ABSTRACT
The aim of this study was to investigate the phenolic acid and carotenoid composition of bitter melon and to evaluate its antioxidant activity. The harvest starts from 5–35 days after fertilization. With increasing maturation, gallic acid, chlorogenic acid, and catechin contents increased, while caffeic acid, p-coumaric acid, and ferulic acid contents decreased. With increasing maturation, all carotenoids contents increased. Although lycopene content was not detected for 20 days after fertilization, its content increased to 842.0 μg/g at 35 days after fertilization. Total polyphenol and flavonoid contents were increased to 639.4 and 203.3 mg/100 g after 35 days. Free radical scavenging activities increased with increasing maturation. This suggests that bitter melon is a good source of natural antioxidants and might have potential health benefits for consumers as a functional food or value-added ingredient.
Introduction
Various properties of plant products are associated with the presence, type, and contents of their phytochemicals.[Citation1] Plants are important sources of natural antioxidants and are commonly used for preparing various herbal health supplements. The search for antioxidant-rich plants or their parts is an ongoing process.
Plants contain a large variety of phenolic derivatives including simple phenols, benzoic acid derivatives, flavonoids, phenylpropanoids, tannins, lignans, and carotenoids.[Citation2] Fruits and vegetables are excellent sources of phenolics and carotenoids; these phytochemicals exhibit various health-promoting effects, such as free radical scavenging,[Citation3–Citation5] reducing blood pressure, and lowering possibilities of cancer and cardiovascular diseases.[Citation6–Citation8] Hence, these phytochemicals are significant for producers and consumers of foods. Antioxidants present in foods or in the body at low concentrations markedly delay or prevent the oxidation of a substrate. The previously mentioned health benefits ascribed to the consumption of phenolics and carotenoids are achieved by preventing lipid oxidation, protein crosslinking, DNA mutation, and tissue damage.[Citation9,Citation10] Antioxidants can be classified as free radical terminators, metal ion chelators, or oxygen scavengers that react with oxygen in closed systems. Phenolics and carotenoids act as free radical terminators.[Citation11,Citation12]
Bitter melon (Momordica charantia L.) is an important cultivated food crop that is widely used as a vegetable in Asia. Fruit pulp, seeds, and the whole plant of bitter melon have been studied for their hypoglycemic effects. Bitter melon also has other medicinal properties, such as anticarcinogenic and hypocholesterolemic effects.[Citation6–Citation8] The ethanolic extract of bitter melon fruit was non-toxic at concentrations of 10–80 µg/mL on the Hela and Siha cells.[Citation13] Also, the water and methanolic extracts did not show cytotoxicity at concentration of 1000 µg/mL against human fibrosarcoma HT 1080 cells.[Citation14] Like many plants, bitter melon may be a source of phytochemicals such as phenolics and carotenoids. Phytochemical contents change during ripening, as has been reported for the fruits of avocado, tomato, pear, apple,[Citation15] and Momordica species,[Citation16] but the extent of change depends on the plant species. Synthesis and concentration of phenolics, carotenoids, and other secondary metabolites in plant tissues are inter-dependent but detailed research is needed to conclude their relationships and the pattern of changes in compound matrix during maturation stages. Although the food value of bitter melon is realized, information on its phenolic constituents is limited; detailed investigation on bitter melon phenolics is needed to provide information regarding their nutraceutical values. The objectives of this study were to determine the total polyphenol and flavonoid contents and the composition of phenolics and carotenoids in bitter melon, and to evaluate the antioxidant properties of bitter melon fruits at different maturation stages.
Materials and methods
Chemical reagents
Analytical grade chemical reagents were used. Folin–Ciocaulteu reagent, sodium carbonate, aluminum chloride hexahydrate, sodium nitrite, sodium hydroxide, 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid; ABTS), potassium persulfate, potassium hydroxide, ascorbic acid, gallic acid, chlorogenic acid, catechin, caffeic acid, p-coumaric acid, ferulic acid, acetic acid, and triethylamine were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Methanol, acetonitrile, water, hexane, chloroform, methyl tertiary butyl ether (MTBE) were purchased from Merck (Darmstadt, Germany), and capsanthin, lutein, zeaxanthin, β-cryptoxanthin, α-carotene, β-carotene, and lycopene were obtained from Extrasynthese (Lyon, France).
