ABSTRACT
In this study, the effects of different pH values and co-pigments, including tannic, caffeic, benzoic, and coumaric acids, in various concentrations on anthocyanin co-pigment complexes (ratio 1:1) were investigated. The anthocyanin was extracted from a round sweet variety of blueberry and subjected to the highest co-pigment concentration (960 mg/l) at different pH values. The results showed that caffeic acid produced the highest stability of anthocyanin compared to the other co-pigments. In addition, benzoic acid had the lowest hyperchromic and bathochromic changes. A positive correlation was found between the co-pigment concentrations and the stability of anthocyanin as well as hyperchromic and bathochromic changes. The results also demonstrated that higher pH values resulted in further destruction of anthocyanin. Furthermore, the absorption rate of anthocyanin in the anthocyanin–co-pigment complex within lower pH values was more compared with the higher ones. The strongest hyperchromic and bathochromic effects for all organic acids were observed in pH values 1 and 4, respectively. It can be concluded that among the tested co-pigments, caffeic acid was the best co-pigment, while benzoic acid was the weakest, which was not suitable for anthocyanin in the round sweet variety of blueberry.
Introduction
Blueberry (Cornus mas L.) belonging to the family Cornaceae is a tall shrub or a short tree ranging from 5 to 8 m in height, with tiny leaves. In Iran, the plant grows in Arasbaran forests, Alborz Mountains, Alamout region, Kuhein of Qazvin, and forests of Mazandaran and Guilan[Citation1]. In some varieties of blueberry, the presence of polyphenol compounds, especially anthocyanin and antioxidants, has been reported[Citation2,Citation3]. Anthocyanins are a subgroup of flavonoids with very high antioxidant activity, which are responsible for the red, purple, and blue colours of many flowers, fruits, and vegetables[Citation4]. Recent studies have shown that anthocyanins can be used as a natural food colourant. However, since anthocyanins are very unstable molecules in food matrices, this application is limited[Citation5]. Increased anthocyanin concentrations result in more stability and intensity of the colour[6,i7]. The colour stability of anthocyanin is significantly affected by pH, solvents, temperature, concentration, structures of anthocyanins, oxygen, light, enzymes, and other substances attached to them[Citation8,Citation9].
Most published reports on colour changes in fruits undergoing processing are based on analytical techniques measuring the anthocyanin content by spectrophotometric techniques[Citation10]. It is well known that the colour and stability of anthocyanin strongly depend on the pH, appearing red in acidic, violet in neutral, and blue in basic aqueous solutions[Citation11].
Co-pigmentation reactions are influenced by pH, concentration, solvent, and molecular structure. Increased pH values result in the destruction of anthocyanin and decreased colour intensity. Cevallos-Casals et al. studied[Citation12] anthocyanin in red carrot, red potato, gooseberry, and red corn and investigated the effects of pH on anthocyanin. They concluded that the absorbance rate in red carrot and red potato was relatively higher in elevated pH values, which might be due to the acylation of anthocyanins. Moreover, they found that in lower pH values, the dominant form of anthocyanins was red flavylum.
Co-pigments are colourless on their own, but when added to anthocyanin solution, they increase the colour intensity and the stability of the solution. Co-pigments are electron-rich systems, which can link to electron-deficient falvylum ion; this linkage protects the flavylum ion from the nucleophilic attack of water molecule[Citation13]. Flavonoids are well known co-pigments among which flavons, flavonols, flavonons, and flavanols have been vastly studied. In addition, phenolic acids, including hydroxynamic and hydroxybenzoic acids, have been investigated in co-pigmentation and have shown strong effects on the stability of anthocyanin[Citation14,Citation15].
Markovic et al.[Citation15] and Gris et al.[Citation16] stated that among the different co-pigments, tannic and ferulic acids showed the strongest hyperchromic effect on grape extracts. In the present study, the effects of different pigments with various concentrations and pH values on the stability of anthocyanin in round sweet variety of blueberry (Cornus mas – Yulyush) were investigated.
