ABSTRACT
Hickory milk and reconstituted milk (about 12 g/100 g total solid) were mixed in the proportion of 3:7 by volume to prepare hickory milk yogurt. Cow milk yogurt was used as control. The acidity and total count of lactic acid bacteria of hickory milk yogurt were not significantly different from cow milk yogurt (p > 0.05). Compared with cow milk yogurt, hickory milk yogurt had higher total solids, fat, crude protein, and amino acids, but lower ash and not-fat solids. Sensory evaluation showed that the appearance and flavor scores of hickory milk yogurt had no significant difference from cow milk yogurt (p > 0.05), but the texture score was significantly higher (p < 0.05). IC50 values in relation to 1,1-diphenyl-2-picrylhydrazyl scavenging activity and inhibition of lipid peroxidation (46.88 and 26.70 g/L) of hickory milk yogurt were significantly lower than those of cow milk yogurt which suggested that the antioxidant activity of hickory milk yogurt was significantly higher than cow milk yogurt (p < 0.05). There were 30 and 28 kinds of volatile compounds identified in hickory milk yogurt and cow milk yogurt, respectively. Compared with cow milk yogurt, the concentration of acetaldehyde, hexanal, nonanal, 2-nonanone, caproic acid, heptylic acid, and nonanoic acid in hickory milk yogurt increased significantly, and the concentration of benzaldehyde, 2,3-pentanedione, 2,3-butanedione, 3-hydroxy-2-butanone, acetic acid, butyric acid, and benzoic acid decreased significantly (p < 0.05).
Introduction
Juglans regia L. (hickory) is a nut with high commercial value and rich nutrition. It contains 90.08% unsaturated fatty acid, accounting for 92.71% of the total fatty acids, among which the monounsaturated fatty acid (oleic acid) is dominant.[Citation1] The market price of Juglans regia L. is about five dollars per kilogram in the domestic market, which is a reasonable price. Taking into account the cost of yogurt, adding with hickory is feasible. Frequently eating hickory nut can help decrease the T CHO level in the human body.[Citation2] In addition, the phenols and fatty acids contained in the hickory nut have promising antioxidant activity.[Citation3]
Yogurt is a fermented dairy product with pleasant flavor which is believed to have function of balancing intestinal flora and anti-aging.[Citation4] In recent years, yogurts that were added with different additives (such as apple polyphenol, paprike extract, etc.) have been reported with antioxidant activity.[Citation5,Citation6] Ye et al.[Citation7] and Condurso et al.[Citation8] have examined the volatile compounds in hawk tea yogurt, fermented cow milk, and cheese by solid-phase microextraction and gas chromatography-mass spectrometry (GC-MS), respectively, and found out that the volatile compounds in them mainly include aldehydes, ketones, acids, and some other compositions.
A particular food release flavor is a major factor determining consumer acceptance.[Citation9] Method of solid-phase microextraction and GC-MS with such advantages of being simple, convenient, quick, highly selective, and sensitive,[Citation10,Citation11] thus been widely used in identification of the volatile compounds in yogurt. This study was intended to prepare hickory yogurt with hickory nut and reconstituted milk, and examine its antioxidant activity and volatile flavor compounds.
Materials and methods
Materials
Hickory nut was provided by Jane’s Food Co., Ltd (Ningguo, China). Skimmed milk powder (Guangming) was purchased from Carrefour in Hefei, China. The starter (Lactobacillus delbrueckii subsp.bulgaricus 1.1480 (Lb) and Streptococcus thermophilus ys14 (St)) was prepared and preserved in the Laboratory of Microbial Resources and Application of Hefei University of Technology.
Methods
Preparation of hickory milk
Plump hickory kernels with no signs of fungal growth were soaked in 2% sodium carbonate solution at 75°C for 15 min and rinsed with running water for decortication. The decorticated hickory kernels were mixed with six times volume water, and sieved with 150-mesh sieve after being ground by a colloid mill, obtaining hickory milk with the solid content about 12%. (Lactobacillus delbrueckii subsp.bulgaricus 1.1480 (Lb)(+) and Streptococcus thermophilus ys14 (St))(+) were expanded cultured to prepare starter (Lb:St = 1:1) after three times activation.
