ABSTRACT
Bouhezza is an Algerian cheese, which is ripened in a goat-skin bag, called Chekoua. The aim of the study was to determine the protein profiles and aromatics, which contribute to sensory properties of Bouhezza cheese. The chemical composition, proteolysis, and volatile profile have been carried out in cheese made from raw goat milk. The results showed that, the dry matter ranged from 23.07 to 51.95%, the fat-in-dry matter ranged from 10.58 to 31.77%, and the protein ranged from 28.27 to 42.09%. Water-soluble and 12% trichloroacetic acid-soluble nitrogen fractions decreased at the beginning of the ripening process and then they increased until the end of ripening. The reverse phase-high-performance liquid chromatography peptide profiles of the cheese showed the modifications occurred during the ripening process. The volatile compounds showed a diversity of odorous components which contribute to give to the cheese its particular organoleptic. There were 109 compounds identified in Bouhezza cheese. Carboxylic acids, esters, and alcohols were the main classes of the volatile components in the cheese.
Introduction
Traditional cheeses are characterized by a strong link with their origin land and reflect the community history and culture of the product.[Citation1] Various studies showed that the enzyme system of the indigenous microflora in raw milk is much more complex than starter bacteria used in cheese manufacture and, therefore, it has an important influence on cheese proteolysis.[Citation2] Beuvier et al.[Citation3] have demonstrated that the role of the raw milk microflora takes an important place in the biochemical and sensory characteristics of cheese. The cheeses microflora from raw milk showed a more extensive proteolysis and acidification, leading to more pronounced flavor.[Citation3] It has been reported that proteolysis is considered as one of the most important biochemical processes involved in manufacturing of many fermented dairy products.[Citation4] Furthermore, the proteolytic or peptidolytic enzymes of lactic acid bacteria (LAB) contribute to sensory properties of the final milk products.[Citation5] In raw-milk cheeses, a diverse microbiota composed of endogenous LAB,[Citation6] and other bacteria; yeasts and molds, contribute to their distinctive sensory characteristics. In order to maintain the traditional characteristics of cheese varieties, there is a need to preserve the biological diversity involved in the ripening process of goats’ milk cheeses. However, current scientific information on many varieties, some of major economic importance, is still scarce and the research for the better understanding and improving of their manufacture and ripening is needed.[Citation7] The flavor of dairy products, especially cheeses, have been extensively studied; however, cheeses made from raw goat milk have not been studied in detail to the same extent as those from cow milk.[Citation8,Citation9] The more powerful and the most important compounds of the flavor in the cheeses are particularly, the aldehydes, alcohols, acids, and esters.[Citation10]
In Algeria, traditional cheeses are not well known and studied. It has been reported that there are only few traditional dairy products including klila, djben, bouhezza, mechouna, and madeghissa in the East of Algeria (Chaouia region), takammèrite and aoules in the South, and Igounanes in the Middle North (Kabily region).[Citation11] Among these products, Bouhezza is originating from the region of Chaouia located at the Northeastern of Algeria. This product is a traditional cheese which produced from different types of raw milk (cow, sheep, and goat) used alone or mixture of them. In order to characterize this traditional cheese, an investigation was conducted; with families that are living in the territory where exists Bouhezza’s manufacturing practice, in the Chaouia and Aures area in the Northern of Algeria. In general, manufacturing of the cheese is carried out in a goat-skin or sheep-skin (Chekoua) for about 2 or 3 months until achieving the final state of preparing the cheese. The objective of this study was to characterize the chemical composition, proteolysis, and volatile compounds of the Bouhezza cheese during ripening. The specific cheese manufacturing process was studied, as well as the evolution of some parameters, which are proteolysis and aromatic profile. This is the first study on the aromatic profile compounds in the Algerian traditional Bouhezza goats’ milk cheese.
Materials and methods
Cheese making
Bouhezza is a local Algerian traditional cheese produced and consumed in the Chaouia region to the Northeast of the country. It is made with raw milk from cows, goats, or their mixture. Its manufacture requires the specific use of the skin of goat or sheep, treated called “Chekoua” used as a container for drainage and refining. The goat-skin is treated with salt and juniper. Before starting fabrication of Bouhezza, a volume of Lben (2 to 3 L) is introduced into the Chekoua during overnight (12–24 h) to allow at the skin-bag to have a good smell, to adapt the bag to the characteristics of the milk product, remove any skin odor, and eliminate the bad smell. This amount of Lben is removed from the Chekoua. Then the Bouhezza preparation begins widely with a volume of salty Lben between the half or three quarts of the Chekoua.
This quantity undergoes biochemical transformations during 3 to 4 days. Then, a further amount of 3 L of salty Lben is added on the 4th day into the Chekoua, and mixed with the cheese. The addition of Lben continuous for 7 weeks (or more). Finally, 2 weeks before the end of the ripening, raw whole milk is added at 4 d intervals (2 at 3 L per Chekoua) until the end, where the cheese is collected and seasoned with red hot pepper. The traditional diagram of Bouhezza, the preparation of the Chekoua and of Lben, were obtained such as a result of the survey conducted among families in the Chaouia region where the Bouhezza cheese is commonly manufactured.
Goat-skin bag preparation
Goat-skin bags (Chekouates) are prepared according to the following steps: The age of goats were between 6-months- and 1-year-old. Skins obtained are left in a nylon bag for 5 to 6 d at ambient temperature, then they are washed and depilated. After washing them with the tap water, they are dried under the shade, and preserved with salt and juniper (Ârâar) for 4 to 8 d until their uses. The juniper is used for its antiseptic and antifungal properties,[Citation12] and due to having its special deodorant effects. Therefore, it eliminates undesired odors of the skin. The skins are then rinsed and turned out (the side hairs takes the interior face). They are tied up to avoid air entrance and the resultant bag is called “Chekoua” in Algeria. Chekouates are used immediately or conserved for future uses. The Chekouates prepared for this work were used directly; their volume was between 10 to 15 L.
Lben preparation
The Lben used for the manufacturing of Bouhezza was obtained according to the following steps: raw milk (3 L) undergoes the fermentation and coagulation processes during 24 to 36 h at ambient temperature (25–30°C). The coagulated milk named “Rayeb” was churned for 30 to 45 min and some warm water (20–25°C); a level of 0.25 L was added to this mixture. Water is added in Rayeb, to maintain a fresh temperature of 20–25°C, which promotes gathering of butter grains, and so, facilitates the skimming operation. After, a partial skimming, the recovered Lben is employed in the manufacturing of Bouhezza.