Sample collection and preparation
Fresh fruits of bitter melon (Momordica charantia L.; Japan green Nakanokoya variety) were grown in the experimental field of the Farming Cooperation Hamyang (Hanmyang, Korea; 35°52’ 44” N, 127°72’ 24” E) and harvested from 5 to 35 days after fertilization at July 2015. The average mean, maximum, and minimum temperature and daily precipitation during development stages were 25.6, 30.3, and 21.5°C and 3.1 mm, respectively. The fruits containing the seeds were washed with tap water and chopped to approximately 5 mm thickness using slicer. The fruits were then freeze-dried (FD5508, Ilshin Biobase Co., Ltd., Seoul, Korea) and pulverized (Micro hammer cutter mill type-3, Culatti AG, Zurich, Switzerland) to a particle size of <180 μm. Five hundred milligrams of the fine ground bitter melon fruit were weighed into a test tube and 5 mL of 80% methanol was added. The tube was then extracted for 1 h at room temperature using the sonication bath (SD-350H, Seong Dong, Seoul, Korea). During the extraction, the dispersion was vortexed every 10 min. After extraction, the dispersion was centrifuged at 3000 rpm for 10 min. The residue was rinsed with 10 mL of 80% methanol, and the clear solution was combined with the previous supernatant. The combined methanolic extract was then evaporated to dryness under a stream of nitrogen at 60°C. The dried methanolic extract was stored at 4°C until further analysis.
Total polyphenol and flavonoid contents
The total polyphenol content of extracts were determined by using the Folin–Ciocalteu assay.[Citation17] In a 1.5 mL micro tube, 1 mL of 2% Na2CO3 solution was mixed with 50 μL of the sample. After 3 min, 50 μL of 2 N Folin–Ciocalteu’s phenol reagent was added and mixed. After exactly 30 min, the absorbance was determined at 750 nm with ultraviolet-visible (UV-Vis) spectrophotometer (UV-1650PC; Shimadzu, Kyoto, Japan). Total polyphenol contents of the extracts were expressed as mg of gallic acid equivalents (GAE) per 100 g of dry weight.
Total flavonoid contents were measured according to a modification of the method of Dewanto et al.[Citation17] An aliquot (125 μL) of extracts or standard solution of (+)-catechin was added to 1.5 mL micro tube containing 500 μL of distilled water. To the tube, 37.5 μL of 5% NaNO2 was added. After 5 min, 75 μL of 10% A1C13 was added. After 6 min, 250 μL of 1 M NaOH was added and mixed. After exactly 11 min, the absorbance was determined at 510 nm with UV-Vis spectrophotometer. Total flavonoid content was expressed as mg (+)-catechin equivalents (CE) per 100 g dry weight.
Phenolic acid composition determination
The determination of major phenolic acids in bitter melon fruit extracts with different maturation stages were carried out on a reversed-phase high-performance liquid chromatography system (Water 2695e system, Waters, USA) coupled with a photodiode array detector, binary pump, and an autosampler.[Citation18] The high-performance liquid chromatography (HPLC) column was a Mightysil RP-18 (250 × 4.6 mm, 5 μm, Kanto Co., Ltd., Kyoto, Japan). The mobile phase consisted of A (0.1% acetic acid in acetonitrile) and B (0.1% acetic acid in water). A gradient established in our laboratory was applied as follows: 0–2 min, 8–10% A; 2–27 min, 10–30% A; 27–50 min, 30–90% A; 50–51 min, 90–100% A; 51–60 min, 100% A; 60–61 min, 100–108% A; 61–70 min, 100–108% A. The injection volume of sample was 20 μL and the flow rate was 1.0 mL/min. The monitor wavelength was set at 280 nm. Before analysis, all samples were filtered through a 0.25 μm membrane filter (Millipore, Billerica, MA, USA). The phenolic acid constituents of the sample were identified by their retention times, while quantification was made by comparing the area ([mAU × s]) of each corresponding peak of the sample and the calibration curves of the standards that were obtained from five varying concentrations.