Preparation of yogurt
The skimmed milk powder was dissolved in 43°C warm water, obtaining reconstituted milk with about 12% solid content, mixed with hickory milk in the proportion of 7:3 by volume, added 6% sucrose and stirred, homogenized under 22 MPa for 15 min by JHG-Q54-P60 homogenizer (Shanghai Fusion Machinery Equipment Co., LTD, Shanghai, China). Milk was heated to 85°C in a stainless steel tank for 15 min, and rapidly cooled to 42°C. Pasteurized milk was inoculated with 6% of starter by the starter culture (Lb:St = 1:1, v/v), incubated at 42°C for 6 h until the pH reached 4.3. After fermentation, yogurt samples were stored at 4°C overnight.
Titratable acidity
Phenolphthalein was used as the indicator. Yogurt was titrated with 0.1 mol/L NaOH, and the titratable acidity of yogurt was expressed as the lactic acid percentage.
Total count of lactic acid bacteria
Ten-fold serial dilution was adopted to determine the total count of lactic acid bacteria. De Man Rogosa Sharpe (MRS) agar medium with sample was cultured for 48 ± 2 h in 36 ± 1°C anaerobic incubator, and the colony count on each plate was recorded.
Main composition and amino acid (AA) composition
The contents of ash, total solids, non-fat milk solids, protein, and fat were determined according to AOAC[Citation12] procedures. Yogurt (1 g) was put into a bottle for acidolysis, adding with 10 mL HCl (6 mol/L). The bottle was sealed for acidolysis in a vacuum drying oven (DZF-6020, Shanghai, China) at 135°C for 6 h after being aerated with nitrogen. Adding with 0.02 mol/L HCl, the acidolysis solution was diluted to 5 mL, and sample of 20 μL was injected into the automatic AA analyzer L-8900 (Hitichi, Japan) for detection of AAs. The chromatographic conditions: sodium ion exchange column; column temperature: 60°C; flow rate: 0.4 mL/min; flowing phase: citric acid buffer; temperature of column response column: 135°C; flow rate: 0.35 mL/min; the detector type: visible (VIS), detection wavelength: 570 and 440 nm, respectively. The content of AA was calculated as,
where C (ng/mL) was the concentration of AA.
Sensory evaluation
Four aspects (appearance, texture, flavor and acceptability) were assessed by eight panelists (sexes were about equally and the panelists ranging in age from 25 to 35) with experience and background in scientific food knowledge. According to the method of Isanga and Zhang.[Citation13] modified slightly. Sensory evaluation scores: Extremely unacceptable = 1; Unacceptable–barely acceptable = 2–4; Acceptable–very acceptable = 5–9; Extremely acceptable = 10.
Antioxidant activity
For the 1,1-Diphenyl-2-picrylhydrazyl (DPPH·) scavenging reaction, method of Chun et al. with minor modification was used. The solution was centrifuged at 1400 rpm for 10 min after reaction in dark at room temperature (25°C) for 30 min.[Citation14] The supernatant was obtained to determine the absorption value at 517 nm,
where A0 was blank absorption value and A1 was sample absorption value.
Inhibition of lipid peroxidation (LPO)
The method of Ye et al.,[Citation15] with minor modifications, was used. Sample solutions of different concentrations (1 mL) and 10% yolk homogenate (w/v; 0.5 mL) prepared by 1.15% KCl solution were evenly blended, added with 2 mL distilled water, 1.5 mL glacial acetic acid (20%, pH 3.5), and 1.5 mL 2-Thiobarbituric acid (0.8%) that contained 1.1 g/100 mL sodium dodecyl sulfate. The mixture solution was evenly mixed, and put in 95°C water bath for 60 min. After being cooled to room temperature, the samples were added to 5 mL butanol, and centrifuged at 3000 rpm for 10 min. The supernatant was obtained to determine the absorption value AT at 532 nm. Using the distilled water instead of the sample solution as control, AC was measured by the same method. Anti-rate Ia = (1 – AT/AC) × 100%.