Cheese manufacturing
Two cheeses were produced at Boukhadra (Fabrications, F1 and F2) and the third one in Cheria (F3), two localities in the town of Tebessa, in the Northeast of Algeria. The goat’s milk is provided from a small farm located at Boukhedra and from the local city of Cheria. Three liters of salted Lben (20 to 25 g NaCl per L of Lben) were put into each Chekoua (three Chekouates). Successive additions of salted Lben were carried out each 4 d for several weeks (7 weeks). During cheese making (), the Chekoua is placed in a well-ventilated place and is cleaned every day and after each addition of raw material. Cleaning is done by scarping the outsider of Chekoua and rinsing with water to avoid any accumulation of whey and/or soluble phase. The whole raw milk (2 L per each Chekoua) was added after 54 d until the end of the production (72 d) at four intervals. Adding the whole raw goat milk directly into the Chekoua and mixed with curd, contribute to bring the fat, and regulate the final acidity and final salt level of the cheese. At the end of cheese making process, the hot pepper powder was blended with the raw milk, and homogenized with the cheese paste; the quantities are added according to the taste and the food habit of each family. The analysis for Lben was done at the first day and cheese analysis was done in eight intervals.
Chemical analysis
Sampling
Samples were taken at regular intervals of eight days (0, 8, 15, 22, 30, 42, 54, 64, and 72 d) during ripening of Bouhezza to follow any changes in physicochemical parameters. Lben and cheese samples of Bouhezza were analyzed in duplicate for moisture or dry matter (DM) by the oven drying method at 102°C;[Citation13] fat by the Van Gulik method[Citation14] and total nitrogen (TN) by the micro-Kjeldahl method.[Citation15] The pH was measured using a digital pH meter (model Seven Compact S220K, Mettler-Toledo, Greifensee, Switzerland). Titratable acidity of samples was measured according to AOAC,[Citation16] the results were expressed as the percentage of lactic acid. The ash rate was also determined.[Citation17]
Proteolysis
Nitrogen Fractions
Water-soluble nitrogen (WSN) and 12% trichloroacetic acid soluble nitrogen (TCA–SN), as the percentage of the TN, and the total free amino acid (FAA) levels in the WSN fraction of the cheeses were determined by the methods described by Hayaloglu et al.[Citation18]
Urea polyacrylamide gel electrophoresis (urea-PAGE) of caseins
Water-insoluble fractions of the cheeses were freeze-dried and then analyzed by urea-PAGE using a Protean II XI vertical slab gel unit (Bio-Rad Laboratories Ltd, Watford, UK) according to the method of Andrews[Citation19] and the gels were stained directly by the method of Blakesley and Boezi[Citation20] with Coomassie Brilliant Blue G-250. After detaining using the pure water, gel slabs were digitized using a scanner (HP Scan Jet software, Scan Jet G4010, Hewlett Packard, Palo Alto, CA).
Reverse-phase-high-performance liquid chromatography (RP-HPLCs) of peptides
The WSN fraction of the cheeses were freeze-dried and analyzed by a RP-HPLC as described in Sulejmani et al.[Citation21] using a Shimadzu LC 20 AD Prominence HPLC system (Shimadzu Corporation, Kyoto, Japan). A Phenomenex Jupiter C18 column 250 × 4.6 mm × 5 μm, 300 Å pore size (Phenomenex Co, Torrance, CA, USA) was used. The solvents were as follows: (A) 0.1% (v⁄v) trifloroacetic acid (TFA, sequencing grade; Sigma-Aldrich Laborchemikalien GmbH, Seelze, Germany) in deionized HPLC-grade water (Milli–Q system; Waters Corp., Molsheim, France) and (B) 0.1% (v⁄v) TFA in acetonitrile (HPLC grade; Merck KGaA, Darmstadt, Germany) at a flow rate of 0.75 mL⁄min. A 10 mg WSN fraction was dissolved in solvent A (10 mg/mL), filtered through a 0.45 μm cellulose acetate filter (Sartorius GmbH, Gottingen, Germany) and an aliquot (60 μL) of filtrate was injected into the column. The samples were eluted initially with 100% solvent A for 5 min, then with a gradient from 0 to 50% solvent B over 55 min, maintained at 50% solvent B for 6 min, followed by a linear gradient from 50 to 60% solvent B over 4 min and finally with 60% solvent B for 3 min. The elute was monitored at 214 nm.
Volatile analysis
Lben as a raw material (d 0) and cheese samples (from 30 to 72 d of ripening in the goat-skin bag) and one sample farm of cow’s milk cheese (>30 d ripened) were sliced into small granules and placed immediately in glass bottles in a freezer at −20°C. Volatiles were determined by solid-phase microextraction (SPME) method using gas chromatography-mass spectrometry system (GC-MS; Shimadzu Corporation, Kyoto, Japan), as described in Sulejmani et al.[Citation21] The results were calculated by the comparison of the peak area of the internal standard and the unknown compounds. The standards used for confirmation in the GC analysis were: Benzyl alcohol, 1-hexanol, 2-octanol, 2-heptanol, 2-ethyl-1-butanol, 1-pentanol, pentanoic acid, nonanoic acid, phenyl acetaldehyde, isopropy butanoate, methyl octanoate, 2-pentanone, propyl butanoate, 2-methyl butyl acetate, 2-heptanone, 1-octanol, 2,5-dimethyl pyrazine, isovaleric acid, hexanoic acid, octanoic acid, methyl hexanoate, proyl hexanoate, acetaldeyde, ethanol, 2-butanone, nonanol, 2-heptanone, 2-butanol, 1-hexanal, 2-nonanone, ethyl acetate, and octyl acetate, etc. Each compound was expressed in a microgram per L of Lben and per kg of cheese.
Statistical analysis
The analysis of variance (ANOVA) was performed and a multiple comparison test was used to compare the means in chemical composition of the three-cheese making. The relationship between the chemical parameters were examined by correlations with the different age of ripening. The least significant difference of the data were reported. The level of significance of differences between treatments was determined (p < 0.05, p < 0.01, and p < 0.001). The logicial Costat 6.400, CoHort Software 1998–2008 was used for ANOVA analysis. The relationship between variables in volatiles was assessed by the principal component analysis (PCA). The statistical treatment was performed using the XLSTAT version 2009.1.02 (Copyright Addinsoft 1995–2009)[Citation22] for Windows for the PCA volatiles analysis.
Results and discussions
Chemical composition
Physico-chemical results are given in . The pH of Lben was 4.70 ± 0.15; this result was similar with those of cow’s milk (4.84) found by Aissaoui Zitoun et al.[Citation11] and slightly higher than the results of the Lben that is obtained from the fermented traditional cow’s milk (4.45).[Citation23] The dry matter was 13.5 ± 2.49%, higher than those of Aissaoui Zitoun et al.[Citation11] (9.06 ± 1.2%) and Samet-Bali et al.[Citation23] (7.05%).
Table 1. Chemical composition (in %) and pH of traditional Algerian Bouhezza goat’milk cheese (n = 3).