Carotenoid composition determination
The extraction and determination of carotenoids were measured according to a modification of the method of Tuan et al.[Citation19] Five hundred milligrams of the finely ground bitter melon fruit were weighed into a test tube and 20 mL of ethanol containing 0.2% ascorbic acid (w/v) was added. This mixture was vortexed for 30 s, and then incubated in a water bath at 80°C for 15 min. Subsequently, 5 mL of potassium hydroxide (80%, w/v) was added to saponify any potentially interfering oils. After vortexing and incubating at 80°C for 10 min, the samples were placed on ice and 5 mL of water and 1 mL of β-Apo-8’-carotenal (25 ppm), an internal standard, were added. Next, the carotenoids were extracted three times with 5 mL of hexane and centrifuged at 1800 rpm for 10 min each time to separate the layers. Then, the extracts were evaporated under a stream of nitrogen gas and resuspended in 50:50 (v/v) MTBE/methanol. For HPLC analysis, the carotenoids were separated on a water 2695e HPLC system coupled with a photodiode array detector, binary pump, and an autosampler. The HPLC column was a YMC carotenoid (250 × 4.6 mm, 5 μm, YMC Co., Ltd., Tokyo, Japan). The mobile phase consisted of methanol, MTBE, water and triethylamine. The ratio of mobile phase A and B was 6:90:4:0.1 and 81:15:4:0.1, respectively. A gradient established in our laboratory was applied as follows: 0–35 min, 0% A; 35–40 min, 0–50% A; 40–45 min, 50–100% A; 45–50 min, 100–100% A; 50–55 min, 0% A; 55–60 min, 0% A. The injection volume of sample was 20 μL and the flow rate was 1.0 mL/min. The monitor wavelength was set at 450 nm. Before analysis, all samples were filtered through a 0.2 μm PVDF membrane filter (Millipore, Billerica, MA, USA). Samples were quantified by comparing the retention times with the known authentic standards.
Antioxidant activities
The antioxidant activity was assessed quantitatively using the DPPH method.[Citation20] The solution of DPPH (0.1 mM) was prepared with ethanol. The DPPH radical solution was diluted with ethanol to obtain an absorbance of 1.4–1.5 at 520 nm. About 0.2 mL of extract solution or standard solution of L-ascorbic acid was added to ethanolic solution of DPPH. The mixture was shaken vigorously and then kept at room temperature for 30 min under the dark for protection from light. The absorbance was determined at 520 nm using UV-Vis Spectrophotometer. DPPH radical scavenging activity was expressed as mg of L-ascorbic acid equivalents per gram of dry weight.
The ABTS radical scavenging activity assay was carried out using a modified method by Lee et al.[Citation20] Briefly, ABTS was dissolved in distilled water to 7 mM concentration. The ABTS stock solution was then obtained by mixing 7 mmol/L of ABTS water solution with 2.45 mmol/L of potassium persulfate solution and leaving it to stand in the dark at room temperature for 12 h before use. The ABTS radical solution was diluted with distilled water and adjusted to an absorbance of 1.500 at 735 nm. The assay was performed using 96-well microplates. The extract (0.05 mL) was added to 1 mL of the ABTS radical solution for use in each reaction. The absorbance was measured at 735 nm. The ABTS antioxidant activity was expressed as mg of L-ascorbic acid equivalents per gram of dry weight.
Statistical analysis
Sampling was conducted at once and then extracted and analyzed at three times. The results were reported as mean ± standard deviation (n = 3 or 30). The significance of differences among the maturation stage means were determined using one-way analysis of variance (ANOVA), calculated by SPSS version 12 (SPSS Institute, Chicago, IL, USA), with a significance level of p < 0.05 by Duncan’s multiple range test.
Results and discussion
Morphological characterization
The morphological characteristics such as length, weight and color of bitter melon fruits at different maturation stages showed in . The average length and weight of bitter melon fruit were significantly increased (p < 0.05) from 6.60 cm and 15.24 g to 18.60 cm and 87.24 g at 20 day after fertilization; however, there are no significant differences among the 25–35 day after fertilization (p > 0.05). The color of bitter melon fruits changed from dark green to light green and yellow green during the maturation stages.
