ABSTRACT
The dry root tuber of Stephania epigaea contained 36.5% starch, indicating a good starch source. In this study, starch was isolated from S. epigaea. Its morphology, physicochemical, and functional properties were investigated and compared with potato and maize starches. S. epigaea starch had small spherical granules with centric hila and large ellipsoidal granules with eccentric hila, and granule sizes varied from 7 to 40 μm. The starch had 33.9% amylose content and B-type crystallinity. The gelatinization onset, peak, and final temperatures were 59.4, 62.3, and 66.2°C, respectively, and were lower than those of potato and maize starches, but the enthalpy (16.3 J/g) was higher than that of potato and maize starches. The peak, hot, final, and breakdown viscosities were 2227, 1623, 2149, and 594 dPa s, respectively, and were significantly higher than those of maize starch and lower than those of potato starch. S. epigaea starch was more susceptible to amylase hydrolysis and in vitro digestion than potato starch and less than maize starch. This study would be useful for the applications of starch from S. epigaea in the food and non-food industries.
Introduction
Starch is the most abundant natural, renewable, and biodegradable polysaccharide in plants. It can be found in plant seeds, fruits, roots, or tubers. Starches from different sources are varied in their morphological, structural and functional properties.[Citation1–Citation8] These differences result in different applications in food and non-food industries. However, most commercially available starches are isolated from cereal grains (rice, maize, and wheat) and some plant tubers and roots (potato, cassava, and yam). With the development of food and non-food industries, more and more attentions have been paid on finding new starches with different properties.[Citation1]
Stephania epigaea Lo, belonging to the family Menispermaceae, is an herbaceous liana mainly growing in Yunnan and Sichuan provinces, China. Its root tubers have been utilized as folk medicine for sedation and treating fever by local people.[Citation9] Previous researches are mainly focused on the isolation, purification and structure identification of some small-molecule active ingredients from root tubers.[Citation10–Citation12] To our knowledge, there is no published data on root tuber starch from S. epigaea.
In this study, the new starch was isolated from the root tubers of S. epigaea and its physicochemical properties were investigated and compared with potato and maize starches. Our objective was to characterize the morphology, physicochemical, and functional properties of starch from S. epigaea and provide some information for its utilization in food and non-food industries.
Materials and methods
Plant materials
The fresh root tubers of Stephania epigaea Lo were collected from Baidi Village, Sanba Town, Xianggelila County, Yunnan Province, China, in August, 2015, and were identified by Yu Zhang, Kunming Institute of Botany, Chinese Academy of Sciences. The tubers of potato were bought from a local natural food market (Yangzhou City, China). Normal maize starch (S4126) was purchased from Sigma-Aldrich.
Measurement of starch and soluble sugar contents
The fresh root tubers of S. epigaea were washed, peeled, and sliced into small pieces. The pieces were dried at 105°C, extensively ground, and then passed through a 100-mesh sieve to obtain the flour. The starch and soluble sugar contents in the flour were determined as described previously.[Citation13]
Starch isolation
The fresh root tubers of S. epigaea and tubers of potato were washed, peeled, and sliced into small pieces. The pieces were homogenized in water using a home blender, squeezed through five layers of cotton cloth, and then filtered with 100-, 200-, and 300-mesh sieves, successively. The starch precipitation was obtained through centrifuging at 5000 × g for 10 min. The dirty gel-like layer on top of the packed white starch was carefully scraped off and discarded. The starch was washed five times with distilled water and two times with anhydrous ethanol. Finally, the starch was dried at 40°C, ground into powder, and passed through a 100-mesh sieve.
Morphology observation of starch
For light microscope observation, the starch suspension in 50% glycerol was observed with an Olympus BX53 polarized light microscope under normal and polarized light. For electron microscope observation, the starch suspension in anhydrous ethanol was applied to an aluminum stub, and the sample was dried and coated with gold before viewing with an environmental scanning electron microscope (ESEM; Philips XL-30).
