ABSTRACT
The effect of brewing process on 5-hydroxymethylfurfural, 2-furylmethylketone, and 2-furoic acid levels of traditionally prepared and instant Turkish coffee samples containing different amounts of table sugar (0, 2, 4, or 8 g in 20 mL of coffee) were analyzed by high pressure liquid chromatography with diode array detector. The highest change at the levels of furfurals was observed in sample of traditional and instant Turkish coffee named T4 and S4 both of containing 8 g of sugar, respectively. The results showed that 5-hydroxymethylfurfural, 2-furylmethylketone, and 2-furoic acid concentrations in both traditionally prepared and instant Turkish coffee samples increased with increasing sugar concentration. The brewing method and sugar concentration had a significant effect on furfural contents of Turkish coffee (p < 0.05). Daily intakes of furfurals for Turkish population were calculated as 8.14–13.54 and 9.36–10.25 µg kg−1 body weight for traditionally prepared and instant Turkish coffee samples, respectively, and daily intakes of furfurals were lower than the acceptable daily intake value of 0.5 mg kg−1 body weight.
Introduction
Thermal processes like cooking, frying, toasting, sterilization, and roasting are used in the food industry for different purposes, such as prolonged shelf life and better quality for the final product.[Citation1] The roasting stage used in coffee production plays a major role in palatability of the final products. During the coffee roasting process, which results in pleasant aroma characteristics, some reactions occur affecting the organoleptical properties of the coffee because of the heat treatment.[Citation2] One of these reactions is the Maillard Reaction (MR) that occurs between the amino groups of amino acids and the carbonyl groups of reducing sugars.[Citation3] The MR is responsible for aroma and color improvement of foods with heat application.[Citation4] The formation of some substances is desired for flavor and aroma development and color improvement during this reaction. For example, melanoidins are responsible for the color of some foods like coffee, malt, cacao, and bread.[Citation5] During the MR, there are not only desirable substances but also some undesirable substances that can be formed. Undesired compounds are responsible for the end product quality as a quality factor and these substances including furans, which occurrs during the roasting process applied to green coffee beans in different time and temperature conditions.[Citation6] Some undesirable furfurals are also formed both from Amadori products forming throughout the MR or lactose isomerization named Lobry De Bruyn-Alberda van Ekenstein transformation (L-A) and subsequent degradation reactions during the thermal process and/or during storage.[Citation7,Citation8] 5-hydroxymethylfurfural (HMF), 2-furylmethylketone (FMC), and 2-furoic acid (FA) are known as the major furfurals. HMF is naturally formed by direct dehydration of sugars under acidic conditions (caramelization) and formed during the MR.[Citation9] FMC is a compound formed as a by-product of the reaction’s most advanced states or forming by inter-conversion as a result of higher temperatures or longer storage periods. Another substance, FA is created by oxidation of furfurals.[Citation10,Citation11] HMF and other substances like furanic aldehydes and acids (FMC, FA, fumaric acids, and furaic acids) exhibit some genotoxic, cytotoxic, and carcinogenic effects in the liver, skin, and colon/rectum.[Citation12,Citation13]
Coffee brewing methods are important for calculating exposure due to differences in the social and cultural habits of different countries. There are common different brewing procedures for coffee preparation such as filter, plunger, mocha, and espresso coffee brew.[Citation14,Citation15] Preparation methods depends on different factors as filtering, boiling, or ground coffee:water ratio.[Citation10,Citation16]
Turkish coffee is one of the traditional coffee drinks in Turkey and it has some differences like special brewing steps and coffee grounds.[Citation17,Citation18] The best traditional Turkish coffee is made freshly medium roasted coffee beans due to the strong aroma and flavor. The coffee beans are ground to obtain a fine coffee powder by pounding in a mortar or mill. Turkish coffee is prepared with four degrees of sweetness as plain (no sugar), little sugar (half a leveled teaspoon), medium sugar (one leveled teaspoon), and a lot of sugar (one and a half or two leveled teaspoons). The traditional Turkish coffee is prepared with a brewing process consisting of stirring the coffee mix in a special coffee pot called a cezve, removing the spoon from it because the spoon makes much more foam, putting it on a low degree of fire, waiting for it to boil, removing the cezve from the fire, and, finally, to service with special Turkish coffee cups.[Citation18] Technological development led to the introduction of instant Turkish coffee which is just mixing the instant powder with hot water because of its fast and simple preparation.