A variation of the mean pH value of the three cheese making trials was observed during ripening. Its value ranged from 3.64 to 4.44 (). The pH decreased to 3.64 and increased until 4.24 at the end of the ripening process (d 53 and 72, respectively). This result was in accordance with those obtained by Aissaoui Zitoun et al.,[Citation11] slightly different with the soft goat cheese which had pH values 4.12 and 4.27,[Citation8] and lower than in Gokceada goat cheese (pH 4.71)[Citation9] and Darfiyeh (pH 4.87 to 5.10).[Citation24] The titratable acidity increased from 0.99% for Lben to 1.15% for cheese at the 72 d. This is probably due to the continuous activity of LAB which are brought with raw materials (Lben and the whole raw milk). The same trend and also with a high final acidity was observed by Aissaoui Zitoun et al.[Citation11] in Bouhezza cow’s milk (from 0.84 to 3.08 g/100 g). The level of dry matter increased from 13.50 % (Lben) to 51.95% at the end of ripening of cheese (72 d). The level of ash in dry matter decreased from 7.63% (Lben) to 6.03% (72 d; level of ash increased from 1.03% in Lben to 3.13 % in the ripened cheese at 72 d. The results of dry matter were higher than those of referred by Aissaoui Zitoun et al.,[Citation11] when the level in Bouhezza cheese from cow’s milk ripened for 10 weeks, ranged from 9.06% (in Lben) to 35.86% (at the 10th week). The fat in DM increased from 14.81% in Lben to 28.87% in the 72 d-old cheese (final phase in the ripening). The addition of Lben every 4 d during 7 weeks and the whole raw milk, from the 54th d until the end of cheese ripening, contribute at this evolution. This is probably due to the draining of whey (soluble phase), where minerals are lost through the pores of Chekoua which generates the increase of the cheese’s DM and decreasing ash in DM. Aissaoui Zitoun et al.[Citation11] have reported the same allegations. At 72 d of the ripening, Bouhezza goat’s milk is classified, according to FAO,[Citation25] with moisture in non-fat substance (MNFS) and fat-in-DM (FDM) about 67.55% and 28.87% respectively, as soft and mid-fat cheese. This result is similar as those reported by Aissaoui Zitoun et al.,[Citation11] for 10-week-old Bouhezza. The ANOVA results during ripening of Bouhezza cheese showed that there is a very strongly significant effect of the ripening time factor, on evolution of measured parameters; such as pH; acidity/DM, and DM (p < 0.001). Unlike the rate of ash and protein in the dry matter where, there is no significant effect of maturation time factor in their evolution (p > 0.05). Between the three manufacturing the acidity in the dry matter and ash content in the dry matter, showed very significant differences (p < 0.001), between the two manufacturing F1 and F2 with F3. This is because both F1 and F2 fabrications was carried out simultaneously with the same raw materials and under the same conditions. Conversely, F3 was produced with other raw materials which come from a different place where feed goat’s is different with the fabrications F1 and F2.
Proteolysis
Changes of protein fractions during ripening
The total proteins in DM and the TN increase in all cheeses, reaches an appreciable value in final cheese (72 d), 33.75 ± 0.92% and 2.75 ± 0.11% cheese, respectively. The levels of WSN decreased during 45 d of ripening. At the end of the ripening, there was an increase from 7.89% until 14.44% of TN at 64 and 72 d (), respectively. These results are lower than those of the results presented by Aissaoui Zitoun et al.[Citation11] in final Bouhezza cow’ cheese, in Gokceada goat cheese,[Citation9] and then Darfiyeh.[Citation24] Also, slightly higher than those obtained in Xinotyri goat cheese.[Citation26] The same evolution was observed for TCA-SN, a decrease from the beginning of the production to 45 d. Also, at the end of ripening, there was an increase 10.27% of TN. These results are slightly higher than those of Gokceada goat cheese[Citation9] and Xinotyri goat cheese[Citation26] at the end of ripening, but the same rate was obtained at 45 d in both of cheeses Bouhezza and Xinotyri. The level of this fraction was higher in Darfiyeh at 60 d,[Citation24] than in Bouhezza goat cheese at the end of ripening.
Table 2. Evolution of protein fraction (Mean ± SD); total nitrogen (TN); water-soluble nitrogen (WSN/% of TN); 12% Trichloroacetic acid-soluble nitrogen (TCA-SN/% of TN) and total free amino acids (FAA) during ripening of Algerian traditional Bouhezza goat’s milk cheese (n = 3).
showed that there is an increase of the content in FAAs during the ripening, from 0.24 at 1.10 mg Leu.g−1 Bouhezza cheese (at 8 and the 45 d) and reaches 0.70 and 1.38 mg Leu.g−1 cheese, respectively, at 64 and 72 d. These results are higher than Xinotyri goat cheese after 45 d until the end of ripening,[Citation26] and then in Kashar cheese made with raw goat milk at 90 d of ripening.[Citation27] The mean square of the variance of the measured parameters of the protein fractions showed that the significant effect of ripening time factor on these parameters is different, where the effect on the evolution of TN, is very strongly significant (p < 0.001), the effect on the evolution of amino acids is highly significant (p < 0.01) and finally on the TCA-SN/TN is significant (p < 0.05). No effect was observed on the development of WSN/TN and also between the three different productions during cheese ripening of Bouhezza.
Urea-PAGE of caseins (CN)
showed the urea-PAGE of the water-insoluble fractions of the cheese Bouhezza from goat’s milk. It has been established from highest to lowest molecular weight; γ-CN, β-CN, αs-CN, and Pre-αs-CN.[Citation28,Citation29] These four areas of electrophoretic bands showed that there is a proteolysis in the water-insoluble fraction of cheese. In cheese making of the Algerian traditional Bouhezza made with goat’s milk, neither coagulant nor starter were used to coagulate the milk. The process takes place spontaneously by indigenous proteinase and LAB’s enzymes, consequently the proteolysis and the hydrolysis of β-CN fraction is certainly due to the action of plasmin. The intermediate size of peptides are degraded subsequently by the enzymes from non-starter microflora existing in the cheese and originate from raw milk. The extracellular proteinase of lactococci contribute to the formation of small peptides in cheese by hydrolyzing the larger peptides produced from αs1-CN or from β-CN. While the peptidases (intracellular) are released after lysis of bacterial cells, these are responsible for the degradation of short peptides and the production of FAAs.
Figure 2. Urea-polyacrylamide gel electrophoresis (urea-PAGE) of the water-insoluble nitrogen fractions Lben and 15, 30, 45, 64, and 72 d-old Bouhezza cheese. F1, F2, or F3 refer to fabrications (trials) of Bouhezza cheese.
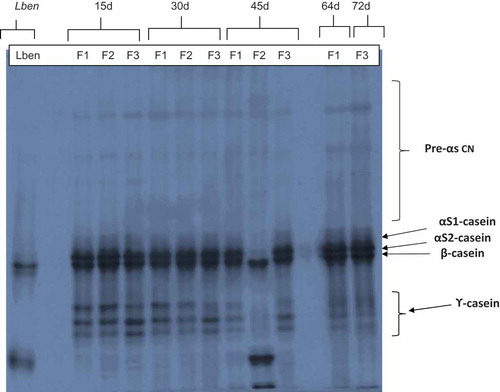
The profile of the urea-PAGE gel shows two main bands with different electrophoretic mobility, which corresponds to native CN; the groups αs-CN and the β-CN. Little bands with lower mobility appear under β-CN fraction, which are released under action of plasmin on this fraction. As reported by Sousa et al.[Citation30] and other previous studies, the cleavage of the plasmin on β-CN be happens at the level of three sites; at Lys28-Lys29, Lys105-His106, and Lys107-Glu108 which gives three peptide fractions as β-CN (f29–209; γ1-CN), β-CN (f106–209; γ2-CN), and β-CN (f108–209; γ3-CN). The same profile was reported by Aissaoui Zitoun et al.[Citation31] in Bouhezza cheese made with cow’s milk. The intensity of these γ-CN bands (peptides of β-CN), appeared with high intensity at 15 and 30 d, and decreased at 45 d until 64 and 72 d, but not completely disappeared. It is probably due to the cleavage of those peptides with the enzymes liberated from cells of the lactic bacteria, also because there is an adding of first matter continuously until the fourth days before recovery of cheese.
