ABSTRACT
The effect of sprouting on the instrumental texture, antioxidant activity, and flavonoid profile of the paste prepared from four Indian onion varieties (Punjab White, Punjab Naroya, PRO-6, and Commercial) was studied. The significant (P ˂ 0.05) effect of sprouting on microstructure, firmness, adhesiveness, gumminess, and chewiness in paste samples from all varieties was observed. There was significant decrease (P ˂ 0.05) in lightness with consequent significant (P ˂ 0.05) increase in redness, greenness, and yellowness in paste samples which was due to the increase in anthocyanin content with sprouting. The paste samples from sprouted onion varieties also showed an increase in phenol content, flavonoid content, antioxidant capacity, and DPPH radical scavenging activity. The HPLC analysis revealed an increase in total flavonoids in pastes from PRO-6 and Punjab Naroya varieties. Thus, present study implied that sprouting could be beneficial as it enhanced the functional potential of onion pastes.
Introduction
Onion (Allium cepa L.) crop of commercial importance in India is characterised by strong aroma and flavour which makes it an essential ingredient in almost all the food preparations in various processed forms such as essential oils or oleoresins, paste, powder, juice, and flakes.[Citation1] Onion of particular origin has specific nutritional profile which consists of protein, carbohydrate, fat, fibre, ash content, ascorbic acid, flavonoid compounds that determine its functional potential in food and therapeutic applications.[Citation2] Onion is an abundant source of phenols, flavonols (quercetin, isorhamnetin, kaempferol, and their glycosides) and anthocyanins (cyanidin and peonin) and their concentration depends upon the geographical location, cultivar, climatic conditions, and storage conditions.[Citation3] Several onion varieties in India have been characterized for their chemical composition and antioxidant properties.[Citation1,Citation4]
In recent years, increasing attention has been paid to the role of bio-molecules which possess antioxidant activity that plays a potential role in human health and are associated with reduced risk of a number of chronic diseases. The major antioxidants of vegetables are vitamins C and E, carotenoids, and phenolic compounds, especially flavonoids.[Citation5,Citation6] These days, consumers also demand mildly processed or treated vegetables without compromising their nutritional value and enhancing quality in terms of texture, flavour, and taste. These considerations have made characterization as a key element in the food industry, since it provides information about the enhancement of quality parameters. Sprouted vegetables contain significantly greater concentrations of bioactive compounds (flavonoids, polyphenols, glucosinolates, and anthocyanins) than mature plants.[Citation7]
Onion crop like every other agricultural commodity is required to be stored properly to prolong its availability with minimum qualitative and quantitative losses.[Citation8] The optimum conditions for storage of onion bulbs are 30°C–35°C temperature and 65%–70% relative humidity under ambient condition. The storage of onion bulbs in cold store at optimal storage temperature of 0°C with 65%–70% relative humidity extends shelf life of onion bulbs and reduces post-harvest storage losses.[Citation9] The onion bulb as a living system is a natural food store for the plant that undergoes a natural biological process of sprouting which is a major contributor of the total post-harvest storage losses.[Citation8] It has been reported that 50%–90% storage losses occur depending upon genotype and storage conditions. Out of total storage losses, 20%–40% comprised sprouting in case of onions. On the other hand, sprouting has been widely used as an inexpensive, fruitful, and easy mechanism convenient for enhancing the nutritional and functional potential of cereals, pseudo cereals, cruciferous, and legumes.[Citation10] Sprouting was found to be associated with the development of phytochemical components, viz. glucosinolates, natural antioxidants that play significant role in prevention of cancer and thus can be utilized for the development of functional foods.[Citation11]
As compared with other vegetables (broccoli, cauliflower, kale, radish),[Citation12–Citation14] sprouting of onions has received little attention. We have recently reported[Citation15] that sprouting in onions led to increase in protein content, ascorbic acid content, anthocyanins content, total phenol content, total flavonoid content, and antioxidant activity. Similarly, in other studies it has been reported that sprouted onion samples were found to contain high level of phenolics,[Citation7] total quercetin,[Citation8] and quercetin-4′-O-monoglucoside.[Citation16] It is clear from aforementioned findings that sprouting of onion is beneficial in terms of functional properties, but no study has ever been carried out to explore the enhancement of functional potential with sprouting in paste from the four onion varieties of Indian origin.