Although many researches related to furfurals and coffee have been reported,[Citation19–Citation21] there has been no study conducted to determine changes in furfurals during brewing of Turkish coffee. Coffee is the one of the most consumed daily drinks,[Citation22] but studies related to its hazardous contents are very limited. Another point to keep in mind is that the hazardous compounds are formed during not only the roasting process, but also at different brewing processes.[Citation4,Citation23] Therefore, researches on brewing effects for coffee substances should be studied and this investigation aims to determine the effect of the brewing method and sugar concentrations on furfural (HMF, FMC, and FA) levels in traditionally prepared and instant Turkish coffee. Additionally, the daily intake of furfurals from Turkish coffee samples was estimated for the Turkish population.
Materials and methods
Chemicals and reagents
Acetonitrile, glacial acetic acid, zinc acetate dehydrate, and potassium ferrocyanide were purchased from Merck (Darmstadt, Germany). HMF, FMC, FA, and acetic acid were supplied by Sigma-Aldrich (St. Louis, MO, USA). Ultra-pure water was purified with a Milli-Q ultra-pure water system (Millipore, Bedford, MA, USA).
Coffee samples
Traditional and instant Turkish coffee (Coffea Arabica L. cultivated in the region of Campinas SP, Brazil) samples were purchased from local markets. Traditionally, Turkish coffee samples were labeled as without sugar (T1), little sugar (T2: adding 2 g sugar), medium sugar (T3: adding 4 g sugar), and a lot of sugar (T4: adding 8 g sugar). The instant Turkish coffee samples were similarly classified as S1, S2, S3, and S4 according to their sugar content as without, little, medium, and a lot of sugar, respectively. A total of 48 coffee samples (three brands with two replications for all samples) were used for furfural determination.
Brewing of traditional and instant Turkish coffee
Traditionally, Turkish coffee samples (T1, T2, T3, and T4) were prepared using the following procedure: 5 g of coffee was weighed and put into “cezve” (a traditional Turkish coffee kettle). Then, 20 mL of water (one portion of Turkish coffee) and sugar (for coded as T2, T3, and T4 which contain 2, 4, and 8 g of sugar, respectively) were added and the water–coffee mix was heated with slight agitation at 80°C for 3 min using magnetic stirrer with digital thermoregulator (Velp Scientifica, Italy). Then the mix was heated without agitation at 95°C for 3 min and all coffee samples were cooled to room temperature.
For the preparation of instant Turkish coffee samples, 5 g of the instant coffee sample was weighted in a conical flask and 20 mL of boiling water (about 100°C) was incorporated. The mixture was stirred for 2 min with magnetic stir at 400 rpm, and quickly cooled to room temperature (labeled as S1). For preparation of S2, S3, and S4 sample, 5 g of instant coffee samples and 2, 4, and 8 g of sugar, respectively, were weighted and added 20 mL of boiling water. Then they were cooled to room temperature after 2 min of stirring. For the determination of furfural formation during brewing process, we analyzed the coffee samples before the brewing process without heating.
HMF, FMC, and FA extraction
HMF, FMC, and FA extraction procedure was adapted from Oral et al.[Citation24] with some modifications. Nine milliliters of each of the coffee samples before and after the brewing process were taken into a centrifuge tube and incorporated with 0.5 mL of Carrez I (dissolving 21.9 g of crystallized zinc acetate and 3 mL of glacial acetic acid in 100 mL of distilled water) and 0.5 mL of Carrez II (dissolving 10.6 g of potassium hexacyanoferrate [Fe+2] in 100 mL of distilled water). The tubes were shaken using a vortex (Dragon Lab, Mx-S, China) for 1 min and then centrifuged for 7 min at 7000 rpm (Sigma, 3K-30, UK) at room temperature. About 5 mL of supernatant was filtered through with 0.45 µm filters (Macharey-Nagel, PTFE 45/25, Germany) into tubes and diluted five-fold with added ultra-pure water, then injected into high-pressure liquid chromatography (HPLC).