Granule size analysis of starch
The size of starch granule was analyzed with a laser diffraction instrument (Mastersizer 2000, Malvern). The starch was suspended in distilled water and stirred at 2000 rpm. The obscuration in the measurement was approximately 12%.
Measurements of iodine absorption spectrum, iodine blue value, and amylose content of starch
The starch-iodine absorption spectrum was performed as described previously.[Citation14] The λmax was the wavelength at which the absorbance was the highest, the iodine blue value was the absorbance at 680 nm, and the apparent amylose content was evaluated from absorbance at 620 nm by reference to a standard curve prepared with amylopectin from corn (Sigma-Aldrich 10120) and amylose from potato (Sigma-Aldrich A0512).
Crystalline structure analysis of starch
The dry starch powder was moistened in a desiccator, where a saturated solution of NaCl maintained a constant humidity atmosphere for 1 week at 25°C. The sample was exposed to the X-ray beam at 40 mA and 40 kV, and scanned from 3 to 40° 2θ with a step size of 0.02° using an X-ray powder diffractometer (XRD; D8, Bruker).
Short-range ordered structure analysis of starch
The starch was analyzed using a Varian 7000 Fourier transform infrared (FTIR) spectrometer with a DTGS detector equipped with an attenuated total reflectance (ATR) single reflectance cell containing a germanium crystal (45° incidence-angle; PIKE Technologies). The 64 scans with a 4 cm−1 resolution were coadded before Fourier transformation. The spectrum was corrected by a baseline in the region from 1200 to 800 cm−1 before deconvolution was applied using Resolutions Pro. The assumed line shape was Lorentzian with a half-width of 19 cm−1 and a resolution enhancement factor of 1.9. The IR absorbance values at 1045, 1022, and 955 cm−1 were extracted from the deconvoluted spectrum.
Swelling power and water solubility determination of starch
The swelling power and water solubility of starch were determined by heating starch–water slurries (2%, w/v) at temperatures ranging from 50 to 95°C at 5°C intervals following the modified procedures of Lin et al.[Citation15] according to the method of Konik-Rose et al.[Citation16]
Thermal properties analysis of starch
The thermal properties of starch were investigated with a differential scanning calorimetry (DSC; 200-F3, NETZSCH). Briefly, 3 mg of starch was precisely weighted and mixed with 9 μL of distilled water. The mixture was sealed in an aluminum pan and equilibrated for 2 h at room temperature. The sample was then heated from room temperature to 130°C at a rate of 10°C/min.
Pasting properties analysis of starch
The pasting properties of starch were evaluated with a rapid visco analyzer (RVA; RVA-3D, Newport Scientific). Briefly, 2 g of starch was dispersed in 25 mL of distilled water and subjected to gelatinization analysis. The temperature program was set as follows: holding at 50°C for 1 min, heating to 95°C at 12°C/min, maintaining at 95°C for 2.5 min, cooling to 50°C at 12°C/min, and holding at 50°C for 1.4 min.
Enzyme hydrolysis analysis of starch
Starch was hydrolyzed by porcine pancreatic α-amylase (PPA) or Aspergillus niger amyloglucosidase (AAG) alone following the previous method of Huang et al.[Citation17] The first-order kinetics was used to investigate the hydrolysis kinetics, and the linear-least-squares fitting of decay plot was performed as described previously[Citation18] using the first-order rate equation of:
where t is the hydrolysis time, C is the fraction of hydrolyzed starch at hydrolysis time t, and K is the hydrolysis rate constant.