Materials and methods
Chemicals, raw material, and storage conditions
The chemicals Folin Ciocalteau reagent, gallic acid, and sodium carbonate were purchased from Loba Chemie Pvt. Ltd., Mumbai; 2,2-diphenyl picryl hydrazyl (DPPH) was purchased from Fluka Goldie, Mumbai; acetone and methanol (HPLC grade) were purchased from Ranbaxy, New Delhi; and reference standards quercetin, myricetin, Kaempferol, quercetin 3′-glucoside (3,3′,4′,5,7-pentahydroxyflavone 3-β-glucoside), and quercetin 4′-glucoside (3,3′,4′,5,7-pentahydroxyflavone 4′-glucoside) were from Acros Organics, NJ, USA.
Three Rabi season onion varieties (Punjab Naroya, Punjab White, and PRO-6) () were procured from Department of Vegetable Science, P.A.U., Ludhiana, India, which were sown in January and harvested in month of May 2014. Onion bulbs (150) of each variety were subsequently stored in jute bags under dark storage conditions for a period of 7 months till December in order to break dormancy at an average temperature of 26.3°C and average relative humidity of 64.3%, and after that they were moved to ambient conditions for sprouting. The average light intensity of 64 Lumen, average relative humidity of 80.2%, average temperature of 15.22°C was observed for 3 weeks and after 3 weeks the onions attained the sprout length in the range of 7–10 cm. The varieties free of any microbial growth were subsequently utilized for the preparation of onion paste and analysed for physico-chemical, textural, antioxidant properties, and flavonoid profile. One commercial variety () bought from local market was also stored, utilized, and analysed under similar conditions.
Preparation of paste
Raw and sprouted onion bulbs (varieties: Punjab Naroya, Punjab White, PRO-6, and commercial) were peeled, cut, and immediately processed into puree using a laboratory size grinder (Morphy Richards, Swinton, UK). The purees were passed through a 14-mesh sieve to obtain the product of uniform consistency and held at room temperature (25°C ± 1°C) for 1 h in a covered container in order to facilitate enzymatic action for colour and flavour development. The pastes from raw and sprouted onion varieties were prepared by adding 10% common salt (sodium chloride) to the purees in order to increase their total soluble solids (TSS). The pH of the pastes was adjusted by adding desired quantity of 0.3% citric acid solution which acts as an antioxidant, and an acidified food (pH < 4.6) requires only mild heat treatment to become shelf stable.[Citation17]
Physico-chemical properties
Total soluble solids (°Brix) were determined using a hand-held digital refractometer (Erma Hand Refractometer, Erma, Inc., Tokyo, Japan) at 20°C and total solids using a vacuum oven at 70°C to constant weight.[Citation18] pH is regarded as an important index for onion paste, since it could be an indirect means to demonstrate internal changes of the product such as microbial activations. This index was measured by a digital pH metre (Eutech Instruments Pvt. Ltd., Singapore) at 25°C. Before the experiment, the pH metre was calibrated with commercial buffer solutions at pH 7.0 and 4.0. The per cent titratable acidity was measured in terms of citric acid by titrating the diluted paste against 0.1 N NaOH solution using phenolphthalein indicator.[Citation19] Sodium chloride was determined by titration with silver nitrate.[Citation19] Water activity (aw) of the product was determined using water activity meter (Aqualab CX2T, Decagon Devices, USA) according to the method of AOAC.[Citation20]
Particle size distribution measurement
The particle size distribution (PSD) was measured using a laser light diffraction particle size analyser (Shimadzu SALD-2300, M/s. Shimadzu Corporation, Kyoto, Japan). Each sample was run in duplicate. In the instrument, laser light (wavelength 720 nm) was scattered by the suspended particles and the generated diffraction pattern (a composite of the diffraction patterns for all particles) was measured. The instrument converted this composite diffraction pattern into particle size distributions. The percentage number of particles with different sizes was evaluated and the median, mean, and modal was calculated as given by the software.[Citation18]
Microstructural study
The changes in cell wall material distribution and form were studied using light microscopy (Olympus CX21i, Japan) with a magnification of about 100×, in at least six pictures for each sample.[Citation18]
Instrumental textural measurement
Textural measurements of the paste samples were performed at 25°C through a back extrusion technique using the texture analyser (Stable Micro Systems, Surrey, UK). The tests were performed at a pre-test speed of 2.0 mm/s, a test speed of 1.0 mm/s, and a post-test speed of 5.0 mm/s with a target mode strain 70.0% at a high calibration of 100 mm. The samples were carefully scooped into cylindrical containers to minimize the presence of air pockets. The depth of the samples in the cylinder was 70 mm and the probe was calibrated to a starting distance 30 mm above the top of the sample surface. The probe, which was positioned centrally over the sample container, gradually moved through the paste while readings were taken by the sensors.[Citation21]
Colour evaluation
Colour of both raw and sprouted paste samples was determined using a colorimeter (Model CR-10, Konica Minolta Sensing, Inc., Japan) equipped with D 65 illuminant. Three colour values, namely lightness (L*), redness/greenness (a*), and blueness/yellowness (b*) values were noted.