Hayaloglu et al.[Citation18] have also found that the cheese without starter had a slightly higher concentration of ϒ-CN because of plasmin activity. The proteolysis involved in the Bouhezza goat’s cheese has two sources. Indigenous proteinases in milk; the plasmin and cathepsine, with proteinases enzymes from the native microflora of the milk. In addition, non-starter lactic bacteria contribute to the production of short peptides and FAAs.[Citation30,Citation32] Aissaoui Zitoun et al.[Citation31] have reported the same allegations, where the native enzymes of the milk and the endogenous proteinases brought by the indigenous bacteria which are the source of proteolysis in Bouhezza made with cow’s milk.
Three bands with different higher electrophoretic mobilities corresponded to the peptides released during proteolysis of αs1-CN, probably by cathepsin D with formation of αs1-CN (24-199) which is attributed to the acid milk proteinase.[Citation33] The three bands appear at the first step of ripening at 15 d and two bands disappearing at 30 and 45 d, probably by a cleavage by peptidases of LAB, and appearing at the end of ripening after adding goat’s raw milk, at 64 and 72 d, where indigenous enzymes plays their role of primary proteolysis. These bands are showed as αs1-CN (24-199), αs1-CN (102-199), and αs1-f (*-*).[Citation31] Also, the plasmin had an action on αs2-CN in solution at the level of eight sites.[Citation30]
RP-HPLCs of water-soluble fractions
indicated the peptide profiles of the WSN fractions of the cheeses. The peptides eluted between 10 and 35 min, were hydrophilic (HI) peptides, whereas the peptides eluted from 35 to 80 min were considered as hydrophobic (HO) peptides. It has been reported that RP-HPLC chromatograms of soluble fractions at pH 4.6, can be divided into three areas; when the FAAs are eluted between 0 to 10 min, HI and HO peptides are then eluted in the previous order.[Citation34–Citation36]
Figure 3. RP-HPLC profile of water-soluble nitrogen fraction of Bouhezza raw goat’s milk cheese during ripening. Eluted at 214 nm (arbitrary units).
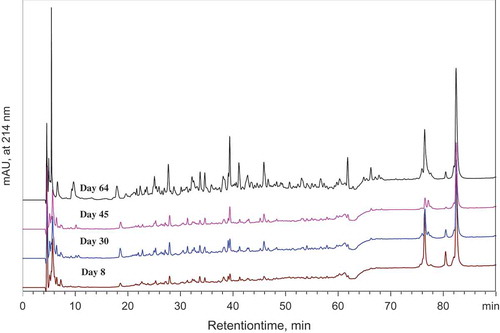
The RP-HPLC chromatogram was rich in some FAAs with the first 15 min for all cheeses productions (F1, F2, and F3). It was appeared that the HI peptides eluted between 15 and 35 min. It was an abundance and diversity of peaks representing the different peptides released by proteolysis and casein degradation. As shown in , urea-PAGE of the cheeses revealed some fractions obtained following the proteolysis of β- and αs-CN giving those bands of peptides, which are liberated in the water-soluble phase. The more interesting is that the intensity of the peaks, which represents the concentration, increases during the ripening time, after 30 d of ripening, there is an increase of the number of peaks, which means that new peptides are generated. At 45 d, there is a profusion of HI peptides, which stills appeared to enrich the Bouhezza. The peptide fractions following proteolysis may, in turn, be metabolized into amino acids, which helps to provide the specific sensory properties to the cheese.
Finally, the third step of the chromatogram shows that this phase from 35 to 80 min emerging from the HO peptides which are important as the HI ones. The new peaks, emerging represent new peptides, which mean that the proteolysis continues to take place in enriching cheese with new molecules. Proteolysis of the CN to a range of small- and intermediate-sized peptides and FAAs probably only contributes to the flavor of most cheese varieties, but FAA are important precursors for a volatile compounds.[Citation33] In Bouhezza cheese, the initial hydrolysis of CN is caused by the plasmin, which results in the formation of large and intermediate-sized peptides, which are degraded subsequently by the enzymes from the non-starter microflora of the cheese. In addition, it is going to be very rich with aromatic substances, which may derive from proteolysis.
Volatile composition
A total of 109 volatile compounds were determined in the different samples analyzed. In Lben, 55 compounds found, and during the manufacture of the Algerian traditional cheese Bouhezza made from raw goat’s milk, 87 volatile compounds at 30 d, 92 compounds at 72 d, and in the sample of cow’s milk Bouhezza cheese, whose age is greater than 30 d, 69 volatile compounds was detected. The relative concentrations of volatile compounds including 31 esters, 14 carboxylic acids, 14 alcohols, 8 ketones, 8 aldehydes, 21 terpenes, and 13 miscellaneous compounds detected in Bouhezza cheese were given in through .
Table 3. Esters (Mean ± SD µg.L−1 for Lben and µg.Kg−1 for cheese) in Lben from goat milk and Bouhezza raw goat’s milk cheese.