In vitro digestion analysis of starch
Native starch was digested in vitro by both PPA and AAG following the method of Carciofi et al.[Citation19] with some modifications. Briefly, 10 mg of starch was digested in 2 mL of enzyme solution (20 mM sodium phosphate buffer, pH 6.0, 6.7 mM NaCl, 0.01% NaN3, 2.5 mM CaCl2, 4 U PPA [Sigma A3176], 4 U AAG [Megazyme E-AMGDF]) in a thermomixer at 37°C with continuous shaking (1000 rpm). The digestion was terminated by adding 240 μL of 0.1 M HCl and 2 mL of 50% ethanol and centrifuged (14,000 × g, 5 min). The glucose content in the supernatant was measured using the D-Glucose assay kit (Megazyme, K-GLUC), and then transformed to the digested starch. The total starch content in starch was determined using the total starch assay kit (Megazyme, K-TSTA). The rapidly digestible starch (RDS) and slowly digestible starch (SDS) was the percentage of the digested starch within 20 min and between 20 and 120 min to the total starch, respectively. The resistant starch (RS) was the percentage of the undigested starch within 120 min to the total starch.
Statistical analysis
The XRD and ATR-FTIR analyses were replicated twice, and the other experiments were replicated thrice. The mean values and standard deviation values were reported. The one-way analysis of variance (ANOVA) by Tukey’s test was evaluated using the SPSS 16.0 Statistical Software Program.
Results and discussion
Starch and soluble sugar contents of root tuber
The S. epigaea dry root tuber contained 36.54% starch and 14.82% soluble sugar. This starch content was within the range reported for most tuber starches.[Citation20] The high starch content showed that S. epigaea was a good resource for starch.
Morphology and size distribution of starch
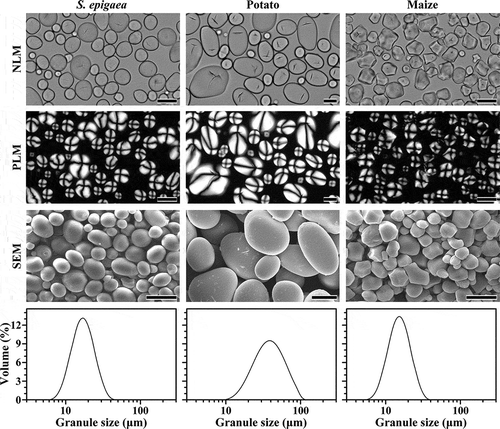
The morphology of starch is presented in . S. epigaea starch had small spherical granules with centric hila and large ellipsoidal granules with eccentric hila. The morphology was similar to that of potato starch, but significantly different from that of maize starch. Maize starch had polygonal granules with centric hila. The starches from S. epigaea, potato and maize all exhibited smooth surface with no evidence of cracks and pores. Starch granule size was analyzed via laser diffraction instrument. They all showed unimodal size distribution, and size ranged from 7 to 40 μm for S. epigaea starch, from 10 to 100 μm for potato starch, and from 6 to 30 μm for maize starch. The mean diameter of starch granules is listed in . The granule size of S. epigaea starch was markedly smaller than that of potato starch, but larger than that of maize starch, which was in agreement with the morphology observation. The granule morphology and size have significant effects on functional properties of starch, such as thermal, pasting, and hydrolysis properties. The differences in starch morphology, hilum position, and granule size may be attributed to the biological origin, biochemistry of the amyloplast, and physiology of the plant.[Citation21]
Table 1. Granule sizes and iodine absorption parameters of starches.