Phytochemical analysis
Sample extraction
Paste sample (0.5 g) was weighed and centrifuged for 15 min at 5000 rpm at 4°C. The supernatant was separated and stored at 20°C for further studies.
Determination of total flavonoid content
To 1 ml of methanolic extract, 0.5 ml of 2% w/v AlCl3 in methanol, and 0.5 ml potassium acetate (120 mM) were added and incubated at room temperature for 30 min. Absorbance was read at 415 nm using UV spectrophotometer (HACH DR 6000, Dusseldorf, Germany). Quercetin was used as a standard and the results were expressed as milligrams of quercetin equivalents per gram of fresh weight sample.[Citation22]
Determination of total phenolics
Total phenolics were determined by the method described by Sobhi et al.[Citation23] using gallic acid as a standard and results were expressed as milligrams of gallic acid equivalents per gram of fresh weight of sample.
Total antioxidant capacity
Total antioxidant capacity determination is based on the reduction of Mo (VI) to Mo (V) by the extract, and subsequent formation of a green phosphate–Mo (V) complex at acidic pH. The total antioxidant capacity was expressed based on GAE as described by Ruanma et al.[Citation24]
DPPH radical scavenging activity
The percentage of DPPH scavenging activity was determined as follows:
DPPH radical scavenging activity (%) = [(A0 − A1)/A0], where A0 is the absorbance of control and A1 is the absorbance of sample.[Citation15]
High-performance liquid chromatography (HPLC) analysis for flavonoids
From onion paste samples (2.5 g) flavonoids were extracted using 60% ethanol (10 ml) for 24 h at room temperature in the dark. The solution was filtered through a 0.45 µm PTFE membrane filter and was kept in the refrigerator until the chromatographic analysis. The flavonoid contents of the paste samples were analysed according to a modified method by Lee et al.[Citation25] Analyses of the extracts were carried out by HPLC with 1290 infinity LC system equipped with a quaternary pump (G4204A) and UV absorbance detector. The separation was performed by using Phenomenex Luna C18 100 Å column (150 × 4.6 mm) with a particle size of 5 µm. The mobile phase consisted of the following linear gradient of acetonitrile (A) and 0.5% formic acid in water (B): 0–10 min, 20% B; 10–15 min, 20%–80% B; 15–22 min, 80%–20% B. Detection was set to 360 nm for flavonoids with flow rate of 0.8 ml/min at 30°C and the injection volume was 20 µl. Identification of flavonoids was carried out by comparing their retention time and spectral characteristics of unknown analytes with those of the reference standards.
Statistical analysis
The analysis was carried out in four replicates for all determinations and the average values were used in the data analysis. A two-way analysis of variance (ANOVA) was carried out to assess the significant main effects for variety and sprouting on various analysed parameters. Duncan’s test was further used to estimate least significant range between means by using Statistica, version 12 (StatSoft India Pvt. Ltd., New Delhi, India).
Results and discussion
Two-way ANOVA of data revealed significant varietal differences (P ˂ 0.05) in paste samples for all the determined parameters except per cent acidity, moisture content, water activity (aw), and salt content. The significant differences (P ˂ 0.05) due to sprouting on onion pastes were also found for all the determined parameters except per cent acidity, moisture content, and salt content in all the varieties.
Physico-chemical properties
The physico-chemical characteristics of paste samples from raw and sprouted Punjab White, Punjab Naroya, PRO-6, and commercial variety are summarized in . The pH value and per cent acidity of paste samples from raw onion varieties did not vary significantly (P > 0.05). However, paste samples from sprouted onion varieties showed significant differences (P ˂ 0.05) in pH value and per cent acidity. It was reported that there was increase in ascorbic acid content with sprouting in onion varieties which led to increase in per cent acidity and subsequent decrease in pH of pastes from sprouted onion varieties.[Citation15]
Table 1. Effect of sprouting on physic-chemical composition of paste of four onion varieties.