Determination of volatile flavor compounds
Extraction of flavor compounds: The yogurt sample of 5 mL was put into a 20 mL glass bottle (Supelco, Bellefonte, PA, USA), which was sealed on the top. Then a syringe needle with a Carboxen/Polydimethylsiloxane (CAR/PDMS) fiber (Supelco, Bellefonte, PA, USA) coated with 75 μm coating was used to penetrate the seal for extraction at 60°C for 30 min.
GC-MS analysis of flavor compounds. A C4000 gas chromatograph-mass spectrometer (Varian Inc., Walnut Creek, CA, USA) was used for analysis of the components of the hickory milk yogurt (HMY) and cow milk yogurt (CMY) extracted by solid phase micro-extraction. The injection temperature was 250°C, splitless injection mode was adopted and a DB-5MS column (30 m × 0.25 mm × 0.25 μm) was used as the gas chromatographic column. The gas chromatographic oven was kept at 45°C for 5 min, then heated to 80°C at the rate of 10°C/min, then heated to 240°C at the rate of 5°C/min. Helium gas was the carrier gas, whose velocity was constant at 1 mL/min. The temperature of the transmission line was 250°C, and samples were ionized by 70 eV electron impact, with the capture range of 30–400 m/z and the scanning rate of 4.5 scans/s. The flavor compounds in the yogurts were determined by comparing the retention time with Mainlib mass spectrum database. And the contents of the volatile flavor compounds were expressed by the relative peak area of each compound.
Results and discussions
Acidity
There was no significant difference in the acidity between HMY (91.5) and CMY (90.0; p > 0.05). A suitable acidity of yogurt was an important indicator to ensure the quality of fermented yogurt.[Citation16]
Total count of lactic acid bacteria
The total count of lactic acid bacteria in HMY (8.94 log cfu/mL) was 0.02 log cfu/mL less than that in CMY (8.96 log cfu/mL), showing no significant difference (p < 0.05), which indicated that Lactobacillus delbrueckii subsp.bulgaricus and Streptococcus thermophilus all grew well during the fermentation process of yogurt.[Citation17,Citation18]
Main composition and AA composition
The ash contents in HMY (0.64 g/100 g) was significantly lower than those in CMY (0.98 g/100 g; ). The total solid content and non-fat solid of HMY and CMY were no significant difference, respectively (p < 0.05). Both fat and protein contents in HMY were significant higher than those in CMY, because hickory contained higher amount of fat and protein.[Citation19] Essential amino acids, for which can not be synthesized or synthetic speed can not meet the needs of the human body, included leucine, histidine, threonine, isoleucine, methionine, lysine, phenylalanine, arginine, and valine. They need to get from food but also to have a proper proportion.[Citation20] In both HMY and CMY, 17 kinds of AAs were detected (). The contents of arginine, isoleucine, and lysine in HMY were significantly higher than those in CMY (p < 0.05), whereas there was no significant difference in the contents of threonine, valine, methionine, leucine, and phenylalanine between the two yogurts (p > 0.05). Besides, the contents of various nonessential AAs in HMY were all significantly higher than those in CMY (p < 0.05). The HMY should be considered as a high nutritional yogurt with abundant good protein.
Table 1. Analysis of main composition of CMY and HMY (g/100 g).
Table 2. Analysis of amino acids composition of CMY and HMY (mg/g).