Chromatographic analysis
Chromatographic analysis of HMF, FMC, and FA were carried out using the methods described by Arribas-Lorenzo and Morales[Citation10] and Oral et al.[Citation24] The quantification of HMF, FMC, and FA were simultaneously conducted with a Shimadzu HPLC system (Kyoto, Japan) equipped with a pump (Shimadzu LC-20AT), photodiode array detector (Shimadzu SPD-M20A), column oven (Shimadzu CTO-10AS VP) set 20°C, auto sampler (Shimadzu SIL-10A, Japan), and data station (Shimadzu LC-20AT). The chromatography column was an ACE 5 C18, 5 µm, 250 mm × 4.6 mm (Scotland). Isocratic elution was performed using water:acetonitrile:acetic acid (89:10:1, v:v:v) as the mobile phase at a flow rate of 1 mL/min. Injection volume was assayed as 20 µL. The detection of HMF, FMC, and FA was carried out at the wavelengths of maximum absorption of the compounds to be 284, 274, and 277 nm, respectively
Validation and quantification
HMF, FMC, and FA stock solutions (100 mg L−1) were prepared using pure reagents. Five different concentrations of each calibration solutions (ranged from 1 to 100 mg L−1) were prepared based on diluting the stock solutions. The curves obtained with five standard solutions were linear with R2 values higher than 0.999 for all compounds. Spiked samples (n = 3 replicates) at three concentrations (25, 50, and 100 mg L−1) of standards were used for calculation of average percentage of recovery (94–98%). Relative standard deviation (RSD %) from parallel measurements of standards were used for repeatability of method and found between 2.25–10.04%. The limit of detection (LOD) and limit of quantification (LOQ) were calculated from the standard deviation (s) of responses and the slopes (S) of calibration of furfurals using the formula 3.3s/S and 10s/S, respectively. The determined LOD and LOQ ranges for the study are 0.34–3.17 and 1.03–10.59 mg kg−1, respectively.
Dietary exposure estimates
Intakes were estimated by the average of HMF, FMC, and FA present in the coffee samples. Using average HMF concentration for intake calculations ensures an appropriate and realistic estimation of long-term exposure.[Citation25] Annual consumption data of Turkish coffee by the general population of Turkey was 0.5 kg per person obtained from Turkish Food Industry Report.[Citation26] An average body weight of 70 kg was used to estimate the daily intake of furfural to total population and expressed as mg kg−1 body weight. For the calculation of daily intake, the data obtained after the brewing procedure in traditionally and instant Turkish coffee samples was used. The daily intakes of furfurals were estimated using the following formula:
Statistical analysis
Statistical data analyses were performed with SPSS 21.0 (SPSS Inc., Chicago, IL, USA). Each value is the average of a duplicate analysis on six separate samples. The data were analyzed as a completed randomized design procedure using the general linear model procedure. The model included brewing method and sugar concentration as main effects, and their interactions. The Tukey’s honestly significant difference (HSD) test was performed for the differences among means within the 95% confidence interval.
Results
The HMF, FMC, and FA levels of traditionally prepared and instant Turkish coffee samples before and after brewing are shown in and . Before brewing, the levels of HMF for traditionally and instant Turkish coffee samples were found between 213.02–238.99 and 336.03–362.05 mg kg–1, respectively. After the traditional brewing process, the percent increase of HMF in T1 was 74.12% and the sample coded as T4 showed the highest percent increase (224.75%; p < 0.05). However, the percent change of HMF in instant Turkish coffee samples were ranged from 32.29 to 55.83% and the highest change was determined in the sample coded as S4 ().
Table 1. Percent change of HMF, FMC, and FA in traditionally prepared and instant Turkish coffee samples at the end of the brewing process (%).
Figure 1. Effects of brewing method on HMF, FMC and FA levels of traditionally prepared Turkish coffee samples have different sugar content (n = 6); a–b: means with different letters within brewing process are significantly different (p < 0.05); A-D: means with different letters within sugar contain are significantly different (p < 0.05).
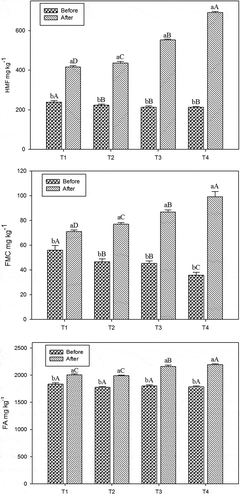
Figure 2. Effects of brewing method on HMF, FMC, and FA levels of instant Turkish coffee samples have different sugar content (n = 6); a–b: means with different letters within brewing process are significantly different (p < 0.05); A–D: means with different letters within sugar contain are significantly different (p < 0.05).