TSS (°B) content significantly (P ˂ 0.05) varied in paste samples from raw onion varieties except paste from Punjab White and PRO-6 varieties. Sprouting led to decrease in total soluble solids in all the four onion varieties, and significant differences (P ˂ 0.05) were observed in paste samples from Punjab White and Punjab Naroya variety. In the study of Chope et al.,[Citation26] rapid utilization of soluble sugars during sprouting was found to decrease TSS concentration in cv. SS1 onion variety of United Kingdom origin. Total solids in paste samples from raw onion variety were found to be statistically (P ˂ 0.05) similar in Punjab White and PRO-6 varieties and also in pastes from Punjab Naroya and commercial varieties. Paste from sprouted onion varieties showed statistically significant differences (P ˂ 0.05) except in paste samples from Punjab White and PRO-6 varieties. TSS in paste samples from all sprouted onion varieties was less than the pastes from corresponding raw onion varieties. According to Zeilenski,[Citation27] the decrease in total solid content could be explained by the synergistic effect of increase in moisture content and utilization of constituents during sprouting.[Citation28]
Salt content (NaCl), water activity, and moisture content showed significant differences (P < 0.05) in pastes from both raw and sprouted onion varieties. Pastes from sprouted onion varieties possessed higher moisture and water activity in contrast to pastes from raw onion varieties. Khalil et al.[Citation29] reported an increase in moisture content due to sprouting in chick pea cultivars, and the increased moisture content was due to absorption of water by the samples from surroundings in order to continue their metabolic processes.[Citation30]
Colour evaluation
Colour analysis revealed significant differences (P ˂ 0.05) in all the three colour values (L*, a*, b*) in pastes from both raw and sprouted onion varieties. From the results () it is clear that sprouting resulted in decrease in L* value (lightness), increase in a* value (green/red) and b* value (yellow). Paredes-lopez and Mora-escobedo[Citation31] have investigated the effect of sprouting on colour values in amaranth seeds and mentioned that sprouting was found to decrease lightness and enhance the red and yellow colours.
Table 2. Effect of sprouting on colour and particle size of paste of four onion varieties.
In the present study, sprouting led to significant decrease (P ˂ 0.05) in lightness in all the varieties except commercial variety, with significant (P ˂ 0.05) enhancement in greenness in Punjab White and redness in Punjab Naroya, PRO-6, and commercial varieties. The increase in b* value, that is pronounced yellowness with sprouting, was also observed with significant increase (P ˂ 0.05) in case of Punjab Naroya and PRO-6 varieties. Majid et al.[Citation15] indicated that decrease in lightness and consequent increase in redness, greenness, and yellowness with sprouting might be attributed to increased anthocyanin content with sprouting in onion varieties.
Particle size distributions
The mean, median, mode, and diameter on cumulative (10% and 90%) of paste samples for both raw and sprouted onion varieties are listed in . Median, mean, and cumulative diameter (10% and 90%) of pastes from raw onion varieties did not vary significantly (P > 0.05) except in the case of paste from raw Punjab Naroya variety. Similarly, the modal diameter did not show significant difference (P > 0.05) in pastes from both raw and sprouted onion varieties. Mean, median, and modal diameter of paste samples from sprouted onion varieties did not significantly (P > 0.05) vary except median in sprouted Punjab White variety. The cumulative 10% diameter of paste samples from sprouted Punjab White and Punjab Naroya onion varieties significantly (P ˂ 0.05) varied, and cumulative 90% diameter did not show significant differences in paste samples from Punjab White, Punjab Naroya, and commercial varieties. From the results of particle size distributions (), it is clear that sprouting led to decrease in particle size in all the four onion varieties, and significant (P ˂ 0.05) decrease was observed in median of Punjab White variety and modal of commercial variety. The cumulative 10% diameter showed significant (P ˂ 0.05) decrease in Punjab Naroya variety, and cumulative 90% diameter also was found to decrease significantly (P ˂ 0.05) in all the varieties except PRO-6 variety. Since in the present study the only difference between the pastes developed from onion varieties is due to the treatment of sprouting, so it can be inferred that sprouting resulted in structural breakdown in onions that resulted in production of smaller particles under similar processing conditions. The results were consistent with those reported on soy butter prepared from sprouted soya beans which was found to have smaller values of particle size diameter and volume than butter prepared from raw soya beans.[Citation32]
Microstructural study
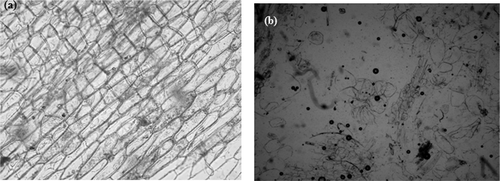
In representative microscopic pictures depict the structure of the pastes from raw and sprouted Punjab White variety. (a) revealed that the structure of paste from raw Punjab White variety consisted mostly of whole cells with apparently intact cell walls. (b) showed that the paste from sprouted Punjab White variety consisted of only few intact cells, with several broken cells and cell wall material suspended in an aqueous media. This implied that significant changes were induced in the structure due to sprouting which resulted in fewer small-sized whole cells and consequently decrease in the particle size of pastes from sprouted onion varieties. Yan et al.[Citation33] reported that during sprouting the intrinsic enzymes (e.g., proteases, amylases, and lipases) are activated that act on protein and cell walls resulting in its breakdown and disruption of whole cells.