Sensory evaluation
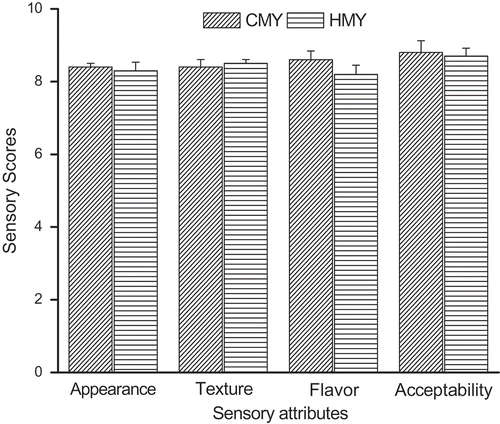
Scores of sensory evaluation of yogurt on the four aspects of appearance, texture, flavor, and acceptability by eight panelists were summarized in . The mean values of all evaluation indicators fell in the acceptable range (5–9).[Citation21] Results showed that the acceptability of HMY and CMY were almost the same, and there was no significant difference in the appearance and flavor scores between the two yogurts (p > 0.05). The texture score of CMY was significantly higher than that of HMY (p < 0.05), it might resulted from the improving effect of higher fat content on the texture of HMY.[Citation22]
Antioxidant activity
Mass concentration of yogurt with the scavenging rate of 50% (IC50) was used as the indicator to evaluate the DPPH· scavenging activity and inhibition of LPO of yogurt (). The smaller IC50 value, the higher the scavenging rate.[Citation23] DPPH· was a stable organic free radical. Free radical scavengers can pair with its single electron and weaken the amaranthine dye, so the DPPH·scavenging activity can be quantitatively analyzed by spectroscopy.[Citation24] IC50 value of HMY in relation to DPPH·scavenging activity (46.879 g/L) was significant lower than that of CMY (48.704 g/L), which means the DPPH·scavenging ability of HMY was significantly higher than that of CMY (p < 0.05).
Figure 2. IC50 values of scavenging DPPH radical and inhibiting of lipid peroxidation of HMY and CMY.
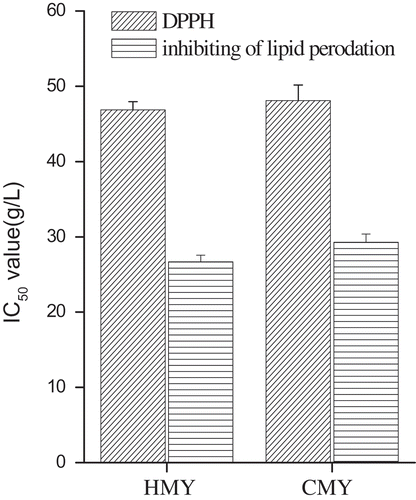
LPO refers to a series of chain reactions of unsaturated fatty acids induced by free radicals or other active oxygens, producing toxic compounds.[Citation25] IC50 of HMY to inhibit LPO (26.697 g/L) was significantly lower than that of CMY (29.284 g/L ; p < 0.05). Results showed that HMY and CMY both had antioxidant activity, and the antioxidant activity of HMY was significantly higher than that of CMY (p < 0.05), which might resulted from the effect of phenols and small peptides in Juglans regia L.
Volatile flavor compounds
Flavor is an important quality characteristic for the determination of yogurt quality. Some flavor compounds occur naturally in milk, while others are produced during the fermentation process. The flavor of yogurt is formed by enzymatic and chemical reactions, including lipid oxidation, fermentation of carbohydrates, lipolysis, catabolism of AAs, and proteolysis.[Citation26,Citation27] In various types of foods around 12,000 volatile compounds have been identified so far, it is estimated that only about 5% of them play an important role in the formation of flavor of these products.[Citation28] If present in concentrations exceeding their odor threshold they can be perceived by human olfactory system. Flavor compounds are characterized by odor threshold sometimes of very low values in a ng L−1 or ng kg−1 range, so it is a challenge both to isolate them from food and then quantify and identify.[Citation29] Non-volatile acids (lactic acid or pyruvic acid), volatile acids (butyric acid, acetic acid, etc), carbonyl compounds (acetaldehyde and butanedione), and other compounds such as AA are the main compounds to constitute the aroma of yogurt.[Citation23]
There are 30 and 28 kinds of volatile compounds that were identified in HMY and CMY, respectively (), which mainly were aldehydes, ketones and acids and a small amount of alcohols and esters. These compounds formed the unique fermentation aroma of HMY and CMY. Most of these compounds were found in other types of fermented milk: kefir,[Citation30] Lebanese Leben,[Citation31] and cheese.[Citation32,Citation33] Dimethyl benzene was detected in CMY, but not in HMY, meanwhile, butyraldehyde, ethyl acetate, and 2-ethyl furan were identified in HMY, but not in CMY, which was in agreement with the report of Elmore et al.[Citation34]
Table 3. Flavor compounds of HMY and CMY.