Esters
The major esters found in Bouhezza cheese () were ethyl esters (14; ethyl acetate, butanoate, haxanoate, octanoate and decanoate, and 2-phenethyl acetate), which are determined in the Lben with appreciable levels (4.670, 4.65, 14.97, 15.83, 8.12, and 0.90 µg L−1, respectively) and in the Bouhezza cheese. The others were methyl (5), propyl (7), butyl (3), and isoamyl and isopentyl esters (2). Their concentrations increased during the ripening, in particular, after 30 and 72 d. The butanoate (65.95 µg·kg−1), haxanoate (345.87 µg kg−1), octanoate (625.37 µg kg−1), and decanoate ester (594.72 µg kg−1), appeared at the highest levels after 72 d, except for ethyl acetate, when the level was higher (59.53 µg kg−1) at the first step of ripening in the d 30 and it decreases (20.81 µg kg−1). The levels of these compounds in Bouhezza goat’s cheese were higher than those found in Bouhezza cheese of the cow’s milk (age >30 d of the ripening) and, than those reported by Aissaoui Zitoun.[Citation37] Propyl and butyl esters have low levels or they were absent in Lben, at d 30, and they increased in the d 72. Hayaloglu et al.[Citation9] reported that the most important esters encountered in the goat’s cheese were ethyl haxanoate, octanoate, decanoate in Turkish cheeses of the goat’s milk Gokceada and Saanen. Ethyl acetate is an important flavor compound in many cheeses; it is formed from lactose by lactic bacteria; citrate and lactate metabolism, or as a product of the catabolism of amino acids.[Citation33]
Carboxylic acids
The carboxylic acids were found at high concentrations in the Bouhezza cheese of the goat’s milk (), as reported in other works on cheeses made with goat’s milk (raw or pasteurized milk).[Citation8,Citation9,Citation33,Citation38] The acetic (59.9 µg L−1), hexanoic (51.5 µg.L−1), octanoic (111.2 µg L−1), and decanoic (60.1 µg L−1) acids are the most abundant acids in Lben, compared with other carboxylic acids. Butyric acid has a high content (7.8 µg L−1) compared to the rest of other acids but it remains below these four mentioned acids. In the Lben of cow’s milk and the Bouhezza cheese, the acids; butyric, caproic and capric are presented with a highest level.[Citation37] The level of low-chain carboxylic acids (i.e., C2-C10 acids) in goat’s milk Bouhezza cheese increased during ripening. At the d 72, the increase of the levels of short-chain carboxylic acids was very important for all carboxylic acids, ranging from three to over twenty fold than at d 30. The five principal acids including acetic (606.3 µg kg−1), butanoic (778.9 µg kg−1), hexanoic (2,063.7 µg kg−1), octanoic (1,660.3 µg kg−1), and decanoic (1,054.2 µg kg−1) acids are shown in the . Among those acids, the hexanoic was present at the highest level in the final cheese, followed by octanoic, decanoic, butanoic, and acetic acids. Those were the principal acids presented in many goat cheeses made using raw or pasteurized milk.[Citation8,Citation9,Citation38,Citation40] The reason of this increase is probably due to the contribution of fat by the addition of the whole raw milk during the manufacturing of Bouhezza from d 54. Hayaloglu et al.[Citation9] have obtained similar results, and demonstrated that acetic, decanoic, hexanoic, and octanoic acids were the most abundant acids in cheese, especially after d 90 of the ripening. The increase in hexanoic acid, which is formed by lipolysis and contributes to the goat cheese odor, was noticeable in the cheeses from Turkish Gokceada goat’s milk that having higher levels during the ripening.[Citation9] Acetic acid was the most abundant carboxylic acid in a Greek Xinotyri cheese of the goat’s milk.[Citation26]
Table 4. Carboxylic acids (Mean ± SD µg.L−1 for Lben and µg.Kg−1 for cheese) in Lben from goat milk and Bouhezza raw goat’s milk cheese.
In Bouhezza cheese produced with cow milk (age >30 d), the same acids were present with lower levels. The order of these acids is hexanoic followed by acetic, octanoic, butanoic, and decanoic with lower concentrations. In the Bouhezza cheese at d 75, the level of hexanoic acid was the most abundant, followed by octanoic, the lowest level was the decanoic.[Citation37] The content of short-chain FA had a significant impact on the development of the aroma characteristics of Lebanese Darfiyeh cheese; the acids C6-C12 concentrations were significantly higher throughout the ripening.[Citation24] The concentrations of caprylic and capric acids were significantly higher in Teleme cheese made from goats’ milk than in cheeses made from ewes’ milk at the ripening.[Citation41] Butyric acid is also an important component of Greek Feta cheese, which contributes greatly to its flavor and piquant taste.[Citation42] In semi-hard Spanish goat’s cheese, fatty acids (FAs) were the predominant compounds. Decanoic acid was present at the highest concentrations followed by octanoic and hexanoic acids.[Citation43] The short- and medium-chain FAs, contribute directly to cheese flavor, are released into the cheese by lipolysis.[Citation44] They are also precursors of molecules of flavor and aroma compounds (methyl-ketones, lactones, esters, alkanes and the secondary alcohols).[Citation43,Citation45] According to Le Quéré et al.,[Citation39] some FAs including hexanoic, octanoic, nonanoic, decanoic, 3-methylbutanoic, 4-methyloctanoic, and 4-ethyloctanoic acids were identified in a traditional soft goat cheese, and the cheese characterized as goaty aroma.
Alcohols
Fourteen alcohols were determined in the Bouhezza cheese made from goat’s raw milk () during 72 d of ripening. In Lben, the most abundant alcohol was ethanol (12.1 µg L−1), followed by 3-methyl-1-butanol (2.8 µg L−1) and 1-hexanol (1.1 µg L−1). Ethanol was also a principal component in the Tunisian traditional Lben of the cow’s milk.[Citation23] The other compounds were of lower levels; including 2-nonanol, 1-octanol, benzyl alcohol and phenethyl alcohols. showed the variation of ethanol, 3-methyl-butanol, phenethyl-alcohol, 2-nonanol, and 1-octanol at all the stages of the cheese ripening (in Lben, to the end of the ripening at d 72). Also, in Bouhezza cheese made using cow’s milk (after 30 d) except the last two, where the 2-methyl-1-propanol which was present.
Table 5. Alcohols (mean ± SD µg.L−1 for Lben and µg.Kg−1 for cheese) in Lben from goat milk and Bouhezza raw goat’s milk cheese.
Large quantities of ethanol were detected in Majorero cheeses of the goat milk[Citation34] and in Greek Xinotyri goat cheese.[Citation26] Ethanol has a limited aromatic role in cheeses, but it is the precursor of several esters and the conditions in the cheese can promote the rapid reduction of aldehydes and ketones to their primary and secondary alcohols.[Citation38] The concentrations of 3-methyl-1-butanol decreased from d 30 to d 72; they were lower than in the Bouhezza of cow’s milk (age of sample >30 d) and in that reported by Aissaoui Zitoun.[Citation37] at 75 d. The level of 3-methyl-1-butanol was higher in the Teleme cheeses of the goat milk than in a ewe’s milk cheeses as reported by Massouras et al.[Citation46] The phenethyl alcohol increases at d 30, and higher at d 72, its content is lower in the Bouhezza cheese of the cow’s milk (age of sample > 30 d), and it was found by Aissaoui Zitoun.[Citation37] at 75 d. Some compounds appeared at d 30 of ripening and then disappeared, such as 2-butanol, 2.6-dimethyl-4-heptanol, 2,3-butanediol, and 2-furanemethanol. Benzyl alcohol were present in Lben, increased in Bouhezza cheese at d 30 and then disappeared at d 72. The other compounds found are, 4-methyl-2-pentanol (absent in the Lben), 1-hexanol (absent at d 72), 2-nonanol, and 1-octanol (present at 30 and 72 d). The most abundant alcohols were 1-phenylethanol, octanol, 3-methylbutanol and 1-propanol in Darfiyeh cheese which is ripened in the goat-skin bag[Citation24] and alcohols were the main group of the chemicals found in the volatile fraction of Greek Xinotyri, a goat’s cheese.[Citation26] Ethanol, pentan-1-ol and 3-methyl-1-butanol contributed to the aroma of Turkish Gokceada and Saanen goat’s milk cheese.[Citation9]
The sample of Bouhezza made with the goat’s milk was richer with alcoholic aromatic compounds than the Bouhezza from the cow’s milk. Probably, the whole raw milk added at d 54 of manufacture, have an effect on aromatic alcohols profile in the final cheese Bouhezza goat’s milk. Aissaoui Zitoun[Citation37] reported that the profile of alcohols in Bouhezza made with cow’s milk could be due to the addition of the raw milk at the end of the manufacturing process of the cheese or due to the use of the raw milk in the production process.[Citation26]
Aldehydes
Eight aldehydes were identified in Bouhezza cheeses produced with goat’s milk, and six in the cheese produced with cow’s milk (). Hexanal, was the principal one, and nonanal were found in Lben and at all the stages of manufacturing of Bouhezza goat’s cheese. The high concentration was at d 30, for hexanal (from 3.2 decreased to 2.0 µg kg−1 at d 72) and for heptane (from 3.0 µg kg−1 disappeared at 72 d). Hayaloglu et al.[Citation9] reported that aldehydes are not the major volatile compounds of goat milk cheeses; however, hexanal was abundant in all the samples of Gokceada and Turkish Saanen. 3-Methyl-1-butanal, appeared at d 72 of ripening (1.1 µg kg−1) which, provided malt, oil, or aroma butter to cheese[Citation24] and it was main aldehyde in Gokceada and Turkish Saanen cheeses.[Citation9] The aldehyde compounds identified in Darfiyeh cheese ripened in goat-skin including octanal and 3-methyl-1-butanal. The latter was the major aldehyde found and it plays an important role in the flavor’s development because of its low perception threshold.[Citation24] The aroma compounds identified in the Majorero goat’s milk cheese included 3-methyl-1-butanal (which was the major aldehyde), hexanal, and heptanal. Those three compounds would provide malty and green aroma notes.[Citation38]
Table 6. Aldehydes (mean ± SD µg.L−1 for Lben and µg.Kg−1 for cheese) in Lben from goat milk and Bouhezza raw goat’s milk cheese.