Iodine absorption spectrum and amylose content of starch
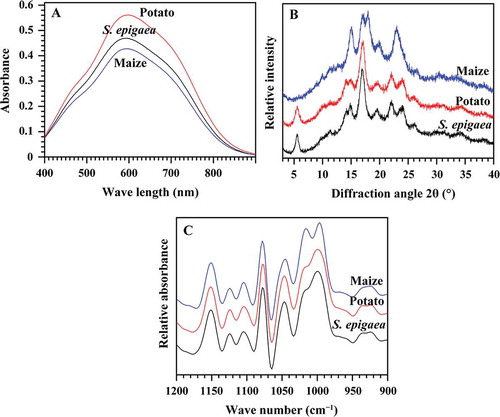
The iodine absorption spectra of starches are presented in , and their maximum absorption wavelength, iodine blue value, and apparent amylose content are summarized in . The S. epigaea starch had 33.9% apparent amylose content, which was higher than maize starch (30.8%) and lower than potato starch (42.6%). The amylose content was within the range reported for most tuber starches.[Citation20]
Crystalline structure of starch
The crystalline structure of starch is often investigated using XRD pattern, which contains A-, B-, and C-type crystallinity. Usually, cereal starches have an A-type crystallinity, most tuber starches exhibit a B-type crystallinity, and legume and rhizome starches present a C-type crystallinity.[Citation22–Citation24] The XRD spectra of S. epigaea, potato, and maize starches are shown in . The S. epigaea and potato starches showed characteristic peaks at 5.6°, 15°, 17°, 20°, 22° and 23° of 2θ, indicating that they had B-type crystallinity, while maize starch exhibited A-type crystallinity with strong reflection at about 15° and 23° 2θ, and an unresolved doublet at 17° and 18° 2θ. The A-, B-, and C-type crystallinity all has been reported in tuber and root starches.[Citation20]
Short-range ordered structure of starch
The ATR-FTIR spectrum of starch is sensitive to the short-range ordered structure, defined as the double-helical order, in the external region of granule. The intensity of absorbance at 1045, 1022, and 995 cm−1 is sensitive to changes in starch conformation. The bands at 1045 and 1022 cm−1 are associated with ordered/crystalline and amorphous regions in starch, respectively. The ratio of absorbance 1045/1022 cm−1 is used to quantify the ordered degree, and that of 1022/995 cm−1 can be used as a measure of the proportion of amorphous to ordered carbohydrate structure in the starch.[Citation25] The ATR-FTIR spectra of three starches from S. epigaea, potato, and maize starches in the 1200−900 cm−1 region are shown in , and the ratios of 1045/1022 and 1022/995 cm−1 of starches are shown in . The results showed that potato and maize starches had the highest and lowest short-range ordered degree. Usually, B-type starch has higher IR ratio of 1045/1022 and lower IR ratio of 1022/995 cm−1 than A-type starch,[Citation25] which is in agreement with the present result.
Table 2. IR ratios and thermal parameters of starches.
Swelling power and water solubility of starch
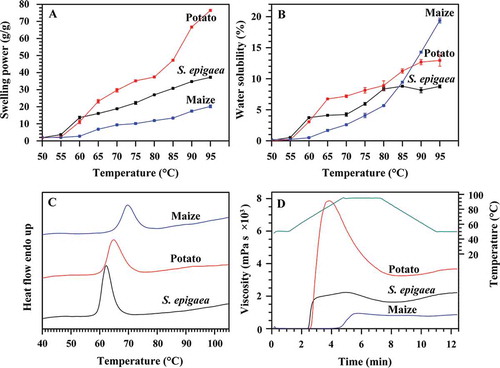
The swelling powers and water solubilities of starches were determined from 50 to 95°C at 5°C intervals ( and ). Swelling power and water solubility gradually increased with increasing temperature after 60°C for S. epigaea and potato starches and after 65°C for maize starch. The starch molecules start to integrate with water as the temperature increases, then the amylose and amylopectin are dissociated in suspension, and the solubility of starch is increased. The insoluble starch granules start to swell because of the hydration.[Citation26] Potato starch swelled quickly from 80 to 95°C, and maize starch dissolved quickly from 80 to 95°C. During heating, the swelling power in S. epigaea starch was higher than in maize starch and lower than in potato starch. Among three starches, S. epigaea starch had intermediate water solubility between 65 to 80°C but exhibited the lowest water solubility after 90°C. The swelling power and water solubility provide measures of the magnitude of interaction between starch chains within the amorphous and crystalline domains. The extent of this interaction is influenced by amylose content, amylopectin fine structure, granule size, crystalline structure, and protein and lipid content.[Citation27–Citation30] The difference in morphological structures of granules may also be responsible for the difference in swelling power and water solubility of starch.[Citation31] In the present study, the different swelling powers and water solubilities of three starches during heating might result from the different morphology, granule size, molecular structure, crystallinity, and botanical source.