Aldehydes are important compounds that constitute the characteristic flavor of yogurt. Aldehydes, including acetaldehyde, hexanal, benzaldehyde, furfural, and nonanal were found in both yogurts. The contents of acetaldehyde, hexanal, and nonanal in HMY increased significantly by 0.66, 0.72, and 0.56%, respectively, compared with those in CMY (p < 0.05). Acetaldehyde is one of the most important flavor compounds in yogurt,[Citation35] mainly occurs as a natural product of AAs (especially threonine, methionine, and valine) and as a result of pyruvate and nucleotide catabolism of starter cultures. Acetaldehyde provides a pungent, fresh, and fruity aroma to fermented milk and yogurt.[Citation36–Citation38] When its concentration is in the range of 23–41 mg/kg, the yogurt has the best flavor.[Citation39] The furfural contents in the two yogurts were not significantly different (p > 0.05), whereas the benzaldehyde content in HMY was significantly lower than that of CMY (p < 0.05). No butyraldehyde was identified in CMY.
Ketones are produced by metabolism of lactose by Lactobacillus delbrueckii subsp.bulgaricus via the EMP pathway in the pre-fermentation stage of yogurt.[Citation40] Some studies suggested that only when the acetaldehyde content is low, butanedione is the main flavor of yogurt.[Citation39] Butanedione is lactic acid bacteria by the use of lactose metabolism by EMP pathway, but also by the formation of aspartate metabolism.[Citation40] In HMY, the content of 2-nonanone increased by 1.42% compared with CMY (p < 0.05), contents of 2-heptanone, 1-hydroxy-2-propanone, and acetone were not significantly different from those in CMY (p > 0.05), and contents of 2,3-pentanedione, 2,3-butanedione and 3-hydroxy-2-butanone were significantly lower than those in CMY (p < 0.05).
Acids are an important quality parameter of milk and dairy products. They serve as a precursor to formation of methyl ketones, alcohols, lactones, and esters.[Citation41] The acid compounds are also the major components causing the sour taste of yogurt. The detected volatile acids included acetic acid, butyric acid, caproic acid, benzoic acid, caprylic acid, heptylic acid, nonanoic acid, and capric acid. Caproic acid and butyric acid is produced by lactose fermentation, and can also be formed by free fatty acid fatty acid decomposition.[Citation42] Acetic acid occurs due to citric acid, lactic acid, AA, and lactose catabolism of bacteria in yogurt. Formation of a high concentration of acetic acid is not desirable because it provides a vinegary and pungent flavor to the final product.[Citation41,Citation43] Caproic acid, heptylic acid, and nonanoic acid contents in HMY were 0.98, 0.19, and 0.18% higher than those in CMY, respectively (p < 0.05), contents of caprylic acid and capric acid were not significantly different (p > 0.05), whereas contents of acetic acid, butyric acid, and benzoic acid in HMY were significantly lower than those in CMY (p < 0.05).