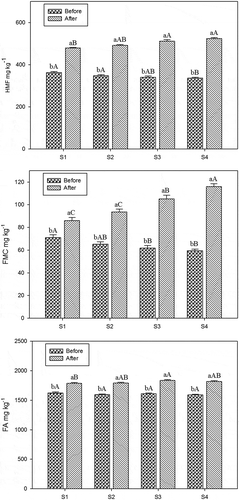
Before brewing procedure, the FMC levels of instant Turkish coffee samples were between 59.61 and 71.13 mg kg–1 while it was found ranging from 35.82 and 55.97 mg kg–1 for traditionally prepared Turkish coffee samples. After the brewing process, the highest percent change (177.20%) of FMC in Turkish coffee samples was observed in the sample coded as T4 with the highest FMC levels (99.28 mg kg−1; p < 0.01; ). Similarly, the highest FMC level was found in S4 sample (p < 0.01; ).
Before the brewing of Turkish coffee, the FA levels of traditionally and instant Turkish coffee samples were varied from 1776.89 to1835.23 mg kg–1 and 1590.42 to 1624.95 mg kg–1, respectively. After the brewing, the FA levels of Turkish coffee samples increased in the ratios varying from 9.22 to 22.78% while the increase in instant coffee ranged from 9.89 to 14.45%. The levels of this substance in brewed and unbrewed coffee samples were statistically different from each other (p < 0.01).
The dietary intake values of HMF, FMC, and FA after brewing method were calculated as 0.57–0.95, 0.1–0.14, and 2.75–3.01 mg/person per day, respectively, for traditional Turkish coffee samples and 0.65–0.72, 0.12–0.16, and 2.44–2.51 mg/person per day, respectively, for instant coffee samples ().
Table 2. Daily intakes of HMF, FMC, and FA in traditionally prepared and instant Turkish coffee samples after brewing process (µg kg−1 bw).
Discussion
Coffee preparation methods can be divided into different categories in terms of roasting and brewing methods. Although there have been several studies on HMF, FMC, and FA content in roasted coffee,[Citation19–Citation21] any researches about the formation of HMF, FMC, and FA during Turkish coffee brewing have not been carried out.
The brewing process caused significant increase in the HMF, FMC, and FA levels of traditionally prepared and instant Turkish coffee samples (p < 0.01; ). Before the brewing process, the HMF and FMC levels in traditionally prepared Turkish coffee samples were lower than in the instant ones. However, the HMF and FMC levels in traditionally prepared Turkish coffee samples were found higher than in instant Turkish coffee samples depending on sugar concentration after the brewing step. Whereas, the FA levels were similar in traditionally prepared and instant Turkish coffee samples after brewing step and the percent changes of the samples were similar depending on the sugar concentration. The furfural levels were correlated positively with increasing temperature (>110°C) and extending time (30 min).[Citation27,Citation28] However, Murkovic and Bornik[Citation29] found that the highest HMF level in roasted coffee was measured after 3 min and then the degradation was rather quick.
HMF is recognized as an indicator compound of quality deterioration in many food products, formed as a result of excessive heating or storage.[Citation20] In the case of coffee, it is formed during roasting process of green beans.[Citation10,Citation20] Murkovic and Bornik[Citation29] reported that HMF could be formed during the roasting process up to 900 mg kg−1. In addition, in this study, increasing the temperature of hot water used to prepare the beverages resulted in significant raises of HMF levels during brewing. However, the HMF formed in coffee was also depended on brewing method in this study. In the case of preparation of traditionally Turkish coffee, preparation method led to HMF formation at higher levels compared with the instant Turkish coffee preparation method. Arribas-Lorenzo and Morales[Citation10] stated that the levels of HMF in espresso and Italian coffee cup were 0.52 and 1.79 mg/cup, respectively. Although Italian coffee is prepared in coffeemakers at 0.5–1 bar, espresso machine works at a pressure of 15 bar.[Citation10] Similarly, Altaki et al.[Citation6] examined that roasting conditions and brewing process affect the furan occurrence of heat-treated foods, such as coffee.
