly correlated with the DPPH radical scavenging activities; (2) the total polyphenol and GA content were significantly correlated with the ABTS radical scavenging activities; and (3) only GA content was correlated with anti-cancer abilities. Anyway, this study could provide useful information of chemical constituents and bioactivity of aged Pu-erh teas with different storage years for consumers to understand the value of aged Pu-erh teas deeply.
Acknowledgments
The authors would like to thank Ji-Duan Qiu at Xiamen Kemingyuan Tea Shop for supplying the Pu-erh tea samples.
Funding
The authors are grateful for financial support from the Special Fund of the Fujian Key Science and Technology Special Projects—Key Agricultural Science and Technology Special Project (No. 2015NZ0003) and the Fujian Special Fund for Scientific Research Institutes in the Public Interest (No. 2016R1017-3).
Additional information
Funding
References
- Duh, P.D.; Yen, G.C.; Yen, W.J.; Wang, B.S.; Chang, L.W. Effects of Pu-Erh Tea on Oxidative Damage and Nitric Oxide Scavenging. Journal of Agriculture and Food Chemistry 2004, 52, 8169–8176.
- Fan, J.P.; Fan, C.; Dong, W.M.; Gao, B.; Yuan, W.; Gong, J.S. Free Radical Scavenging and Anti-Oxidative Activities of an Ethanol-Soluble Pigment Extract Prepared from Fermented Zijuan Pu-Erh Tea. Food and Chemical Toxicology 2013, 59, 527–533.
- Zhao, X.; Qian, Y.; Zhou, Y.L.; Wang, R.; Li, G.J. Pu-Erh Tea Has in Vitro Anticancer Activity in TCA8113 Cells and Preventive Effects on Buccal Mucosa Cancer in U14 Cells Injected Mice in Vivo. Nutrition and Cancer 2014, 66, 1059–1069.
- Wu, C.W.; Yen, C.Y.; Wang, B.S; Chiu, C.K.; Yen, W.J.; Chang, L.W.; Duh, P.D. Antimutagenic and Antimicrobial Activities of Pu-Erh Tea. LWT–Food Science and Technology 2007, 40, 506–512.
- Kuo, K.L.; Weng, M.S.; Chiang, C.T.; Tsai, Y.J.; Lin-Shiau, S.Y.; Lin, J.K. Comparative Studies on the Hypolipidemic and Growth Suppressive Effects of Oolong, Black, Pu-Erh, and Green Tea Leaves in Rats. Journal of Agriculture and Food Chemistry 2005, 53, 480–489.
- Yamashita, Y.; Wang, L.; Wang, L.; Tanaka, Y.; Zhang, T.; Ashida, H. Oolong, Black and Pu-Erh Tea Suppresses Adiposity in Mice Via Activation of AMP-Activated Protein Kinase. Food & Function 2014, 5, 2420–2429.
- Braud, L.; Peyre, L.; de Sousa, G.; Armand, M.; Rahmani, R.; Maixent, J.M. Effect of Brewing Duration on the Antioxidant and Hepatoprotective Abilities of Tea Phenolic and Alkaloid Compounds in a t-BHP Oxidative Stress-Induced Rat Hepatocyte Model. Molecules 2015, 20, 14985–15002.
- Xie, G.X.; Ye, M.; Wang, Y.G.; Ni, Y.; Su, M.G.; Huang, H.; Qiu, M.; Zhao, A.; Zheng, X.J.; Chen, T.; Jia, W. Characterization of Pu-Erh Tea Using Chemical and Metabolic Profiling Approaches. Journal of Agriculture and Food Chemistry 2009, 57, 3046–3054.
- Shao, W.F.; Clifford, M.N.; Powell, C.A. Preliminary Study on the Differences Between the Black Tea and Pu-Erh Tea by HPLC. Journal of Yunnan Agriculture University 1995, 10, 285–291.
- Qian, Z.M.; Guan, J.; Yang, F.Q.; Li, S.P. Identification and Quantification of Free Radical Scavengers in Pu-Erh Tea by HPLC-DAD-MS Coupled Online with 2,2′-Azinobis(3-ethylbenzthiazolinesulfonic acid) Diammonium Salt Assay. Journal of Agriculture and Food Chemistry 2008, 56, 11187–11191.