Instrumental textural measurement
The textural properties () of pastes developed from raw onion varieties varied significantly (P ˂ 0.05) except firmness in Punjab White and commercial varieties, adhesiveness in PRO-6 and commercial varieties, and springiness in Punjab Naroya and commercial varieties. The textural properties (firmness, adhesiveness, gumminess, and chewiness) of pastes developed from sprouted onion varieties showed significant difference (P ˂ 0.05). However, cohesiveness and springiness did not show significant difference (P > 0.05) except in paste from sprouted Punjab White variety. It is clear from the results () that sprouting led to significant (P ˂ 0.05) effect on the textural properties of pastes except cohesiveness in paste from PRO-6 variety and springiness in pastes from Punjab Naroya and commercial varieties. These results were similar to the findings of Murugkar,[Citation34] who reported decrease in firmness and increase in cohesiveness, springiness, and chewiness in tofu prepared from sprouted soya bean. In another study, it has been reported that noodles prepared from sprouted buckwheat were less firm than those prepared from raw buckwheat, and the decrease in firmness was attributed to the action of various degrading enzymes which get activated by sprouting.[Citation35]
Table 3. Effect of sprouting on phenolic content, antioxidant activity, and textural characteristics of paste of four onion varieties.
Total phenol
From the raw onion varieties, paste from PRO-6 and Punjab Naroya varieties possessed statistically (P ˂ 0.05) highest content of total phenol content followed by paste from commercial and Punjab White varieties. Pastes from sprouted onion varieties showed significant (P ˂ 0.05) differences and higher content of total phenol content than the pastes from their corresponding raw onion varieties (). Results were well supported by previous studies which showed increase in total phenol content with sprouting in onion.[Citation36] Tian et al.[Citation37] reported that during sprouting product moisture increases which increases the susceptibility for injury by oxidation and as a result of which hydrolytic enzymes produce phenolic compounds having more antioxidant activity. Increased total phenol content can also be explained by the action of endogenous esterases activated during sprouting which releases cell wall–bound phenolic compounds.[Citation38]
Total flavonoid
Total flavonoid content was found to vary significantly (P ˂ 0.05) in pastes from raw onion varieties and reported to be highest in PRO-6 variety. For sprouted onion varieties, pastes from Punjab White, Punjab Naroya, and commercial varieties had similar content of total flavonoids with PRO-6 variety, which has the highest total flavonoid content. The significant increase (P ˂ 0.05) in total flavonoid content with sprouting was observed in pastes from Punjab White and Punjab Naroya varieties. Sharma et al.[Citation36] studied the effect of sprouting in onion and reported increase in total flavonoid content. The flavonoid biosynthesis during sprouting follows the pathway similar to that followed in anthocyanin biosynthesis that converts precursor l-phenylalanine (l-Phe) to dihydrokaempferol, and this biosynthesis is regulated by several environmental factors, such as light, temperature, and internal factors including plant hormones, nutrients, other secondary metabolites, and multiple regulatory genes.[Citation39]
Total antioxidant capacity
Total antioxidant capacity in pastes from raw onion varieties was found to differ significantly (P ˂ 0.05) with highest in PRO-6 variety (7.14 mg GAE/g). The pastes from sprouted onion varieties Punjab Naroya and PRO-6 significantly varied (P ˂ 0.05) in their total antioxidant capacity with similar content of total antioxidant capacity in paste from Punjab White and commercial varieties. From the results, it is evident that total antioxidant capacity was higher in pastes from sprouted onion varieties with significant increase (P ˂ 0.05) in Punjab White variety (). The higher total antioxidant capacity can be ascribed to the increased total phenol and total flavonoid content as reported earlier due to sprouting and heating process involved in paste making. Further, Randhir et al.[Citation40] reported that thermal processing of sprouted wheat resulted in increase in the total phenol content thereby increasing the total antioxidant capacity.