Materials and method
Sample preparation and anthocyanin extraction
The primary sample of C. mas – Yulyush was obtained from the agriculture research centre of Kaleybar in East Azerbayjan province, Iran, in September 2015. Pharmacognostic verification of the plant was performed by the Department of Botany and Herbal Medicine, Urmia University, Urmia, Iran. The voucher specimens were deposited at the herbarium of the institute and received code no: 4559. The anthocyanin was extracted using 96% ethanol and 0.1% HCl[Citation17]. One hundred gram of the specimen was mixed with 100 ml of extraction solvent until it was totally crushed and became homogenous. Then, it was stirred with a magnetic stirrer for 4 h. The solution was filtered using Buchner funnel and Whatman No.1 filter and diluted to 500 ml. The resultant solution was centrifuged at 8000 g for 30 min and the upper coloured and clear solution was collected for the experiments. The samples were kept for 24 h at 4°C in sealed vials[Citation18].
Preparation of anthocyanin–co-pigment complex and pH treatment
Four types of co-pigments, including benzoic, tannic, caffeic, and coumaric acids, were utilized in this study. The effect of the type of co-pigment on anthocyanin stability was assessed using similar concentration (960 mg/l) and co-pigment–anthocyanin ratio (1:1) for all of the co-pigments. Five groups were considered, including the control group (Ι), which received no co-pigment, and the treatment groups (ΙΙ–IV). Various kinds of co-pigments were administered. Co-pigmentation reactions were determined through measuring the optical absorption rate spectrophotometerically, at wavelengths of 450 and 650 nm (Biowave, S2100, UK)[Citation19,Citation20]. Five different concentrations of each of the co-pigments (0, 120, 240, 480, and 960 mg/l) with a ratio of 1:1 to anthocyanin were used[Citation20]. The mixture of co-pigments and anthocyanin was prepared and after 30 min, the absorption rate was measured at pH 3.5. The impacts of various pH values including 1, 2, 3, and 4 on the co-pigmentation reaction with diverse concentrations of each co-pigment also were investigated [Citation6,Citation21,Citation22].
Statistical analysis
All experiments were repeated three times. The values were reported as the mean values and standard deviation (SD) of the means. The difference between the treatments was measured using one-way ANOVA and Tukey’s test with the probability of p ≤ 0.05. In order to plot the diagrams and statistical analysis, SPSS Version 21 and Excel software were used.
Results and discussion
Types and concentrations of the co-pigment are the most effective factors in co-pigmentation reactions. The intensity of the reaction depends on the molar ratio of the anthocyanin to co-pigment. Furthermore, the concentrations of both anthocyanin and co-pigment are influential in co-pigmentation[Citation23]. In the present study, the anthocyanin concentration was kept constant, and the effects of various concentrations of the co-pigments on co-pigmentation reaction were analysed. Since the constant concentration of the anthocyanin was utilized, it could be concluded that the amount of bathochromic shift and hyperchromic effect could be attributed to the concentration of the co-pigments. (A–D) represents the effects of various co-pigments, including tannic, caffeic, benzoic, and coumaric acids, on the anthocyanin stability, respectively.
Figure 1. Absorption spectra of various anthocyanins with different concentrations at pH 3.5 for round sweet variety blueberry. (A) Tannic acid, (B) caffeic acid, (C) benzoic acid, (D) coumaric acid. The data are the mean value for three replications ± standard deviation.
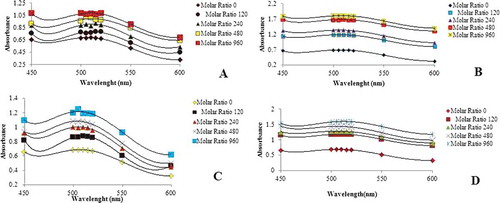
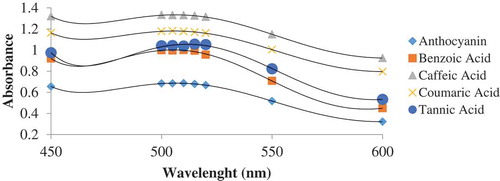
The obtained results revealed that the stability of anthocyanin as well as hyperchromic and bathochromic changes depend on the concentration of the co-pigments. In all of the anthocyanin–co-pigment complexes, the best stability was observed at the highest concentration (960 mg/l) of the co-pigment. Moreover, the effects of the highest concentrations of each co-pigment (960 mg/l) on anthocyanin stability were determined (). It was found that caffeic acid resulted in more stability compared with the other co-pigments. In addition, benzoic acid was found to have the lowest hyperchromic and bathochromic effects. Co-pigmentation reaction occurred better in pH values between 2 and 5 in which the quinoidal form is dominant[Citation24]. The co-pigmentation phenomenon arises from a molecular connection between pigments and non-coloured organic molecules. Such a connection causes the pigments to have more intense colours[Citation14]. In very acidic solutions (pH values 3 and 4), most hemiketals are colourless, and chalcone forms may transform to flavylum cations, which are quinoid through the formation of co-pigmentation complexes and therefore produce significant colour. However, in very acidic solutions, all anthocyanins are in flavylum coloured form[Citation25].