- Chen, Y.S.; Liu, B.L.; Chang, Y.N. Bioactivities and Sensory Evaluation of Pu-Erh Teas Made from Three Tea Leaves in an Improved Pile Fermentation Process. Journal of Bioscience and Bioengineering 2009, 109, 557–563.
- Wang, Q.; Gong, J.; CHisti, Y.; Sirisansaneeyakul, S. Bioconversion of Tea Polyphenols to Bioactive Theabrownins by Aspergillus Fumigatus. Biotechnology Letters 2014, 36, 2515–2522.
- Ahmad, R.S.; Butt, M.S.; Huma, N.; Sultan, M.T.; Arshad, M.U.; Mushtaq, Z.; Saeed, F. Quantitative and Qualitative Portrait of Green Tea Catechins (GTC) Through HPLC. International Journal of Food Properties 2014, 17, 1626–1636.
- Gaulejac, N.S.C.; Provost, C.; Vivas, N. Comparative Study of Polyphenol Scavenging Activities Assessed by Different Methods. Journal of Agriculture and Food Chemistry 1998, 47, 425–431.
- Antolovich, M.; Prenzler, P.D.; Patsalides, E.; McDonald, S.; Robards, K. Methods for Testing Antioxidant Activity. Analyst 2002, 127, 183–192.
- Van de Loosdrecht, A.A.; Beelen, R.H.J.; Ossenkoppele, G.J.; Broekhoven, M.G.; Langenhuijsen, M.M.A.C. A Tetrazolium-Based Colorimetric MTT Assay to Quantitate Human Monocyte Mediated Cytotoxicity Against Leukemic Cells from Cell Lines and Patients with Acute Myeloid Leukemia. Journal of Immunological Methods 1994, 174, 311–320.
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Polyphenolic with Phosphomolybdic-Phosphotungstic Acid Reagents. American Journal of Ecology and Viticulture 1965, 16, 144–158.
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the Total Phenolic, Flavonoid and Proline Contents in Burkina Fasan Honey, as Well as Their Radical Scavenging Activity. Food Chemistry 2005, 91, 571–577.
- Liang, Y.R.; Wu, Y.; Lu, J.L.; Zhang, L.Y. Application of Chemical Composition and Infusion Color Difference Analysis to Quality Estimation of Jasmine-Scented Tea. International Journal of Food Science and Technology 2007, 42, 459–468.
- Zhang, H.M.; Wang, C.F.; Shen, S.M.; Wang, G.L.; Liu, P.; Liu, Z.M.; Wang, Y.Y.; Du, S.S.; Liu, Z.L.; Deng, Z.W. Antioxidant Phenolic Compounds from Pu-Erh Tea. Molecules 2012, 17, 14037–14045.
- Lu, C.H.; Hwang, L.S. Polyphenol Contents of Pu-Erh Teas and Their Abilities to Inhibit Cholesterol Biosynthesis in Hep G2 Cell Line. Food Chemistry 2008, 111, 67–71.
- Huang, Y.Y.; Liu, C.; Xiao, X.D. Quality Characteristics of a Pickled Tea Processed by Submerged Fermentation. International Journal of Food Properties 2016, 19, 1194–1206.
- Wang, D.M.; Lu, J.L.; Miao, A.Q.; Xie, Z.Y.; Yang, D.P. HPLC-DAD-ESI-MS/MS Analysis of Polyphenols and Purine Alkaloids in Leaves of 22 Tea Cultivars in China. Journal of Food Composition and Analysis 2008, 21, 361−369.
- Lin, L.Z.; Chen, P.; Harnly, J.M. New Phenolic Components and Chromatographic Profiles of Green and Fermented Teas. Journal of Agriculture and Food Chemistry 2008, 56, 8130–8140.
- van der Hooft, J.J.J.; Akermi, M.; Ünlü, F.Y.; Mihaleva, V.; Roldan, V.G.; Bino, R.J.; de Vos, R.C.H.; J. Vervoort, Structural Annotation and Elucidation of Conjugated Phenolic Compounds in Black, Green, and White Tea Extracts. Journal of Agriculture and Food Chemistry 2012, 60, 8841–8850.