Thermal properties of starch
The thermal properties of three starches were determined by DSC, and their DSC thermograms and parameters are given in and . Among three starches, S. epigaea starch had the lowest gelatinization temperature and the highest enthalpy, maize starch had the highest gelatinization temperature and the lowest enthalpy. Usually, A-type starch has higher gelatinization temperature than B-type starch.[Citation32] In the present study, the differences in thermal properties of three starches might be attributed to the differences in starch morphology and size, amylose content, crystalline structure, and the internal arrangement of starch fractions within the granule.[Citation33]
Pasting properties of starch
The pasting properties of starch dispersions are presented in and , and were significantly different among S. epigaea, potato, and maize starches. Potato and maize starch had the highest and lowest peak, hot, breakdown, final viscosity, respectively. The setback viscosity was the lowest in maize starch and the highest in S. epigaea starch. Pasting properties are influenced by the granule size, amylopectin fine structure, the rigidity of starch granules that affect the granule swelling potential, the amount of amylose leaching out in the solution, and the interactions between starch and other components present in the starch suspension during heating.[Citation34,Citation35] The increase in viscosity during the heating cycle is influenced by the granular swelling and the extent of friction between swollen granules. The starches with larger granules may occupy more volume and thus enhance viscosity. The high breakdown viscosity suggests the sample has undergone a higher degree of swelling and subsequent disintegration.[Citation35,Citation36] In the present study, the very high peak viscosity of potato starch might be attributed to their large granule size and high swelling power, the very high breakdown viscosity was due to susceptibility of the highly swollen granules to shear.
Table 3. Pasting properties of starches.
Enzyme hydrolysis properties of starch
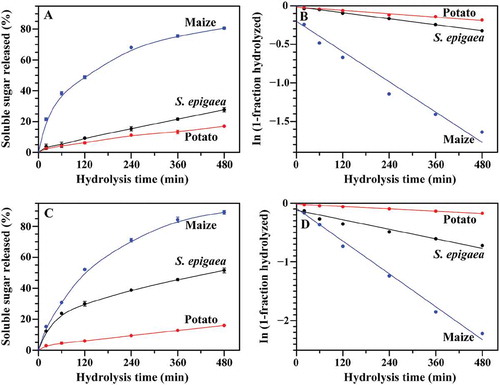
The hydrolysis of three starches by PPA or AAG is shown in and . The hydrolysis kinetics was fitted by the first-order rate equation: C = 1‒e‒Kt,[Citation18] and is shown in , , and . The R-values (>0.963) of the fitting to hydrolysis kinetics indicated that the hydrolysis of three starches from 0 to 480 min well followed the first-order behavior. The kinetics of enzymatic hydrolysis showed that the resistance of starch to PPA and AAG hydrolysis in S. epigaea was higher than in maize and lower than in potato. When starch granules are hydrolyzed by PPA, PPA pits the granule surface first, then penetrates into the interior and hydrolyzes the granule from the inside out. However, AAG hydrolyzes starch from the outer surface of the granule.[Citation37] The hydrolysis of starch by PPA and AAG is influenced by starch size, amylose content, crystalline structure, short-range ordered degree.[Citation17,Citation37,Citation38] Compared with maize starch, S. epigaea starch had large granule size, high amylose content, high short-range ordered degree, and B-type crystallinity, resulting in that it had higher resistance to PPA and AAG hydrolysis than maize starch. But compared with potato starch, S. epigaea starch had small granule size, low amylose content, and low short-range ordered degree, thus leading to that it had lower resistance to PPA and AAG hydrolysis than potato starch.