Ketones
Eight ketones were detected in the Bouhezza cheese from goat’s milk (). In Lben, there were only four ketones including 2-pentanone, 2-heptanone, 4-octanone, and 2-nonanone and their levels were between 0.2 and 0.8 µg L−1. The same compounds were also identified in Bouhezza cheese during the ripening. Their respective levels were 2.3, 4.2, 0.2, and 5.1 µg kg−1 of cheese at the end of the ripening, respectively. These values are higher than those obtained at d 30, except the 4-octanone. 2-Propanone was found to be a high level (3.6 µg kg−1) at the end of the ripening, and the levels of 3-methyl-2-pentanone and butyrolactone were 2.8 and 1.7 µg kg−1, respectively. Ketones contribute to the aromatic properties of goat’s cheese such as 2-heptanone (mushroom), 2-nonanone (sour), and 3-hydroxy-2-butanone (buttery) with appreciable concentrations.[Citation43] 3-hydroxy-2-butanone was identified in the goat cheese varieties,[Citation39] fresh goat cheese;[Citation8] artisanal fresh goat’s cheese.[Citation40] The methyl-ketones detected in Bouhezza cheese, are probably formed by the degradation of lipids and followed a metabolic pathway of β-oxidation.[Citation45] When the corresponding FAs have been released in cheese,[Citation39] the FAs are oxidized into β-ketoacids[Citation43] and decarboxylated to alkan-2-ones.[Citation33]
Table 7. Ketones (mean ± SD µg.L−1 for Lben and µg.Kg−1 for cheese) in Lben from goat milk and Bouhezza raw goat’s milk cheese.
Terpenes
Twenty terpenes were identified in Bouhezza cheese from goat’s milk and 12 terpenes in Lben (). The volatiles appear or disappear at different stages of ripening. dl-Limonene is the product with the highest content in the Lben reaching 131.7 µg·L−1, followed by β-myrcene with 4.2 µg·L−1, and then α-pinene and β-phellandrene at levels of 2.3 and 0.9 µg·L−1, respectively. Levels of linalool (0.7 µg·L−1) was higher than other compounds (sabinene, delta-3-carene, cymol, para-cymene, alpha-terpinolene, (+)-2-carene and gamma-cadinene) and their levels were lower than 0.5 µg.L−1. The group of terpenes is widely varied in Bouhezza cheese. As the level of β-pinene ranged from 0.5 to 0.9 µg kg−1 at d 72. dl-Limonene decreased to 5.6 µg.kg−1 at d 30 and increases until 45.0 µg kg−1 at d 72 and as well as gamma-terpinene (0.4 to 5.9 µg kg−1 at 30 and 72 d, respectively). Aissaoui Zitoun[Citation37] has reported the presence of d-limonene and α-pinene in Bouhezza cheese from the cow milk at 75 d.
Table 8. Terpenes (mean ± SD µg.L−1 for Lben and µg.Kg−1 for cheese) in Lben from goat milk and Bouhezza raw goat’s milk cheese.
Miscellaneous compounds
Among the identified benzenes (); toluene, 1,4-dimethylbenzene, 1,2-dimethylbenzene are present at low quantities in the Lben and continue to appear during the manufacturing. The high level of toluene at d 72 in Bouhezza cheese was the same as that which has been found by Hayaloglu et al.[Citation9] in goat’s milk Gokceada cheese at d 90 of the ripening. Benzene, ethyl benzene,-1,3,5-trimethylbenzene, and m-chlorotoluene either they are absent in Lben, and appear at d 30 or at the end of ripening. The same compounds (benzene, dimethylbenzene, trimethylbenzene, and toluene) were found in the Camembert cheese.[Citation45] Methyl groups are present at d 72. Dimethyl sulfone was present with a stable level at d 30 to 72. Eight various compounds were present in the Bouhezza cow’s milk (age >30 d); the group of identified benzenes with moderate levels, exception of toluene which is present with a high level.
Table 9. Miscellaneous (mean ± SD µg.L−1 for Lben and µg.Kg−1 for cheese) in Lben from goat milk and Bouhezza raw goat’s milk cheese.
PCA of volatiles
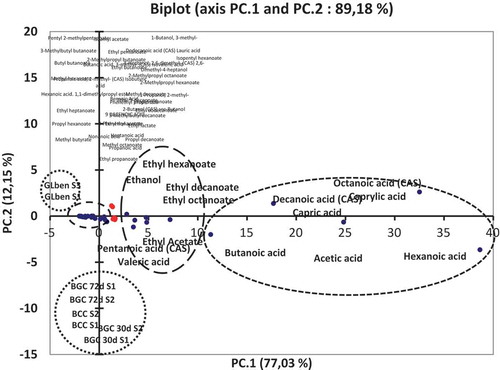
Two principal components are used in most cases of PCA analysis, which is sufficient to explain a great proportion of the variation into the original parameters. A biplot of sample scores is shown in , where the most important loadings and the percentage accounted by the two-first principal components (PC1 and PC2) after analysis. The variability of 77 and 12% were explained respectively by PC1 and PC2. The total variance explained 89% of the GC-MS data. A biplot showed that carboxylic acids contribute strongly as flavoured to the principal components such as octanoic, decanoic, hexanoic, acetic, butanoic and pentanoic acids which are grouped in the positive side of the PC1 axis, they represent the most important acids with high levels in the different samples of Bouhezza cheese goat’s milk (72 d) and in sample made with cow’s milk (>30 d; ). The ethyl esters of octanoate, decanoate, hexanoate, acetate, and ethanol are also grouped in the positive side of the PC1 axis and in the negative side of the PC2 axis except the ethanol who is in the positive side of the PC2, their contribution is important but lesser than the acids. The rest of compounds are grouped together around the center of bi-plot, the most of them are in the negative side of PC1 and on the line zero of the PC2 and then the contribution to the principal component is smaller than the two groups of the acids and their esters. All the samples are grouped in , on the positive side of the PC1 but samples of Lben are grouped on the positive side of the PC2 while the samples of Bouhezza cheese are grouped in the negative side of the PC2 and their contribution is strongly than those of Lben because the high levels of flavored compounds were found in the samples of cheese.