DPPH radical scavenging activity
DPPH radical scavenging activity of pastes from raw onion varieties varied significantly (P ˂ 0.05) with highest activity in PRO-6 variety followed by Punjab Naroya, commercial, and Punjab White. The pastes from sprouted onion varieties followed the same trend in their radical scavenging activity (). Current research results were consistent with results reported in Brassica oleracea varieties.[Citation41] Majid et al.[Citation15] found similar trend of increase in DPPH radical scavenging activity with sprouting and increased antioxidant activity among one of the metabolic change during sprouting is believed to occur as a result of increased activity of the endogenous hydrolytic enzymes.[Citation42]
However, in the present study the greater increase in radical scavenging activity in pastes from both raw and sprouted onion varieties might be due to release of free aglycones or generation of Maillard products as a result of heat processing which act as reducing agents and thus serve as electron donor or transfer a hydrogen atom to the DPPH radical, thus increasing the scavenging-linked antioxidant activity.[Citation24]
Flavonoid profile
The flavonoid profile of pastes from raw and sprouted onion varieties (Punjab White, Punjab Naroya, PRO-6, and commercial) are presented in , and the sample HPLC chromatograms for pastes from raw and sprouted Punjab Naroya variety are presented in and 3(b), respectively. Statistically significant (P < 0.05) irregular trend of increase and decrease in flavonoid profile was observed in specific flavonol components in pastes from raw and sprouted onion varieties (). The irregular trend of increase and decrease in flavonoid components due to sprouting was consistent with the studies reported in mung bean, radish, broccoli, and sunflower sprouts.[Citation43] Quercetin and kaempferol content was found to be maximum in pastes from both raw and sprouted PRO-6 variety. The increase in quercetin and kaempferol in case of PRO-6 variety was well supported by increased content of these flavonols (quercetin and kaempferol) in germinated kidney beans.[Citation44] In all the four onion varieties, except Punjab White, sprouting was observed to lead to significant (P < 0.05) increase in quercetin content. These results about increase in quercetin content were similar to the findings of Sharma et al.[Citation36]
Table 4. Effect of sprouting on flavonoid profile of paste of four onion varieties (mg/kg).
Figure 3. The typical representation of HPLC chromatograms obtained for: (a) Punjab Naroya raw onion variety; (b) Punjab Naroya sprouted onion variety (1: quercetin-3-glucoside; 2: quercetin-4-glucoside; 3: quercetin; 4: myricetin; 5: kaempferol).
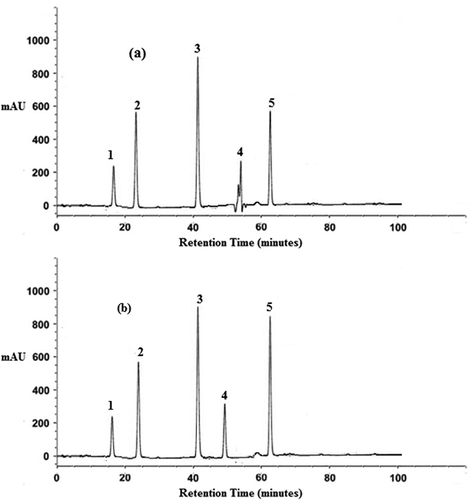
Myricetin content was found to increase with sprouting in pastes from Punjab Naroya and PRO-6 varieties with significant (P < 0.05) increase in Punjab Naroya variety. Sprouting decreased the myricetin content in pastes from Punjab White and commercial variety but this decrease was not statistically significant (P > 0.05) (). Quercetin-3′-glucoside (3,3′,4′,5,7-pentahydroxyflavone 3-β-glucoside) for raw paste samples was highest in PRO-6 variety followed by Punjab Naroya, commercial, and Punjab White varieties. There was an increase in quercetin-3′-glucoside concentration with sprouting in pastes from PRO-6 and commercial variety with significant (P < 0.05) increase in paste from PRO-6 variety, while it decreased in pastes from Punjab White and Punjab Naroya. Quercetin-4′-glucoside (3,3′,4′,5,7-pentahydroxyflavone 4′-glucoside) for raw paste samples was also highest in PRO-6 variety and with sprouting it increased in pastes from Punjab Naroya and PRO-6 varieties. However, a decrease was found with sprouting in pastes from Punjab White and commercial with significant (P < 0.05) decrease in Punjab White variety (). Increase in quercetin-4′-glucoside (3,3′,4′,5,7-pentahydroxyflavone 4′-glucoside) concentration with sprouting in onion was also reported by Sharma et al.[Citation16] Similarly, Albishi et al.[Citation45] reported that sprouted onion possessed higher concentrations of both quercetin and quercetin glucoside as compared with the non-sprouted onion samples and also showed that high level of antioxidant activity in onions was attributed to their flavonoid constituents, namely quercetin, kaempferol, and myricetin.