Table 1. Morphological characteristics of bitter melon (Momordica charantia L.) at different maturation stages.
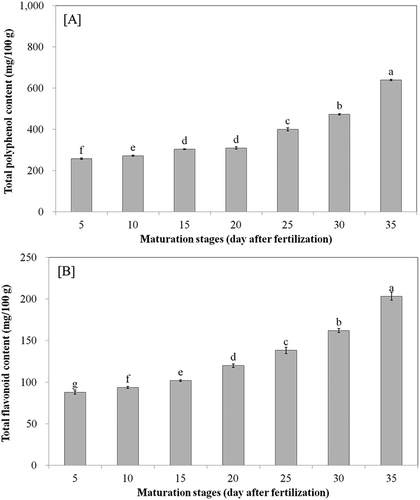
Total polyphenol and flavonoid content
Phenolics including polyphenols and flavonoids have received considerable attention because of their physiological activities like antioxidant, antimutagenic, antidiabetic, and anticancer activities.[Citation3] The total polyphenol and flavonoid contents of bitter melon fruits at different maturation stages are shown in and , respectively. The total polyphenol and flavonoid contents of bitter melon were 257.06 mg GAE/100 g (dry material) and 87.70 mg CE/100 g at 5 days after fertilization, respectively. However, these values were significantly increased to 639.37 mg GAE/100 g and 203.31 mg CE/100 g at 35 days after fertilization, respectively (p < 0.05). Kubola and Siriamompun reported that the total phenolic contents (TPCs) of the leaf, stem, unripe fruit, and ripened fruit of bitter melon were 474, 259, 224, and 324 mg GAE/100 g dry material, respectively.[Citation3] Sirikhwan reported that the total phenolic and flavonoid contents of unripe gac fruit (M. cochinchinesis Spreng) were 117 mg GAB/100 g and 47 mg CE/100 g, respectively; however, these values increased to 518 mg GAE/100 g and 230 CE/100 g of ripened gac fruit, respectively.[Citation21] This difference, even within the same variety, depends on several factors like environmental factors, maturity, location, and soil condition.[Citation1] Higher TPCs of ripened bitter melon fruit extracts than unripened bitter melon fruit extracts could be due to biosynthesis of phenolic compounds caused by enzyme hydrolysis during maturation.[Citation3]
Phenolic acid composition
The changes in phenolic acid composition of bitter melon fruit extracts at different maturation stages are shown in . The major phenolic acids are gallic acid, chlorogenic acid, catechin, caffeic acid, p-coumaric acid, and ferulic acid. Gallic acid, chlorogenic acid, and catechin contents of bitter melon increased from 92.25 to 425.98 µg/g, from 12.26 to 17.35 µg/g, and from 35.26 to 48.68 µg/g of dry material, respectively, upon significant maturation (p < 0.05). However, caffeic acid, p-coumaric acid, and ferulic acid contents of bitter melon significantly decreased from 32.45 to 12.13 µg/g, from 2.76 to 0.17 µg/g, and from 4.57 to 1.28 µg/g of dry material with increasing maturation (p < 0.05). Horax et al.[Citation1] reported that the phenolic acids, including gallic acid, protocatechuic acid, gentisic acid, catechin, vanillic acid, chlorogenic acid, syringic acid, epicatechin, p-coumaric acid, ferulic acid, sinapic acid, benzoic acid, and cinnamic acid, were detected in bitter melon fruit and the major phenolic acids were gallic acid (80.4–397.6 µg/g), gentisic acid (169.9–323.9 µg/g), catechin (230.6–824.5 µg/g), chlorogenic acid (45.5–158.3 µg/g), and epicatechin (161.4–442.8 µg/g). Kubola and Siriamompun reported that the gallic acid, tannic acid, and catechin contents increased from 95.6, 1.08, and 3.95 to 202.0, 1.41, and 4.54 mg/L upon ripening; however, caffeic acid and p-coumaric acid contents decreased from 3.35 and 0.56 to 1.62 and 0.16 mg/L.[Citation3] Phenolic acids having a wide range of structures and molecular weights are widely distributed throughout the plant kingdom. They have various physiological activities like antioxidant, anticancer, antidiabetic, and antibacterial effects through a combination of hydroxyl group with protein and macromolecules.[Citation22] The previously mentioned results indicate that phenolic acid compositions in bitter melon varied widely depending on the type of phenolic acid and the maturation stage of bitter melon fruit. The differences among the types of phenolic acids could be due to the variability of each type against environmental stresses that can induce an increase in phenolic acid contents as a natural survival instinct of the plants. Variation in phenolic acid contents among different maturation stages could be caused by different biochemical mechanisms in their synthesis during maturation.[Citation1]
Table 2. Phenolic acids composition of bitter melon (Momordica charantia L.) at different maturation stages.