Alcohols have little effect on the flavor of yogurt because of its high flavor threshold. 1-hexanol, 3-pentanol, and 1,4-butanediol were detected primarily. 1,4-butanediol content in HMY increased significantly by 0.8% compared with CMY (p < 0.05), 1-hexanol content in the two yogurts were not significantly different (p > 0.05), whereas content of 3-pentanol in HMY was significantly lower (p < 0.05). Octyl formate, propyl benzoate, and butyl acetate were identified in both yogurts, among which content of the propyl benzoate in HMY was significantly higher than that in CMY. No ethyl acetate was detected in CMY. 2-pentyl-furan was detected in HMY. Several aromatic compounds containing furan rings can be derived from carbohydrate precursors. Carbohydrate derived aroma compounds such as 2-pentyl-furan was also found in pandan leaves.[Citation44]
Conclusions
The acidity and total count of lactic acid bacteria in HMY prepared by fermentation of Lactobacillus delbrueckii subsp.bulgaricus and Streptococcus thermophilus were not significantly different from those of CMY (p > 0.05).Texture, AAs, and protein content of yogurt had been improved by adding hickory. Compared with CMY, HMY with rich unsaturated fatty acids has more flavor compounds and stronger antioxidant activity. It will be more popular among consumers than CMY. And the antioxidant activity of HMY was significantly higher than that of CMY (p < 0.05). HMY can be promoted in the dairy market as the higher nutrition and stronger antioxidant activity of this yogurt, and it also will be favored by more consumers. The antioxidant mechanism of HMY will have been studied in the future work.
Funding
This work was financially supported by Science and Technology Major Project of Anhui Province (15czz03106). The authors declare no competing financial interest.
Additional information
Funding
References
- Slatnar, A.; Mikulic-Petkovsek, M.; Stampar, F.; Veberic, R.; Solar, A. Identification and Quantification of Phenolic Compounds in Kernels, Oil and Bagasse Pellets of Common Walnut (Juglans Regia L.). Food Research International 2015, 67, 255–263.
- Abdallah, I.B.; Tlili, N.; Martinez-Force, E.; Rubio, A.G.P.; Perez-Camino, M.C.; Albouchi, A.; Boukhchina, S. Content of Carotenoids, Tocopherols, Sterols, Triterpenic and Aliphatic Alcohols, and Volatile Compounds in Six Walnuts (Juglans Regia L.) Varieties. Food Chemistry 2015, 173, 972–978.
- Salcedo, C.L.; López de Mishima, B.A.; Nazareno, M.A. Walnuts and Almonds As Model Systems of Foods Constituted by Oxidisable, Pro-Oxidant and Antioxidant Factors. Food Research International 2010, 43, 1187–1197.
- Michael, M.; Phebus, R.K.; Schmidt, K.A. Impact of a Plant Extract on the Viability of Lactobacillus Delbrueckii ssp. Bulgaricus and Streptococcus LavorHiles in Nonfat Yogurt. International Dairy Journal 2010, 20, 665–672.
- Tamjidi, F.; Nasirpour, A.; Shahedi, M. Physicochemical and Sensory Properties of Yogurt Enriched with Microencapsulated Fish Oil. Food Science and Technology International 2012, 18, 381–390.
- Halah, M.F.; Mehanna, N.S. Use of Natural Plant Antioxidant and Probiotic in the Production of Novel Yogurt. Journal of Evolutionary Biology Research 2011, 3, 12–18.
- Ye, M.; Liu, D.; Zhang, R.; Yang, L.; Wang, J. Effect of Hawk Tea (Litsea Coreana L.) on the Numbers of Lactic Acid Bacteria and Flavor Compounds of Yogurt. International Dairy Journal 2012, 23, 68–71.
- Lin, J.; Chen, W.; Zhu, H.; Wang, C. Determination of Free and Total Phthalates in Commercial Whole Milk Products in Different Packaging Materials by Gas Chromatography-Mass Spectrometry. Journal of Dairy Science 2015, 98, 8278–8284.
- Fadel, H.H.M.; Taher, M.S.; Gad, A.M. Flavor Release from a Banana Soft Drink Complex Model System: Evaluation of the Efficiency of Different Adsorbents for Trapping the Released Volatiles During Storage. International Journal of Food Properties 2015, 18, 796–807.
- Aghlara, A.; Mustafa, S.; Manap, Y.A. Characterization of Headspace Volatile Flavor Compounds Formed During Kefir Production: Application of Solid Phase Microextraction. International Journal of Food Properties 2009, 12, 808–818.