Total flavonoids detected in all raw and sprouted paste samples significantly (P < 0.05) varied and sprouting significantly increased total flavonoids in pastes from Punjab Naroya and PRO-6 variety. It is evident from results () that with sprouting there was significant (P < 0.05) decrease in total flavonoids in paste from Punjab White and non-significant (P > 0.05) decrease in total flavonoids in paste from commercial onion variety. In the aforementioned findings, irregular pattern of flavonol changes was observed in paste samples as the content of various flavonoid components varied in consistently between varieties due to the process of sprouting. The current study implied that onion varieties behaved differently due to probable varietal difference in metabolism of onion bulbs.[Citation25]
Conclusion
It can be concluded that sprouting led to an enhancement in functional potential of onion pastes which was due to an increase in total phenol content, total flavonoid content, and anti-oxidant activity. The sprouting also resulted in reduction in particle size and decrease in firmness in pastes from all the four Indian onion varieties. Sprouting can thus be a fruitful way to cater the need of substituting synthetic compounds by using sprouted vegetables as natural additives in the present functional food market.
Acknowledgment
The authors thank the Department of Vegetable Science, Punjab Agricultural University, Ludhiana, for providing onion varieties.
Funding
UGC, New Delhi, provided the financial assistance in the form of MANF-2014-15.
Additional information
Funding
References
- Bhattacharjee, S.; Sultana, A.; Sazzad, M.H.; Islam, M.A.; Ahtashom, M.M.; Asaduzzaman. Analysis of the Proximate Composition and Energy Values of Two Varieties of Onion (Allium cepa L.) Bulbs of Different Origin: A Comparative Study. International Journal of Nutrition and Food Science 2013, 2, 246–253.
- Eleazu, C.O.; Ikpeama, A.I.; Amajor, J.U.; Eleazu, K.C. Proximate Composition, Essential Oils and Energy Value of 10 New Varieties of Ginger (zingiber officinale roscoe). International Journal of Biology Pharmacy and Allied Science 2012, 1, 1293–1303.
- Ramos, F.A.; Takaishi, Y.; Shirotori, M.; Kawaguchi, Y.; Kawaguchi, Y.; Tsuchiya, K.; Shibata, H.; Higuti, T.; Tadokoro, T.; Takeuchi, M. Antibacterial and Antioxidant Activities of Quercetin Oxidation Products from Yellow Onion (Allium cepa) Skin. Journal of Agricultural and Food Chemistry 2006, 54, 3551–3557.
- Ashwini, M.; Balaganesh, J.; Balamurugan, S.; Murugan, S.B.; Sathishkumar, R. Antioxidant Activity in Vivo and in Vitro Cultures of Onion Varieties (Bellary and CO 3). Food and Nutrition Science 2013, 4, 918–923.
- Duthie, S.J.; Jenkinson, A.; McE.; Crozier, A.; Mullen, W.; Pirie, L.; Kyle, J. The Effects of Cranberry Juice Consumption on Antioxidant Status and Biomarkers Relating to Heart Disease and Cancer in Healthy Human Volunteers. European Journal of Nutrition 2006, 45, 113–122.