Carotenoid composition
The changes in carotenoid composition of bitter melon fruit extracts at different maturation stages are shown in . The detected carotenoids include capxanthin, lutein, zeaxanthin, β-cryptoxanthin, lycopene, α-carotene, and β-carotene. With increasing maturation, all carotenoid contents increased up to 20 days after fertilization. Especially lycopene, which was not detected for 20 days after fertilization; however, the lycopene content increased to 842.00 μg/g at 35 days. Rodriguez et al.[Citation23] reported that the β-carotene, lycopene, lutein, and zeaxanthin were detected in unripe green bitter melon seed and lycopene content increased from 11.8 to 261.0 μg/g of fully ripe bitter melon seed. Singh et al.[Citation24] reported that the lycopene and total carotenoid content of bitter melon increased with increasing maturation; however, β-carotene content decreased. In many studies, lycopene content in Momordica spp. fruits have been reported as 1800–6200,[Citation16] 408,[Citation25] and 2073 μg/g.[Citation26] The variation between reported values and present findings could be due to different species or genotypes and different maturation stage of the fruit.[Citation9] Vuong et al.[Citation27] reported wide ranges of lycopene (424.6–705.0 μg/g) and total carotenoid contents (406.0–784.0 μg/g) in gac fruits. Further, degradation, inter-conversion, or isomerization of carotenoids during fruit transportation, extraction, analysis, and storage might have also contributed to the variation in lycopene and carotenoid contents because these compounds are sensitive to light and heat.[Citation27] The exponential increase in lycopene and total carotenoid contents was in contrast to the decline in β-carotene content; the extent of change, however, depends on the species.
Table 3. Carotenoids composition of bitter melon (Momordica charantia L.) at different maturation stages.
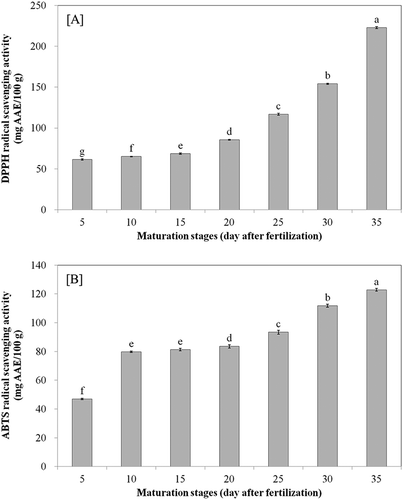
Antioxidant activities
The DPPH and ABTS free radical scavenging activities of bitter melon fruits at different maturation stages are shown in and , respectively. The DPPH and ABTS free radical scavenging activities of bitter melon are 61.57 mg AAE/100 g (dry material) and 46.99 mg AAE/100 g, respectively, at 5 days after fertilization. However, their activities significantly increased to 222.86 mg AAE/100 g and 122.87 mg AAE/100 g, respectively (p < 0.05), at 35 days after fertilization. Kubola and Siriamompun[Citation3] reported that the DPPH and hydroxyl radical scavenging activities of bitter melon increased from 29.3 to 53.9% and from 26.1 to 74.2% upon ripening. Moreover, there is strong correlation between TPC-DPPH (0.711; p < 0.01) and TPC-hydroxyl (0.884; p < 0.01). In the present study, TPC, total flavonoid content (TFC), total phenolic acid content (TPAC), and total carotenoid content (TCC) showed strong positive correlation with DPPH and ABTS. Especially, TPC-DPPH (0.996; p < 0.001) has the highest positive correlation (). In general, the phenolic contents were positively correlated with antioxidant activities because of their hydrogen-donating abilities. The strong positive linear correlations between free radical scavenging activities and polyphenolic concentrations in various food materials are well-known.[Citation1,Citation3] These reports suggest that the radical scavenging capacities of extracts are mostly influenced by the presence and position of the phenolic hydroxyl groups. The radical scavenging activities of phenolic compounds depend on their molecular structures, i.e., on the availability of phenolic hydrogen and the possibility of stabilizing the phenoxyl radicals formed by hydrogen donation.