Humans are potentially exposed to HMF through cigarette smoke and consumption of some beverages and foods ash meat and meat products (below 0.9 mg/kg), bread (up to 410 mg/kg), coffee (5–420 mg/L), dried fruits and juices from dried fruits (25–2900 mg/kg), and caramel products (110–9500 mg/kg).[Citation21] Rufian-Henares and De la Cueva[Citation9] and Husøy et al.[Citation30] estimated the daily intake of HMF in the Spanish and Norwegian population, respectively, and there is no study about the daily intake of HMF in Turkey. However, the daily HMF intake from Turkish coffee has being the most important contributing food item with increasing consumption. Husøy et al.[Citation30] reported that coffee is the most important source for daily intake of HMF increasing the contribution up to 63%. The consumption of coffee per person in Turkey was 0.5 kg and the total coffee consumption was slightly increased year by year.[Citation26]
The substances analyzed in traditionally prepared and instant Turkish coffee samples were significantly affected by from the sugar concentration (p < 0.05). At the same time, an interaction between brewing method and sugar concentration was also observed for HMF, FMC, and FA levels for all samples (p < 0.01). The levels of HMF and FMC in T3 and T4 coffee samples were increased nearly 2–2.5 times after the coffee brewing. Arribas-Lorenzo and Morales[Citation10] estimated the levels of HMF as 110 and 1734 mg kg−1 for natural and torrefacto (coffee roasted with sugar addition), respectively. According the hypothesis specified by Antal et al.,[Citation31] as a result of dehydration of hexoses, a necessary fructofuranosylcation intermediate is produced directly by the hydrolysis of sucrose, which reacts to produce HMF in high yields.[Citation21]
The HMF, FMC, and FA intakes for traditionally prepared and instant Turkish coffee samples increased depending on sugar concentration. The coffee sample with a lot of sugar was the most significant coffee sample for HMF, FMC, and FA intake for traditionally prepared and instant Turkish coffee. Arribas-Lorenzo and Morales[Citation10] discussed the influence of different coffee brewing methods (espresso, filtered, Italian, and soluble) on potential HMF intake and they determined the dietary exposure to HMF to torrefacto, natural, blend and instant coffee consumption in the total Spanish population as 0.24, 0.59, 4.84, and 2.98 mg day−1, respectively. Rufian-Henares and De la Cueva[Citation9] reported a dietary intake of 4.9 mg HMF day−1 for the Spanish population. Considering modified Theoretical Added Maximum Daily Intake (mTAMDI), a dietary intake was estimated for HMF and FA as 1.6 mg/person per day in the Scientific Panel on food additives, flavorings, processing aids, and materials in contact with foods (AFC).[Citation32] This value is far below the threshold of concern of 0.54 mg/person per day derived from a large database containing data on subchronic and chronic animal studies.[32.33] The European Food Safety Authority (EFSA) established an Acceptable Daily Intake (ADI) value of 0.5 mg kg−1 bw for furfural and the furfural component of furfural diethylacetal, but no reference was written for HMF or others.[Citation34] Our daily HMF intake values of traditionally prepared and instant Turkish coffee after the brewing process (8.14–13.54 and 9.36–10.25 µg kg−1 bw, respectively) for 70 kg person were much lower than the ADI value. Arribas-Lorenzo and Morales[Citation10] reported that HMF daily intake for torrefacto, natural, blend, and instant coffee samples as 0.65, 5.44, 43.1, and 25.96 µg kg−1 bw, respectively. In this research, although HMF daily intake values were far below the ADI value, the HMF exposure assessment is a quite important issue due to the low security margin obtained from the dietary intake and the tolerable daily intake (TDI).[Citation9]
Conclusion
Traditional Turkish coffee brewing method caused the higher furfural levels in comparison to instant coffee brewing method. Incorporation of sugar into the Turkish coffee increased HMF, FMC, and FA content. Percent increases in HMF, FMC, and FA in traditionally and instant Turkish coffee samples were correlated with sugar content. Consumers should prefer the coffee with lower sugar or without sugar to reduce daily intake of furfural from the coffee. However, in this study, the daily exposure of furfurals for Turkish coffee was found lower than those found in other studies and below the threshold of concern. On the other hand, the coffee consumption of Turkish population has increased in recent years and coffee is known as one of the most important source of HMF. In addition, there is no regulation for limit of HMF and related substance contents in coffee. Considering all these, first, the dietary exposure of HMF and other undesirable substances should be carefully re-evaluated after the investigation of their presence in the different classes of coffee present in the market, and second, mitigation strategies specifically addressed to reduce furfural levels in coffee should be investigated.