Table 4. The first-order kinetics to PPA and AAG hydrolysis and in vitro digestion properties of starches.
In vitro digestion properties of starch
The in vitro digestion properties of three starches are shown in . S. epigaea starch had higher RDS and SDS and lower RS than potato starch, but lower RDS and SDS and higher RS than maize starch. The differences in the in vitro digestibility among different starches have been attributed to the interplay of many factors such as starch source, morphology, granule size, amylose content, and crystalline structure.[Citation38] Usually, the size and amylose content are negatively correlative with the digestion, and A-type crystallinity is more easily digested than B-type crystallinity.[Citation17,Citation37] The lower short-range ordered structure in the external region of starch granule makes starch to be easily degraded.[Citation25] In the present study, maize starch had the smallest granule size, the lowest amylose content and short-range ordered degree, and A-type crystallinity among three starches, therefore, it was digested the fastest. Though both S. epigaea and potato starches are B-type crystallinity, S. epigaea starch had significantly smaller granule size, lower amylose content and short-range ordered degree, thus leading to that it was digested faster than potato starch.
Conclusion
The new starch was isolated from root tubers of S. epigaea. The starch had small spherical granules with centric hila and large ellipsoidal granules with eccentric hila. The morphology was similar to that of potato starch, but the granule size with unimodal size distribution was significantly smaller than that of potato starch. S. epigaea starch had 33.9% amylose content and exhibited a B-type crystallinity. Compared with potato and maize starches, S. epigaea starch showed the lowest gelatinization temperature, the highest gelatinization enthalpy, and intermediate swelling power, pasting properties, enzymatic hydrolysis, and in vitro digestion rate. These different functional properties might result from the different granule size, amylose content, crystalline structure, and short-range ordered degree. This study indicated that S. epigaea starch could be used to produce the RS enriched food in the food industry.
Funding
This study was financially supported by grants from the National Natural Science Foundation of China (31570324), the National S & T Basic Work Program of China (2012FY110300), the Qing Lan Project of Jiangsu Province, the Talent Project of Yangzhou University, and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Additional information
Funding
References
- Cisneros, F.H.; Zevillanos, R.; Cisneros-Zevallos, L. Characterization of Starch from Two Ecotypes of Andean Achira Roots (Canna Edulis). Journal of Agricultural and Food Chemistry 2009, 57, 7363–7368.
- Jaiswal, P.; Kumar, K.J. Physicochemical Properties and Release Characteristics of Starches from Seeds of Indian Shahi Litchi. International Journal of Biological Macromolecules 2015, 79, 256–261.
- Nasrin, T.A.A.; Noomhorm, A.; Anal, A.K. Physico-Chemical Characterization of Culled Plantain Pulp Starch, Peel Starch, and Flour. International Journal of Food Properties 2015, 18, 165–177.
- Cai, J.; Cai, C.; Man, J.; Xu, B.; Wei, C. Physicochemical Properties of Ginkgo Kernal Starch. International Journal of Food Properties 2015, 18, 380–391.
- Xia, X.J.; Li, G.N.; Liao, F.R.; Zhang, F.S.; Zheng, J.; Kan, J.Q. Granular Structure and Physicochemical Properties of Starches from Amaranth Grain. International Journal of Food Properties 2015, 18, 1029–1037.
- Valencia, G.A.; Moraes, I.C.F.; Lourenco, R.V.; Bittante, A.M.Q.B.; Sobral, P.J.D. Physicochemical Properties of Maranta (Maranta Arundinacea L.) Starch. International Journal of Food Properties 2015, 18, 1990–2001.
- Florence, S.P.; Urooj, A. Isolation and Characterization of Starch from Pearl Millet (Pennisetum Typhoidium) Flours. International Journal of Food Properties 2015, 18, 2675–2687.