Table 4. Correlation coefficients among total polyphenol content (TPC); total flavonoid content (TFC); total phenolic acid content (TPAC); total carotenoid content (TCC); DPPH radical scavenging activity (DPPH); and ABTS radical scavenging activity (ABTS) of bitter melon (Momordica charantia L.) at different maturation stages
Conclusion
The main contribution of this study is that it provides quantitative information on the variation in the contents of important phytochemicals like phenolic acids and carotenoids at different stages of maturation of bitter melon and also correlates these concentration data with the antioxidant activities of bitter melon fruit. This contribution is theoretically and practically relevant because the study suggests that bitter melon is a good source of natural antioxidants and might have potential health benefits as a functional food or value-added ingredient.
Funding
This study was carried out with the support of “Cooperative Research Program for Agricultural Science & Technology Development (Project No. PJ010003),” Rural Development Administration, Republic of Korea.
Additional information
Funding
References
- Horax, R.; Hetttiarachchy, N.; Islam, S. Total Phenolic Contents and Phenolic Acid Constituents in 4 Varieties of Bitter Melons (Momordica Charantia) and Antioxidant Activities of Their Extracts. Journal of Food Science 2005, 70, 275–280.
- Friedman, M. Chemistry, Biochemistry, and Dietary Role of Potato Polyphenols. A Review. Journal of Agricultural Food and Chemistry 1997, 45, 1423–1540.
- Kubola, J.; Siriamornpun, S. Phenolic Contents and Antioxidant Activities of Bitter Gourd (Momordica Charantia L.) Leaf, Stem and Fruit Fraction Extracts in Vitro. Food Chemistry 2008, 110, 881–890.
- Kenny, O.; Smyth, T.J.; Hewage, C.M.; Brunton, N.P. Antioxidant Properties and Quantitative UPLC-MS Analysis of Phenolic Compounds from Extracts of Fenugreek (Trigonella Foenum-Graecum) Seeds and Bitter Melon (Momordica Charantia) Fruit. Food Chemistry 2013, 141, 4295–4302.
- Choi, J.S.; Kim, H.Y.; Seo, W.T.; Lee, J.H.; Cho, K.M. Roasting Enhances Antioxidant Effect of Bitter Melon (Momordica Charantia L.) Increasing in Flavan-3-Ol and Phenolic Acid Contents. Food Science and Biotechnology 2012, 21, 19–26.
- Grover, J.K.; Yadav, S.P. Pharmacological Actions and Potential Uses of Momordica Charantia: A Review. Journal of Ethnopharmacology 2004, 93, 123–132.
- Joseph, B.; Jini, D. Antidiabetic Effects of Momordica Charantia (Bitter Melon) and Its Medicinal Potency. Asian Pacific Journal of Tropical Disease 2013, 3, 93–102.
- Nagarani, G.; Abirami, A.; Siddhuraju, P. Food Prospects and Nutraceutical Attributes of Momordica Species: A Potential Tropical Bioresources—A Review. Food Science and Human Wellness 2014, 3, 117–126.
- Aoki, H.; Kieu, M.T.N.; Kuze, N.; Tomisaka, K.; Chuyen, V.N. Carotenoid Pigments in Gac Fruit (Momordica Cochinchinenensis Spreng). Bioscience, Biotechnology, and Biochemistry 2002, 66, 2479–2482.
- Evans, P.; Helliwell, B. Micronutrients: Oxidant/Antioxidant Status. British Journal of Nutrition 2001, 85, S67–S74.
- Krinsky, N.I.; Johnson, E.J. Carotenoid Actions and Their Relation to Health and Disease. Molecular Aspects of Medicine 2005, 26, 459–516.