Figure 2. Comparison of absorption spectra of anthocyanin with different co-pigments (960 mg/l) and without co-pigment at pH 3.5 for round sweet variety blueberry. The data are the mean value for three replications ± standard deviation.
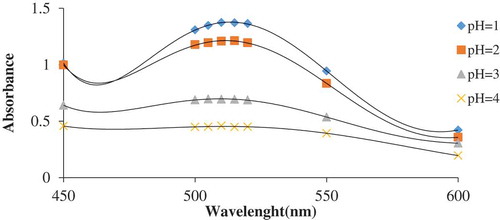
The effects of various pH values including 1, 2, 3, and 4 on co-pigmentation reaction at the highest concentration (960 mg/l) of organic acids with a ratio of 1:2 anthocyanin to organic acids were investigated. The obtained results are depicted in As can be seen, the absorbance rate of anthocyanin and anthocyanin–co-pigment complex was higher in lower pH values and the highest stability for anthocyanin–co-pigment complex was obtained at pH 1. The elevation of pH values from 1 to 4 led to more destruction of anthocyanins in the samples, as evidenced by the decreased absorbance rate due to the reduction of flavylum cation in the raw extract.
Figure 3. Diagram of the effects of different pH values on anthocyanin in blueberry; the data are the mean value for three replications ± standard deviation.
illustrates the effects of various pH values on bathochromic and hyperchromic values. As can be seen, increased pH values resulted in the destruction of anthocyanin and reduction of its colour intensity. At very low pH values, despite the formation of the anthocyanin–co-pigment complex, hyperchromic effects are negligible due to hydration. According to the previous research[Citation26], the best pH for the anthocyanin–co-pigment complex was found to vary between two and five. In very acidic media, flavylum cation is the only species in equilibrium. These salts lose their proton at higher pH values and are transformed to quinoidal, an unstable pigment, which attaches to the water molecule immediately and forms chromend compounds[Citation26].
Table 1. Bathochromic and hyperchromic values in anthocyanin and anthocyanin-co-pigment of round sweet blueberry samples.
Anthocyanins have the highest stability in acidic solvents with low pH values. They have high colour diversity in different pH values ranging from 1 to 14. Parisa et al.[Citation27] showed that in order to achieve the highest anthocyanin stability, the best pH is three. In their study on the effects of pH on the anthocyanins obtained from four berberis species, Laleh et al[Citation28]. reported that increased pH values resulted in less stability of anthocyanins as the destruction of anthocyanins between pH values 1.5 and 3 was twice higher than that of the pH values ranging from 0 to 1.5.
According to the obtained values for the round sweet variety of blueberry, the highest hyperchromic effect in all organic acids was observed at pH 1 (ΔA=0.371), which was not accompanied by bathochromic changes. Furthermore, the highest bathochromic changes were observed at pH 4. Anthocyanin–coumaric acid complex had the lowest hyperchromic effect (ΔA=0.002). The bathochromic effect was not very significant for pH values 1 and 2, but at pH 3 and 4, the highest bathochromic effects were observed. At pHs 1, 2, and 4, the most stable co-pigments were anthocyanin–caffeic and anthocyanin–benzoic acid complexes, respectively. In addition, it is noteworthy that the highest stability among pHs 1 and 2 was obtained for the anthocyanin–caffeic acid complex at pH 1. Between pH values 3 and 4, this belonged to the anthocyanin–benzoic acid complex at pH 4. Gauche., et al and Malaj., et al[Citation20,Citation29]. reported that the complexation of co-pigment with anthocyanins resulted in increased hyperchromic and bathochromic effects.