- Zhao, L.; Huang, J.; Man, J.; Huai, H.; Chen, Y.; Wei, C. Physicochemical Properties of Euryale Ferox Kernel Starches from Two Different Regions. International Journal of Food Properties 2016, 19, 289–299.
- The Editorial Board of Flora of China. Flora of China, Vol. 30(1). Science Press: Beijing, China, 1996; 53 pp.
- Dong, J.W.; Cai, L.; Fang, Y.S.; Xiao, H.; Li, Z.J.; Ding, Z.T. Proaporphine and Aporphine Alkaloids with Acetylcholinesterase Inhibitory Activity from Stephania Epigaea. Fitoterapia 2015, 104, 102–107.
- Lv, J.J.; Xu, M.; Wang, D.; Zhu, H.T.; Rang, C.R.; Wang, Y.F.; Li, Y.; Zhang, Y.J. Cytotoxic Bisbenzylisoquinoline Alkaloids from Stephania Epigaea. Journal of Natural Products 2013, 76, 926–932.
- Ma, Y.M. Research Progress on Chemical Constituents of Stephania Plants. Journal of Northwest Forestry University 2004, 19(3), 125–130.
- Gao, H.; Cai, J.; Han, W.; Huai, H.; Chen, Y.; Wei, C. Comparison of Starches Isolated from Three Different Trapa Species. Food Hydrocolloids 2014, 37, 174–180.
- Man, J.; Lin, L.; Wang, Z.; Wang, Y.; Liu, Q.; Wei, C. Different Structures of Heterogeneous Starch Granules from High-Amylose Rice. Journal of Agricultural and Food Chemistry 2014, 62, 11254–11263.
- Lin, L.; Cai, C.; Gilbert, R.G.; Li, E.; Wang, J.; Wei, C. Relationships Between Amylopectin Molecular Structures and Functional Properties of Different-Sized Fractions of Normal and High-Amylose Maize Starches. Food Hydrocolloids 2016, 52, 359–368.
- Konik-Rose, C.M.; Moss, R.; Rahman, S.; Appels, R.; Stoddard, F.; McMaster, G. Evaluation of the 40 mg Swelling Test for Measuring Starch Functionality. Starch 2001, 53, 14–20.
- Huang, J.; Lin, L.; Wang, J.; Wang, Z.; Liu, Q.; Wei, C. In Vitro Digestion Properties of Heterogeneous Starch Granules from High-Amylose Rice. Food Hydrocolloids 2016, 54, 10–22.
- Zhang, B.; Dhital, S.; Gidley, M.J. Synergistic and Antagonistic Effects of α-Amylase and Amyloglucosidase on Starch Digestion. Biomacromolecules 2013, 14, 1945–1954.
- Carciofi, M.; Blennow, A.; Jensen, S.L.; Shaik, S.S.; Henriksen, A.; Buléon, A.; Holm, P.B.; Hebelstrup, K.H. Concerted Suppression of All Starch Branching Enzyme Genes in Barley Produces Amylose-Only Starch Granules. BMC Plant Biology 2012, 12, 223.
- Hoover, R. Composition, Molecular Structure, and Physicochemical Properties of Tuber and Root Starches: A Review. Carbohydrate Polymers 2001, 45, 253–267.
- Sandhu, K.S.; Singh, N.; Kaur, M. Characteristics of the Different Corn Types and Their Grain Fractions: Physicochemical, Thermal, Morphological and Rheological Properties of Starches. Journal of Food Engineering 2004, 64, 119–127.
- Cheetham, N.W.H.; Tao, L. Variation in Crystalline Type with Amylose Content in Maize Starch Granules: An X-Ray Powder Diffraction Study. Carbohydrate Polymers 1998, 36, 277–284.
- Cai, J.; Cai, C.; Man, J.; Zhou, W.; Wei, C. Structural and Functional Properties of C-Type Starches. Carbohydrate Polymers 2014, 101, 289–300.