- Ziadi, M.; Wathelet, J.P.; Marlier, M.; Hamdi, M.; Thonart, P. Analysis of Volatile Compounds Produced by 2 Strains of Lactococcus Lactis Isolated from Leben (Tunisian Fermented Milk) Using Solid-Phase Microextraction-Gas Chromatography. Journal of Food Science 2008, 73, 247–252.
- AOAC. Association of Analytical Chemists. Official Methods of Analysis, 14th Ed. AOAC: Washington, DC, 2003.
- Isanga, J.; Zhang, G. Production and Evaluation of Some Physicochemical Parameters of Peanut Milk Yoghurt. LWT–Food Science and Technology 2009, 42, 1132–1138.
- Chun, J.; Jeong, W.J.; Kim, J.S. Hydrolysis of Isoflavone Glucosides in Soymilk Fermented with Single Or Mixed Cultures of Lactobacillus Paraplantarum KM, Weissella Sp. 33, and Enterococcus Faecium 35 Isolated from Humans. Journal of Microbiology and Biotechnology 2008, 18, 573–578.
- Ye, M.; Xu, Q.P.; Chen. X.; Yang, L.; Lin, Y.R. Total Phenolic Content and Antioxidant Activities of the Extracellular Melanin from Lachnum sp. YM-223. Mycosystema 2010, 9, 608–611.
- Granata, L.A.; Morr, C.V. Improved Acid, Flavor and Volatile Compound Production in a High Protein and Fiber Soymilk Yogurt-Like Product. Journal of Food Science 1996, 61, 331–336.
- Serra, M.; Trujillo, A.J.; Guamis, B.; Ferragut, V. Flavour Profiles and Survival of Starter Cultures of Yoghurt Produced from High-Pressure Homogenized Milk. International Dairy Journal 2009, 19, 100–106.
- Zare, F.; Boye, J.I.; Orsat, V.; Champagne, C.; Simpson, B.K. Microbial, Physical and Sensory Properties of Yogurt Supplemented with Lentil Flour. Food Research International 2011, 44, 2482–2488.
- Labuckas, D.O.; Maestri, D.M.; Perello, M. Phenolics from Walnut (Juglans Regia L.) Kernels: Antioxidant Activity and Interactions with Proteins. Food Chemistry 2008, 107, 607–612.
- Wang, X.S. Essential Amino Acids for Human Health. Food and Nutrition in China 2005, 7, 48–49.
- Tamime, A.Y.; Robinson, R.K. Yoghurt: Science and Technology. Woodhead Publishing: London, Britain, 1999.
- Oliveira, M.N.; Sodini, I.; Remeuf, F. Effect of Milk Supplementation and Culture Composition on Acidification, Textural Properties and Microbiological Stability of Fermented Milks Containing Probiotic Bacteria. International Dairy Journal 2001, 11, 935–942.
- He, S.M.; Liu, J.L.; Liu, H.M. Study on the Anti-Oxidative of Yogurt by Scavenging DPPH. China Dairy Industry 2006, 38, 18–20.
- Sendra, J.M.; Sentandreu, E.; Navarro, J.L. Reduction Kinetics of the Free Stable Radical 2, 2-Diphenyl-1-Picrylhydrazyl (DPPH•) for Determination of the Antiradical Activity of Citrus Juices. European Food Research and Technology 2006, 223, 615–624.
- Zhou, B.O.; Wu, L.M.; Yang, L.I. Evidence for α-Tocopherol Regeneration Reaction of Green Tea Polyphenols in SDS Micelles. Free Radical Biology and Medicine 2005, 38, 78–84.
- Ott, A.; Fay, L.B.; Chaintreau, A. Determination and Origin of the Aroma Impact Compounds of Yogurt Flavor. Journal of Agricultural and Food Chemistry 1997, 45, 850–858.
- Ott, A.; Hugi, A.; Baumgartner, M.; Chaintreau, A. Sensory Investigation of Yogurt Flavor Perception: Mutual Influence of Volatiles and Acidity. Journal of Agricultural and Food Chemistry 2000, 48, 441–450.