- Fraser, P. D.; Bramley, P. M. The biosynthesis and nutritional uses of carotenoids. Progress in Lipid Research 2004, 43, 228–265.
- Fongmoon, D.; Lalitwongsa, S.; Keyoonwong, W.; Nakong, M.; Iamsaard, S. Antioxidant Activity and Cytotoxicity of Bitter Melon (Momordica Charantia L.) Extract Cultured in Lampang Thailand. NU Science Journal 2013, 10, 18–25.
- Lu, Y.L.; Liu, Y.H.; Liang, W.L.; Chyuan, J.H.; Cheng, K.T.; Liang, H.J.; Hou, W.C. Antibacterial and Cytotoxic Activities of Different Wild Bitter Gourd Cultivars (Momordica Charantia L. Var. Abbreviate Seringe). Bontanical Studies 2011, 52, 427–434.
- Speirs, J.; Brady, C.J. Modification of Gene Expression in Ripening Fruit. Australian Journal of Plant Physiology 1991, 18, 519–532.
- Kubola, J.; Siriamornpun, S. Phytochemicals and Antioxidant Activity of Different Fruit Fractions (Peel Pulp, Aril and Seed) of Thai Gac (Momordica Cochinchinensis Spreng). Food Chemistry 2011, 127, 1138–1145.
- Dewanto, V.; Xianzhong, W.; Liu, R.H. Processed Sweet Corn Has Higher Antioxidant Activity. Journal of Agricultural Food and Chemistry 2002, 50, 4959–4964.
- Xu, J.G.; Tian, C.R.; Hu, Q.P.; Luo, J.Y.; Wang, X.D.; Tian, X.D. Dynamic Changes in Phenolic Compounds and Antioxidant Activity in Oats (Avena Nuda L.) During Steeping And Germination. Journal of Agricultural Food and Chemistry 2009, 57, 10392–10398.
- Tuan, P.A.; Kim, J.K.; Park, N.I.; Lee, S.Y.; Park, S.U. Carotenoid Content and Expression of Phytoene Synthase and Phytoene Desaurase Genes in Bitter Melon (Momordica Charantia). Food Chemistry 2011, 126, 1686–1692.
- Lee, Y.R.; Woo, K.S.; Kim, K.J.; Son, J.R.; Jeong, H.S. Antioxidant Activities of Ethanol Extracts from Germinated Specialty Rough Rice. Food Science and Biotechnology 2007, 16, 765–770.
- Sirikhwan, T. Comparison of Antioxidant and Antimicrobial Activities of Unripe and Ripe Fruit Extracts of Momordica Cochinchinesis Spreng (Gac Fruit). International Journal of Pharmaceutical Sciences Review and Research 2014, 28, 75–82.
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant Properties of Phenolic Compounds. Trends in Plant Science 1997, 2, 152–159.
- Rodriguez, D.B.; Lee, T.C.; Chichester, C.O. Comparative Study of the Carotenoid Composition of the Seeds of Ripening Momordica Charantia and Tomatoes. Plant Physiology 1975, 56, 626–629.
- Singh, S.; Swain, S.; Nisha, M.; Banu, V.S.; Singh, D.R.; Roy, S.D. Changes in Lycopene, Total Carotenoid and Anti-Radical Activity in Teasel Gourd (Momordica Subangulata ssp. Renigera [G. Don] de Wilde) Fruit Fractions at Different Stages of Maturity. Industrial Crops and Products 2015, 73, 154–163.
- Vuong, L.T.; Franke, A.A.; Custer, L.J.; Murphy, S.P. Momordica Cochinchinensis Spreng. (Gac) Fruit Carotenoids Reevaluated. Journal of Food Composition and Analysis 2005, 19, 664–668.
- Ishida, K.B.; Turner, C.; Chapman, H.M.; Mckeon, A.T. Fatty Acid and Carotenoid Composition of Gac (Momordica Cochinchinensis Spreng) Fruit. Journal of Agricultural Food and Chemistry 2004, 52, 274–279.
- Vuong, L.T.; Franke, A.A.; Custer, L.J.; Murphy, S.P. Momordica Cochinchinensis Spreng. (Gac) Fruit Carotenoids Reevaluated. Journal of Food Composition and Analysis 2006, 19, 664–668.