- Huang, J.; Zhao, L.; Man, J.; Wang, J.; Zhou, W.; Huai, H.; Wei, C. Comparison of Physicochemical Properties of B-Type Nontraditional Starches from Different Sources. International Journal of Biological Macromolecules 2015, 78, 165−172.
- Sevenou, O.; Hill, S.E.; Farhat, I.A.; Mitchell, J.R. Organisation of the External Region of the Starch Granule as Determined by Infrared Spectroscopy. International Journal of Biological Macromolecules 2002, 31, 79–85.
- Yuan, Y.; Zhang, L.; Dai, Y.; Yu, J. Physicochemical Properties of Starch Obtained from Dioscorea Nipponica Makino Comparison with Other Tuber Starches. Journal of Food Engineering 2007, 82, 436–442.
- Kaur, L.; Singh, J.; McCarthy, O.J.; Singh, H. Physico-Chemical, Rheological and Structural Properties of Fractionated Potato Starches. Journal of Food Engineering 2007, 82, 383–394.
- Debet, M.R.; Gidley, M.J. Three Chasses of Starch Granule Swelling: Influence of Surface Proteins and Lipids. Carbohydrate Polymers 2006, 64, 452−465.
- Qi, X.; Tester, R.F.; Snape, C.E.; Ansell, R. Molecular Basis of the Gelatinisation and Swelling Characteristics of Waxy Rice Starches Grown in the Same Location During the Same Season. Journal of Cereal Science 2003, 37, 363–376.
- Srichuwong, S.; Sunarti, T.C.; Mishima, T.; Isono, N., Hisamatsu, M. Starches from Different Botanical Sources II: Contribution of Starch Structure to Swelling and Pasting Properties. Carbohydrate Polymers 2005, 62, 25–34.
- Singh, J.; Singh, N. Studies on the Morphological, Thermal and Rheological Properties of Starch Separated from Some Indian Potato Cultivars. Food Chemistry 2001, 75, 67–77.
- Bogracheva, T.Y.; Morris, V.J.; Ring, S.G.; Hedley, C.L. The Granular Structure of C-Type Pea Starch and Its Role in Gelatinization. Biopolymers 1998, 45, 323–332.
- Ma, X.; Chang, P.R.; Zheng, P.; Yu, J.; Ma, X. Characterization of New Starches Separated from Several Traditional Chinese Medicines. Carbohydrate Polymers 2010, 82, 148–152.
- Correa, Z.; Zúñiga, A.; Garfias, C.; Bello-Pérez, L.A. Isolation and Characterization of Alstroemeria Hookeri ssp. Hookeri Starch in Comparison with Potato Starch. Starch 2013, 65, 991–998.
- Zhou, H.; Wang, J.; Fang, X.; Sun, Y.; Dou, X. Physicochemical Properties of New Starches Isolated from Dioscorea Opposita Thunb. Bulbils. Starch 2012, 64, 290–296.
- Srichuwong, S.; Sunarti, T.C.; Mishima, T.; Isono, N.; Hisamatsu, M. Starches from Different Botanical Sources II: Contribution of Starch Structure to Swelling and Pasting Properties. Carbohydrate Polymers 2005, 62, 25–34.
- Li, J.H.; Vasanthan, T.; Hoover, R.; Rossnagel, B.G. Starch from Hull-Less Barley: V. in-Vitro Susceptibility of Waxy, Normal, and High-Amylose Starches Towards Hydrolysis by Alpha-Amylases and Amyloglucosidase. Food Chemistry 2004, 84, 621–632.
- Ambigaipalan, P.; Hoover, R.; Donner, E.; Liu, Q.; Jaiswal, S.; Chibbar, R.; Nantanga, K.K.M.; Seetharaman, K. Structure of Faba Bean, Black Bean and Pinto Bean Starches at Different Levels of Granule Organization and Their Physicochemical Properties. Food Research International 2011, 44, 2962–2974.