- Wojakowska, A.; Perkowski, J.; Góral, T.; Stobiecki, M. Structural Characterization of Flavonoid Glycosides from Leaves of Wheat (Triticum Aestivum L.) Using LC/MS/MS Profiling of the Target Compounds. Journal of Mass Spectrometry 2013, 48, 329–339.
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical Scheme for LC-MSn Identification Of Chlorogenic Acids. Journal of Agriculture and Food Chemistry 2003, 51, 2900–2911.
- O’Coinceanainn, M.; Astill, C.; Baderschneider, B. Coordination of Aluminium with Purpurogallin and Theaflavin Digallate. Journal of Inorganic Biochemistry 2003, 96, 463–468.
- Toshiaki, A.; Ikunori, K.; Danji, F. Antioxidative Properties of Procyanidins B-1 and B-3 from Azuki Beans in Aqueous Systems. Agricultural and Biological Chemistry 1988, 52, 2717–2722.
- Park, J.S.; Rho, H.S.; Kim, D.H.; Chang, I.S. Enzymatic Prepation of Kaempferol from Green Tea Seed and Its Antioxidant Activity. Journal of Agriculture and Food Chemistry 2006, 54, 2951–2956.
- Finger, A.; Engelhardt, U.H.; Victor. W. Flavonol Glycosides in Tea Kaempferol and Quercetin Rhammodiglucosides. Journal of the Science of Food and Agriculture 2006, 55, 313–321.
- Lee, V.S.Y.; Dou, J.P.; Chen, R.J.Y.; Lin, R.S.; Lee, M.R.; Tzen, J.T. Massive Accumulation of Gallic Acid and Unique Occurrence of Myricetin, Quercetin, and Kaempferol in Preparing Old Oolong Tea. Journal of Agriculture and Food Chemistry 2008, 56, 7950–7956.
- Jiang, H.Y.; Engelhardt, U.H.; Thrane, C.; Maiwald, B.; Stark, J. Determination of Flavonol Glycosides in Green Tea, Oolong Tea and Black Tea by UHPLC Compared to HPLC. Food Chemistry 2015, 183, 30–35.
- Suzuki, T.; Honda, Y.; Funatsuki, W.; Nakatsuka, K. Purification and Characterization of Flavonol 3-Glucosidase, and Its Activity During Ripening in Tartary Buckwheat Seeds. Plant Science 2002, 163, 417–423.
- Singab, A.N.; Youssef, D.T.; Noaman, E.; Kotb, S. Hepatoprodective Effect of Flavonol Glycosides Rich Fraction from Egyptian Vicia Calcarala Desf, Against CCI4-Induced Liver Damage in Rats. Archives of Pharmacal Research 2005, 28, 791–798.
- Lu, C.H.; Hwang, L.S. Polyphenol Contents of Pu-Erh Teas and Their Abilities to Inhibit Cholesterol Biosynthesis in Hep G2 Cell Line. Food Chemistry 2008, 111, 67–71.
- Kinjo, J.; Nagao, T.; Tanaka, T.; Nonaka, G.; Okawa, M.; Nohara, T.; Okabe, H. Activity-Guided Fractionation of Green Tea Extract with Antiproliferative Activity Against Human Stomach Cancer Cells. Biological & Pharmaceutical Bulletin 2003, 25, 1238–1240.
- Cerdá, C.; Sánchez, C.; Climent, B.; Vázquez, A.; Iradi, A.; El Amrani, F.; Bediaga, A.; Sáez, G.T. Oxidative Stress and DNA Damage in Obesity-Related Tumorigenesis. Advanced in Experimental Medicine and Biology 2014, 824, 5–17.
- Jie, G.L.; He, P.M.; Ding, R.F. Abecedarian Study on the Antioxidant Activity of Pu-Erh Tea. Cha Ye 2005, 31, 162–165.
- Zhang, L.; Li, N.; Ma, Z.Z.; Tu, P.F. Comparison of the Chemical Constituents of Aged Pu-Erh Tea, Ripened Pu-Erh Tea, and Other Teas Using HPLC- DAD-ESI-MSn. Journal of Agriculture and Food Chemistry 2011, 59, 8754–8760.