Conclusion
Bouhezza cheese is an Algerian traditional product, which represents many years of tradition from a rural civilization. This first characterization of Bouhezza cheese produce from the goat’s raw milk, allowed its classification as soft and mid-fat cheese, and showed a proteolysis and volatile profile of the cheese. The level of proteolysis is limited, in Bouhezza cheese made with raw goat’s milk. Thus, a lower level of the rate of proteolysis occurs in Bouhezza cheese in comparison to the other cheeses made with raw goat’s milk and/or ripened in goat’s-skin bag. However, it was interesting by the liberation of fractions such as peptides and FAAs in the cheese matrix. The volatile profile was rich in carboxylic acids (released by lipolysis) and their esters, which are precursors of other aromatic compounds as well as the volatile compounds derived from the degradation of proteins. It was determined in this cheese, the essential of volatile compounds (levels are higher at d 72 than at d 30), divided into seven groups that constitute the basis of the flavor and the taste of the obtained cheese from raw milk.
References
- Licitra, G. Worldwide Traditional Cheeses: Banned for Business? Dairy Science & Technology 2010, 90, 357–374.
- Dagdemir, E.; Ozdemir, S. Technological Characterization of the Natural Lactic Acid Bacteria of Artisanal Turkish White Pickled Cheese. International Journal of Dairy Technology 2008, 6, 133–140.
- Beuvier, E.; Berthaud, K.; Cegarra, S.; Dasen, A.; Pochet, S.; Buchin, S.; Duboz, G. Ripening and Quality of Swiss-Type Cheese Made from Raw, Pasteurized or Microfiltered Milk. International Dairy Journal 1997, 7, 311–323.
- Fox, P.F. Proteolysis During Cheese Manufacture and Ripening. Journal of Dairy Science 1989, 72, 1379–1400.
- El-Ghaish, S.; Dalgalarrondo, M.; Choiset, Y.; Sitohy, M.; Ivanova, I.; Haertlé, T.; Chobert, J.-M. Screening of Strains of Lactococci Isolated from Egyptian Dairy Products for Their Proteolytic Activity. Food Chemistry 2010, 120, 758–764.
- Cogan, T.M.; Barbosa, M.; Beuvier, E.; Bianchi-Salvadore, B.; Coconcelli, P.H.; Fernandez, P.S.; Gomez, I.; Kalantzoupoulos, G.; Ledda, A.; Medina, M.; Rea, M.C.; Rodriguez, E. Characterization of the Lactic Acid Bacteria in Artisanal Dairy Products. Journal of Dairy Research 1997, 64, 409–421.
- Medina, M.; Nuñez, M. Cheeses Made from Ewes’ and Goats’ Milk. In Cheese: Chemistry, Physics and Microbiology, Vol. 2 Major Cheese Groups, 3rd Ed; Patrick, F.; Fox, P.; McSweeney, L.H.; Cogan, T.M.; Guinee, T.P.; Eds.; Elsevier Ltd., Elsevier Academic Press: Italy, 2004; 279–299, 469.
- Carunchia Whetstine, M.E.; Karagul-Yuceer, Y.; Avsar, Y.K.; Drake, M.A. Identification and Quantification of Character Aroma Components in Fresh Chevre-Style Goat Cheese. Journal of Food Science 2003, 68, 2441–2447.
- Hayaloglu, A.A.; Tolu, C.; Yasar, K.; Sahingil, D. Volatiles and Sensory Evaluation of Goat Milk Cheese Gokceada As Affected by Goat Breeds (Gokceada And Turkish Saanen) and Starter Culture Systems During Ripening. Journal of Dairy Science 2013, 96, 2765–2780.
- Smit, G.; Smit, B.A.; Engels, W.J.M. Flavour Formation by Lactic Acid Bacteria and Biochemical Flavour Profiling of Cheese Products. FEMS Microbiological Review 2005, 29, 591–610.
- Aissaoui Zitoun, O.; Benatallah, L.; Ghennam, El-H.; Zidoune, M.N. Manufacture and Characteristics of the Traditional Algerian Ripened Bouhezza Cheese. Journal of Food, Agriculture & Environment 2011, 9, 96–100.
- Mazari, K.; Bendimerad, N.; Bekhechi, C.; Fernandez, X. Chemical Composition and Antimicrobial Activity of Essential Oils Isolated from Algerian Juniperus Phoenicea L. and Cupressus Sempervirens L. Journal of Medicinal Plants Research 2010, 4, 959–964.
- International Dairy Federation (IDF). Determination of the Total Solid Content (Cheese and Processed Cheese). IDF Standard 4A. International Dairy Federation: Brussels, Belgium, 1982.
- Ardo, Y.; Polychroniadou, A. Laboratory Manual for Chemical Analysis of Cheese COST 95, Luxembourg. Office for Official Publications of the European Communities: Luxembourg, 1999.
- International Dairy Federation (IDF). Standard Method 20B: Milk. Determination of Nitrogen Content, Part 1 and 2. International Dairy Federation: Brussels, Belgium, 1993.
- AOAC. Official Methods of Analysis. Vol. II, 16th Ed; Association of Official Chemists International: Arlington, VA, 1995.
- AFNOR. Collection of French Standards. Product quality control food: Milk and dairy products. Physicochemical analysis. Afnor-dgccrf. 4th Ed. Paris: La Défense, 1993, 562.
- Hayaloglu, A.A.; Guven, M.; Fox, P.F.; McSweeney, P.L.H. Influence of Starters on Chemical, Biochemical, and Sensory Changes in Turkish White-Brined Cheese During Ripening. Journal of Dairy Science 2005, 88, 3460–3474.
- Andrews, A.T. Proteinases in Normal Bovine Milk and Their Action on the Caseins. Journal of Dairy Research 1983, 50, 45–55.
- Blakesley, R.W.; Boezi, J.A. A New Staining Technique for Proteins in Polyacrylamide Gels Using Comassie Brilliant Blue G250. Analytical Biochemistry 1977, 82, 580–581.