Conclusion
The results obtained from this study on round sweet blueberry (C. mas -Yulyush) showed that at pH values 1 and 2, the highest stability was obtained for the anthocyanin–caffeic acid complex and at pH values 3 and 4, maximum stability was reached for the anthocyanin–benzoic acid complex. Moreover, the findings of this study indicated that the anthocyanin–co-pigment complex enjoyed more stability compared with anthocyanin alone, suggesting that the addition of co-pigments resulted in the resistance and stability of anthocyanin in unfavourable conditions. The results also showed that compared to the other co-pigments, caffeic acid had a profound preventive effect and resulted in more stability of anthocyanin. The amount of hyperchromic and bathochromic changes in benzoic acid was the least and through increasing the concentration of the co-pigments, the stability of anthocyanin and the amount of hyperchromic and bathochromic changes went up too. It can be concluded that among the tested co-pigments, caffeic acid was the best co-pigment and benzoic acid was the weakest, which was not suitable for anthocyanin in the round sweet variety of blueberry.
References
- Hassanpour, H.; Hamidoghli, Y.; Samizadeh, H. Some Fruit Characteristics of Iranian Cornelian Cherries (Cornus mas L.). Notulae Botanicae Horti Agrobotanici Cluj-Napoca 2012, 40, 247.
- Ercisli, S.; Yilmaz, S.O.; Gadze, J.; Dzubur, A.; Hadziabulic, S.; Aliman, Y. Some Fruit Characteristics of Cornelian Cherries (Cornus mas L.). Notulae Botanicae Horti Agrobotanici Cluj-Napoca 2011, 39, 255.
- Ersoy, N.; Bagci, Y; Gok, V. Antioxidant Properties of 12 Cornelian Cherry Fruit Types (Cornus mas L.) Selected from Turkey. Scientific Research and Essays 2011, 6, 98–102.
- Harborne, J.B.; Williams, C.A.Advances in Flavonoid Research Since 1992. Phytochemistry 2000, 55, 481–504.
- Andersen, Ø.M.; Jordheim, M. Anthocyanins. Encyclopedia of Life Science; John Wiley & Sons, Ltd, 2005; 597–605 pp.
- Giusti, M.M.; Wrolstad, R.E. Acylated Anthocyanins from Edible Sources and their Applications in Food Systems. Biochemical Engineering Journal 2003, 14, 217–25.
- He, F.; Liang, N-N.; Mu, L.; Pan, Q-H.; Wang, J.; Reeves, M.J.; Duan, C-Q. Anthocyanins and their Variation in Red Wines I. Monomeric Anthocyanins and their Color Expression. Molecules 2012, 17, 1571–601.
- Bakowska, A.; Kucharska, A.Z.; Oszmiański, J. The Effects of Heating, UV Irradiation, and Storage on Stability of the Anthocyanin–polyphenol Copigment Complex. Food Chemistry 2003, 81, 349–55.
- Jiménez, N.; Bohuon, P.; Lima, J.; Dornier, M.; Vaillant, F.; Pérez, A.M. Kinetics of Anthocyanin Degradation and Browning in Reconstituted Blackberry Juice Treated at High Temperatures (100−180 C). Journal of Agricultural and Food Chemistry 2010, 58, 2314–2322.
- Su, G.; Zhu, S.; Xu, M.; Ramaswamy, H.S.; Lin, Y.; Yu, Y. Pressure Degradation Kinetics of Anthocyanin Pigment and Visual Color of Chinese Bayberry Juice. International Journal of Food Properties 2016, 19, 443–453.
- Jiao, Y.; Zhang,Y.; He, Z.; Zhai, W.; Gong, H.; Yang, Z. Effect of Ferulic Acid on the Formation of Pyranoanthocyanins from Purple corn (zea mays l.) Cob in a Model System and their Effects on Color. International Journal of Food Properties 2016, 19, 847–858.
- Cevallos-Casals, B.A.; Cisneros-Zevallos, L. Stability of Anthocyanin-based Aqueous Extracts of Andean Purple Corn and Red-fleshed Sweet Potato Compared to Synthetic and Natural Colorants. Food Chemistery 2004, 86, 69–77.