References
- Capuano, E.; Fogliano, V. Acrylamide and 5-Hydroxymethylfurfural (HMF): A Review on Metabolism, Toxicity, Occurrence in Food and Mitigation Strategies. LWT-Food Science and Technology 2011, 44, 793–810.
- Belitz, H.D.; Grosch. W. Coffee, Tea and Cocoa. In Food Chemistry; Belitz, H.D.; Grosch, W.; Eds.; Springer-Verlag: Berlin, 1987; 682–693.
- Wang, H.Y.; Qian, H.; Yao, W.R. Melanoidins Produced by the Maillard Reaction: Structure and Biological Activity. Food Chemistry 2011, 128, 573–584.
- Tfouni, S.A.V.; Serrate, C.S.; Leme, F.M.; Camargo, M.C.R.; Teles, C.R.A.; Cipolli, K.M.V.A.B.; Furlani, R.P.Z. Polycyclic Aromatic Hydrocarbons in Coffee Brew: Influence of Roasting and Brewing Procedures in Two Coffea Cultivars. LWT-Food Science and Technology 2013, 50, 526–530.
- Lindenmeier, M.; Faist, V.; Hofmann, T. Structural and Functional Characterization of Pronyl-Lysine, a Novel Protein Modification in Bread Crust Melanoidins Showing in Vitro Antioxidative and Phase I/II Enzyme Modulating Activity. Journal of Agricultural and Food Chemistry 2002, 50, 6997–7006.
- Altaki, M.S.; Santos, F.J.; Galceran, M.T. Occurrence of Furan in Coffee from Spanish Market: Contribution of Brewing and Roasting. Food Chemistry 2011, 126, 1527–1532.
- Chávez-Servín, J.L.; De la Torre Carbot, C.; García-Gasca, T.; Castellote, A.I.; López-Sabater, M.C. Content and Evolution of Potential Furfural Compounds in Commercial Milk-Based Infant Formula Powder after Opening the Packet. Food Chemistry 2015, 166, 486–491.
- Morales, F.J.; Jimenez-Perez, S. Study of Hydroxymethylfurfural Formation from Acid Degradation of the Amadori Product in Milk-Resembling Systems. Journal of Agricultural and Food Chemistry 1998, 46(10), 3885–3890.
- Rufián-Henares, J.A.; De la Cueva, S.P. Assessment of Hydroxymethylfurfural Intake in the Spanish Diet. Food Addit. Contam. Part A, 2008, 25, 1306–1312.
- Arribas-Lorenzo, G.; Morales, F.J. Estimation of Dietary Intake of 5-Hydroxymethylfurfural and Related Substances from Coffee to Spanish Population. Food and Chemical Toxicology 2010, 48, 644–649.
- Van Boekel, M.A.J.S. Effect of Heating on Maillard Reactions in Milk. Food Chemistry 1998, 62(4), 403–414.
- Surh, Y.J.; Liem, A.; Miller, J.A.; Tannenbaum, S.R. 5-Sulfooxymethylfurfural As a Possible Ultimate Mutagenic and Carcinogenic Metabolite of the Maillard Reaction Product, 5-Hydroxymethylfurfural. Carcinogenesis 1994, 15, 2375–2377.
- Nassberger, L. Influence of 5- Hydroxymethylfurfural (5-HMF) on the Overall Metabolism of Human Blood Cells. Human and Experimental Toxicology 1990, 9(4), 211–214.
- Lopez-Galilea, I.; De la Pena, M.P.; Cid, C. Correlation of Selected Constituents with the Total Antioxidant Capacity of Coffee Beverages: Influence of Brewing Procedure. J. Agric. Food Chem 2007, 55, 6110–6117.
- Sanchez-Gonzalez, I.; Jimenez-Escrig, A.; Saura-Calixto, F. In Vitro Antioxidant Activity of Coffees Brewed Using Different Procedures (Italian, Espresso, and Filter). Food Chemistry 2005, 90, 133–139.
- Nkondjock, A. Coffee Consumption and the Risk of Cancer: An Overview. Cancer Letters 2009, 277, 121–125.
- Santini, A.; Ferracane, R.; Mikusová, P.; Eged, S.; Srobárová, A.; Meca, G.; Manes, J.; Ritieni, A. Influence of Different Coffee Drink Preparations on Ochratoxin a Content and Evaluation of the Antioxidant Activity and Caffeine Variations. Food Control 2011, 22, 1240–1245.