- Grosch, W. Evaluation of the Key Odorants of Foods by Dilution Experiments, Aroma Models and Omission. Chemical Senses 2001, 26, 533–545.
- Jeleń, H.H.; Majcher, M.; Dziadas, M. Microextraction Techniques in the Analysis of Food Flavor Compounds: A Review. Analytica Chimica Acta 2012, 738, 13–26.
- Beshkova, D.M.; Simova, E.D.; Frengova, G.I. Production of Volatile Aroma Compounds by Kefir Starter Cultures. International Dairy Journal 2003, 13, 529–535.
- Chammas, G.I.; Saliba, R.; Corrieu, G. Characterisation of Lactic Acid Bacteria Isolated from Fermented Milk “Laban.” International Journal of Food Microbiology 2006, 110, 52–61.
- Izco, J.M.; Torre, P. Characterisation of Volatile and Flavor Compounds in Roncal Cheese Extracted by the “Purge and Trap” Method and Analysed by GC–MS. Food Chemistry 2000, 70, 409–417.
- Smit, G.; Smit, B.A.; Engels, W.J.M. Flavour Formation by Lactic Acid Bacteria and Biochemical and Flavor Profiling of Cheese Products. FEMS Microbiology Reviews 2005, 29, 591–610.
- Elmore, J.S.; Nisyrios, I.; Mottram, D.S. Analysis of the Headspace Aroma Compounds of Walnuts (Juglans Regia, L.). Flavour & Fragrance Journal 2005, 20, 501–506.
- Ye, Y.E.; Ji, J.; Ye, M.; He, Y.L.; Yang, L. Physicochemical Properties and Flavor Compounds of Soy Milk Yogurt. Journal of Pure and Applied Microbiology 2013, 7, 317–323.
- Lees, G.J.; Jago, G.R. Role of Acetaldehyde in Metabolism: A Review 2. The Metabolism of Acetaldehyde in Cultured Dairy Products. Journal of Dairy Science 1978, 61, 1216–1224.
- Beshkova, D.; Simova, E.; Frengova, G. Production of Flavor Compounds by Yogurt Starter Cultures. Journal of Industrial Microbiology and Biotechnology 1998, 20, 180–186.
- Gallardo-Escamilla, F.J.; Kelly, A.L.; Delahunty, C.M. Influence of Starter Culture on Flavor and Headspace Volatile Profiles of Fermented Whey and Whey Produced from Fermented Milk. Journal of Dairy Science 2005, 88, 3745–3753.
- Lim, C.M.; Jhoo, J.W.; Kim, G.Y. Determination of Volatile Flavor Compounds During Storage of Cereal Added Yogurt Using HS-SPME. Korean Journal for Food Science of Animal Resources 2013, 33, 646–654.
- Bars, D.L.; Yvon, M. Formation of Diacetyl and Acetoin by Lactococcus Lactis Via Aspartate Catabolism. Journal of Applied Microbiology 2008, 104, 171–177.
- Ott, A.; Fay, L.B.; Chaintreau, A. Determination and Origin of the Aroma Impact Compounds of Yogurt Flavor. Journal of Agricultural and Food Chemistry 1997, 45, 850–858.
- Curioni, P.M.G.; Bosset, J.O. Key Odorants in Various Cheese Types As Determined by Gas Chromatography-Olfactometry. International Dairy Journal 2002, 12, 959–984.
- Ott, A.; Hugi, A.; Baumgartner, M. Sensory Investigation of Yogurt Flavor Perception: Mutual Influence of Volatiles and Acidity. Journal of Agricultural and Food Chemistry 2000, 48, 441–450.
- Ningrum, A.; Minh, N.N.; Schreiner, M. Carotenoids and Norisoprenoids As Carotenoid Degradation Products in Pandan Leaves (Pandanus Amaryllifolius Roxb.). International Journal of Food Properties 2015, 18, 1905–1914.