- Marković, J.M.D.; Petranović, N.A.; Baranac, J.M. The Copigmentation Effect of Sinapic Acid on Malvin: A Spectroscopic Investigation on Colour Enhancement. Journal of Photochemistry and Photobiology B: Biology 2005, 78, 223–228.
- Boulton, R.: The Copigmentation of Anthocyanins and its Role in the Color of Red Wine: A Critical Review. American Journal of Enology and Viticulture 2001, 52, 67–87.
- Markovic, D. – Petranovic, N.A. – Baranac, J.M.: A Spectrophotometric Study of the Copigmentation of Malvin with Caffeic and Ferulic Acids. Journal of agricultural and food chemistry 2000, 48, 5530–5536.
- Gris, E.F.; Ferreira, E.A.; Falcão, L.D.; Bordignon‐Luiz, M.T. Influence of Ferulic Acid on Stability of Anthocyanins from Cabernet Sauvignon Grapes in a Model System and a Yogurt System. International Journal of Food Science & Technology 2007, 42, 992–998.
- Barbero, G.; Liazid, A.; Palma, M.; Barroso, C. Ultrasound-assisted Extraction of Capsaicinoids from Peppers. Talanta 2008, 75, 1332–1337.
- Skrede, G.; Wrolstad, R.; Durst, R. Changes in Anthocyanins and Polyphenolics During Juice Processing of Highbush Blueberries (Vaccinium corymbosum L.). Journal of Food Science 2000, 65, 357–364.
- Asen, S.; Stewart, R.N.; Norris, K.H. Anthocyanin, Flavonol Copigments, and pH Responsible for Larkspur Flower Color. Phytochemistery 1975, 14, 2677–2682.
- Gauche, C.; Malagoli, E.d.S.; Bordignon Luiz, M.T. Effect of pH on the Copigmentation of Anthocyanins from Cabernet Sauvignon Grape Extracts with Organic Acids. Scientia Agricola 2010, 67,41–46.
- Lambert, S.G.; Asenstorfer, R.E.; Williamson, N.M.; Iland, P.G.; Jones G.P. Copigmentation between Malvidin-3-glucoside and Some Wine Constituents and its Importance to Colour Expression in Red Wine. Food Chemistery 2011, 125, 106–15.
- Wang, W-D.; Xu, S-Y. Degradation Kinetics of Anthocyanins in Blackberry Juice and Concentrate. Journal of Food Engineering 2007, 82, 271–75.
- Wang, J.; Shen, X.; Chen, Y. Effect of pH, Temperature and Iron on the Stability of Anthocyanins from Black-Skinned Peanuts (Arachis hypogaea L.). African Journal of Agricultural Research 2013, 8, 2044–47.
- Giust, M.M.; Wrolstad, R.E. Anthocyanins characterization and measurement with UV-visible spectroscopy. In Current protocols in food analytical chemistry;. Wrolstad, R.E., Schwartz, S.J., Eds.; Wiley: New York; 2001.
- Mazza, G.; Brouillard, R. The Mechanism of Co-pigmentation of Anthocyanins in Aqueous Solutions. Phytochemistry 1990, 29, 1097–1102.
- Janna, O.; Khairul, A.; Maziah, M. Anthocyanin Stability Studies in Tibouchina Semidecandra L. Food Chemistry 2007, 101, 1640–1646.
- Parisa, S.; Reza, H.; Elham, G.; Rashid, J. Effect of Heating, UV Irradiation and pH on Stability of the Anthocyanin Copigment Complex. Pakistan Journal of Biological Sciences 2007, 10, 267–272.
- Laleh, G.; Frydoonfar, H.; Heidary, R.; Jameei, R.; Zare S. The Effect of Light, Temperature, pH and Species on Stability of Anthocyanin Pigments in four Berberis Species. Pakistan Journal of Nutrition 2006, 5, 90–92.
- Malaj, N.; De Simone, B.C.; Quartarolo, A. D.; Russo, N. Spectrophotometric Study of the Copigmentation of Malvidin 3-O-glucoside with p-coumaric, Vanillic and Syringic Acids. Food Chemistry 2013, 141, 3614–3620.