- Kucukomurler, S.; Ozgen, L. Coffee and Turkish Coffee Culture. Pakistan Journal of Nutrition 2009, 8(10), 1693–1700.
- Quarta, B.; Anese, M. Furfurals Removal from Roasted Coffee Powder by Vacuum Treatment. Food Chemistry 2012, 130, 610–614.
- Del Campo, G.; Berregi, I.; Caracena, R.; Zuriarrain, J. Quantitative Determination of Caffeine, Formic Acid, Trigonelline and 5-(Hydroxymethyl) Furfural in Instant Coffees by 1H NMR Spectrometry. Talanta 2010, 81(1–2), 367–371.
- Murkovic, M.; Pichler, N. Analysis of 5-Hydroxymethylfurfual in Coffee, Dried Fruits and Urine. Molecular Nutrition and Food Research 2006, 50, 842–846.
- ICO. Historical Coffee Statistics. London: ICO. 2008. http://www.ico.org/historical.asp (accessed date: 16 January 2015).
- Urgert, R.; Katan, M.B. The Cholesterol-Raising Factor from Coffee Beans. J. Roy. Soc. Med 1996, 89, 618–623.
- Oral, R.A.; Mortas, M.; Dogan, M.; Sarioglu, K.; Yazici, F. New Approaches to Determination of HMF. Food Chemistry 2014, 143, 367–370.
- FAO/WHO. Food Consumption and Exposure Assessment of Chemicals. Report of a FAO/WHO Consultation, Geneva, Switzerland, February 10–14, 1997. Geneva (Switzerland): WHO, 1997, 5.
- Republic of Turkey Prime Ministry, Investment Support and Promotion Agency. Turkish Food Industry Report. Republic of Turkey Prime Ministry, Investment Support and Promotion Agency, 2010. 1–22. ( Turkish)
- Kowalski, S.; Lukasiewicz, M.; Duda-Chodak, A.; Zięć, G. 5-Hydroxymethyl-2-Furfural (HMF)–Heat Induced Formation, Occurrence in Food and Biotransformation—A Review. Polish Journal of Food and Nutrition Sciences 2013, 63(4), 207–225.
- Jellum, E.; Børresen, H.C.; Eldjarn, L. The Presence of Furan Derivatives in Patients Receiving Fructose-Containing Solutions Intravenously. Clinica Chimica Acta 1973, 47(2), 191–201.
- Murkovic, M.; Bornik, M.A. Formation of 5-Hydroxymethyl-2-Furfural (HMF) and 5-Hydroxymethyl-2-Furoic Acid During Roasting of Coffee. Molecular Nutrition and Food Research 2007, 51, 390–394.
- Husøy, T.; Haugen, M.; Murkovic, M.; Jöbstl, D.; Stølen, L.H.; Bjellaas, T.; Rønningborg, C.; Glatt, H.; Alexander, J. Dietary Exposure to 5-Hydroxymethylfurfural from Norwegian Food and Correlations with Urine Metabolites of Short-Term Exposure. Food and Chemical Toxicology 2008, 46, 3697–3702.
- Antal, M.J.J.; Mok, W.S. Mechanism of Formation of 5-(Hydroxymethyl)-2-Furaldehyde from D-Fructose and Sucrose. Carbohydrate Research 1990, 199, 91–109.
- EFSA. Scientific Opinion on Flavouring Group Evaluation 13, Revision 2 (FGE.13Rev2): Furfuryl and furan derivatives with and without additional side-chain substituents and heteroatoms from chemical group 14. EFSA Journal, 2011, 9(8), 2313, 1–126.
- EFSA. Toxicological Evaluation of Certain Food Additives. The 44th meeting of the Joint FAO/WHO Expert Committee on Food Additives and contaminants. In WHO Food Additives Series, Vol. 35. Geneva: IFCS, WHO, 1996.
- EFSA. Opinion of the Scientific Panel on Food Additives, flavourings, Processing Aids and Materials in Contact with Food (AFC) on Request from the Commission Related to Flavouring Group Evaluation 13: Furfuryl and Furan Derivatives with and Without Additional Side-Chain Substitutes and Heteroatomes from Chemical Group 14 (Commission Regulation (EC) No. 1565/2000 of 18 July 2000). EFSA J 2005, 215, 1–73.