- Sulejmani, E.; Rafajlovska, V.; Guneser, O.; Karagul-Yuceer, Y.; Hayaloglu, A.A. Volatile Compounds and Proteolysis in Traditional Beaten (Bieno Sirenje) Ewe’s Milk Cheese. International Journal of Dairy Technology 2014, 67, 584–593.
- XLSTAT, version 2009.1.02 for Windows. Copyright Addinsoft 1995–2009. https://www.xlstat.com. (accessed on 17 March 2016)
- Samet-Bali, O.; Bellila, A.; Ayadi, M.A.; Marzouk, B.; Attia, H. A Comparison of the Physicochemical, Microbiological and Aromatic Composition of Traditional and Industrial Leben in Tunisia. International Journal of Dairy Technology 2010, 63, 98–104.
- Serhan, M.; Linder, M.; Hosri, C.; Fanni, J. Changes in Proteolysis and Volatile Fraction During Ripening of Darfiyeh, a Lebanese Artisanal Raw Goat’s Milk Cheese. Small Ruminant Research 2010, 90, 75–82.
- FAO. Milk and dairy products. Codex Alimentarius. FAO and WHO, Rome, 2007, 258.
- Bontinis, T.G.; Mallatou, H.; Pappa, E.C.; Massouras, T.; Alichanidis, E. Study of Proteolysis, Lipolysis and Volatile Profile of a Traditional Greek Goat Cheese (Xinotyri) During Ripening. Small Ruminant Research 2012, 105, 193–201.
- Temizkan, R.; Yasar, K.; Hayaloglu, A.A. Changes During Ripening in Chemical Composition, Proteolysis, Volatile Composition and Texture in Kashar Cheese Made Using Raw Bovine, Ovine Or Caprine Milk. International Journal of Food Science and Technology 2014, 49, 2643–2649.
- Tejada, L.; Abellan, A.; Cayuela, J.M.; Martınez-Cacha, A.; Fernandez-Salguero, J. Proteolysis in Goats’ Milk Cheese Made with Calf Rennet and Plant Coagulant. International Dairy Journal 2008, 18, 139–146.
- Pino, A.; Prados, F.; Galán, E.; McSweeney, P.L.H.; Fernández-Salguero, J. Proteolysis During the Ripening of Goats’ Milk Cheese Made with Plant Coagulant Or Calf Rennet. Food Research International 2009, 42, 324–330.
- Sousa, M.J.; Ardo, Y.; McSweeney, P.L.H. Advances in the Study of Proteolysis During Cheese Ripening. International Dairy Journal 2001, 11, 327–345.
- Aissaoui Zitoun, O.; Pediliggieri, C.; Benatallah, L.; Lortal, S.; Licitra, G.; Zidoune, M.N.; Carpino, S. Bouhezza, a Traditional Algerian Raw Milk Cheese, Made and Ripened in Goatskin Bags. Journal of Food, Agriculture & Environment 2012, 10(2), 289–295.
- McSweeney, P.L.H.; Fox, P.F. Chemical Methods for Characterization of Proteolysis in Cheese During Ripening. Lait 1997, 77, 41–76.
- McSweeney, P.L.H.; Sousa, M.J. Biochemical Pathways for the Production of Flavour Compounds in Cheeses During Ripening: A Review. Lait 2000, 80, 293–324.
- De Llano, D.G.; Polo, C.M.; Ramo, M. Study of Proteolysis in Artisanal Cheeses: High Performance Liquid Chromatography of Peptides. Journal of Dairy Science 1995, 78, 1018–1024.
- Laborda, M.A.; Rubiolo, A.C. Proteolysis of Fynbo Cheese Salted with Nacl/Kcl and Ripened at Two Temperatures. Journal of Food Science 1999, 64, 33–36.
- Hayaloglu, A.A.; Guven, B.M.; Fox, C.P.F.; Hannonc, J.A.; McSweeney, P.L.H. Proteolysis in Turkish White Brined Cheese Made with Defined Strains Lactococcus. International Dairy Journal 2004, 14, 599–610.
- Aissaoui Zitoun, O. ép. Hamama. Manufacture and characterization of a traditional Algerian cheese “Bouhezza”. Doctoral Thesis in Science, Specialty: Food Science. Institute of Nutrition, Food and Agro-Food Technology (INATA-A), Constantine University 1-Algeria, 2014, 160.
- Castillo, I.; Calvo, M.V.; Alonso, L.; Juarez, M.; Fontech, J. Changes in Lipolysis and Volatile Fraction of a Goat Cheese Manufactured Employing a Hygienized Rennet Paste and a Defined Strain Starter. Food Chemistry 2007, 100, 590–598.
- Le Quéré, J.-L.; Pierre, A.; Riaublanc, A.; Demaizières, D. Characterization of Aroma Compounds in the Volatile Fraction of Soft Goat Cheese During Ripening. Lait 1998, 78, 279–290.
- Guillen María, D.; Ibargoitia María, L.; Sopelana, P.; Palencia, G. Components Detected by Headspace-Solid Phase Microextraction in Artisanal Fresh Goat’s Cheese Smoked Using Dry Prickly Pear (Opuntia Ficus Indica). Lait 2004, 84, 385–397.
- Mallatou, H.; Pappa, E.; Massouras, T. Changes in Free Fatty Acids During Ripening of Teleme Cheese Made with Ewes’, Goats’, Cows’ Or a Mixture of Ewes’ and Goats’ Milk. International Dairy Journal 2003, 13, 211–219.
- Georgala, A.; Moschopoulou, E.; Aktypis, A.; Massouras, T.; Zoidou, E.; Kandarakisand, I.; Anifantakis, E. Evolution of Lipolysis During the Ripening of Traditional Feta Cheese. Food Chemistry 2005, 93, 73–80.
- Poveda, J.M.; Sanchez-Palomo, E.; Perez-Coello, M.S.; Cabezas, L. Volatile Composition, Olfactometry Profile and Sensory Evaluation of Semi-Hard Spanish Goat Cheeses. Dairy Science and Technology 2008, 88, 355–367.
- Collins, Y.F.; McSweeney, P.L.H.; Wilkinson, M.G. Lipolysis and Free Fatty Acid Catabolism in Cheese: A Review of Current Knowledge. International Dairy Journal 2003, 13, 841–866.
- Molimard, P.; Spinnler, H.E. Review: Compounds Involved in the Flavor of Surface Mold-Ripened Cheeses: Origins and Properties. Dairy Food. Journal of Dairy Science 1996, 79, 169–184.
- Massouras, T.; Pappa, E.C.; Mallatou, H. Headspace Analysis of Volatile Flavour Compounds of Teleme Cheese Made from Sheep and Goat Milk. International Journal of Dairy Technology 2006, 59, 250–256.

![Figure 1. Flow diagram of traditional manufacture of Bouhezza goat’s raw milk cheese (re-arranged by the data from Aissaoui Zitoun et al. (2011) and Aissaoui Zitoun [2014]).](/cms/asset/16ef7c37-9fc8-4974-9773-d7b4371e9e61/ljfp_a_1222588_f0001_b.gif)