- Singh, J.; Rai, M.; Upadhyay, A.K.; Bahadur, A.; Chaurasia, S.N.S.; Singh, K.P. Antioxidant Phytochemicals in Broccoli (Brassica oleracea L. var. italica Plenck) Cultivars. Journal of Food Science and Technology 2006, 43, 391–393.
- Hanlon, P.R.; Barnes, D.M. Phytochemical Composition and Biological Activity of 8 Varieties of Radish (Raphanus sativus L.) Sprouts and Mature Taproots. Journal of Food Science 2011, 76, 185–192.
- National Bank for Agriculture and Rural development. 2016. Onion storage structures. https://www.nabard.org/english/onion.aspx. (accessed March 14, 2016).
- ICAR-Directorate of Onion and Garlic Research. 2016. Storage. http://www.dogr.res.in/index.php?option=com ( accessed September 19 2016).
- Swieca, M.; Gawlik-Dziki, U. Effects of Sprouting and Postharvest Storage Under Cool Temperature Conditions on Starch Content and Antioxidant Capacity of Green Pea, Lentil and Young Mung Bean Sprouts. Food Chemistry 2015, 185, 99–105.
- Sangronis, E.; Machado, C.J. Influence of Germination on the Nutritional Quality of Phaseolus vulgaris and Cajanus Cajan. LWT - Food Science and Technology 2007, 40, 116–120.
- Abadias, M.; Usall J.; Anguera, M.; Solsona, C.; Vinas, I. Microbiological Quality of Fresh, Minimally-Processed Fruit and Vegetables and Sprouts from Retail Establishments. International Journal of Food Microbiology 2008, 123, 121–129.
- Tournas, V.H. Moulds and Yeasts in Fresh and Minimally Processed Vegetables, and Sprouts. International Journal of Food Microbiology 2005, 99, 71–77.
- Matera, R.; Gabbanini, S.; Berretti, S.; Amorati, R.; De Nicola, G.R.; Iori, R.; Valgimigli, Luca. Acylated Anthocyanins from Sprouts of Raphanus Sativus cv. Sango: Isolation, Structure Elucidation and Antioxidant Activity. Food Chemistry 2015, 166, 397–406.
- Majid, I.; Dhatt, A.S.; Sharma, S.; Nayik, G.A.; Nanda, V. Effect of Sprouting on Physicochemical, Antioxidant and Flavonoid Profile of Onion Varieties. International Journal of Food Science & Technology 2016, 51, 317–324.
- Sharma, K.; Assefa, A.D.; Ko, E.Y.; Lee, E.T.; Park, S.W. Quantitative Analysis of Flavonoids, Sugars, Phenylalanine and Tryptophan in Onion Scales During Storage Under Ambient Conditions. Journal of Food Science and Technology 2015, 52, 2157–2165.
- Ahmed, J.; Shivhare, U.S. Thermal Kinetics of Colour Degradation and Storage Characteristics of Onion Paste. LWT - Food Science and Technology 2001, 34, 380–383
- Bayoda, E.; Pilman, E.; Willers, Tornberg, E. Rheological and Structural Characterization of Tomato Paste and Its Influence on the Quality of Ketchup. LWT - Food Science and Technology 2008, 41, 1289–1300.
- Rangana, S. Handbook of Analysis and Quality Control for Fruit and Vegetable Products; New Delhi: Tata McGraw-Hill Publishers, 1986.
- AOAC. Official Methods of Analysis; Association of Official Analytical Chemists: Washington, D.C., U.S.A, 2003.
- Sobhi, B.; Adzahan, N.M.; Shamsudin, R.; AbKarim, S.; Rahman, R.A.; Bakar, J.; Ghazali, Z. Development of Instrumental Methods for Textural Evaluation of Chili Paste. Kasetsart Journal-Natural Science 2013, 47, 122–131.
- Chanda, S.; Dave, R. In Vitro Models for Antioxidant Activity Evaluation and Some Medicinal Plants Possessing Antioxidant Properties: An Overview. African Journal of Microbiology Research 2009, 3, 981–996.
- Sobhi, B.; Noranizan, M.; Ab Karim, S.; Abdul Rahman, R.; Bakar, J.; Ghazali, Z. Microbial and Quality Attributes of Thermally Processed Chili Shrimp Paste. International Food Research Journal 2012, 19, 1705–1712.
- Ruanma, K.; Shank, L.; Chairote, G. Phenolic Content and Antioxidant Properties of Green Chilli Paste and its Ingredients. Maejo International Journal of Science and Technology 2010, 4, 193–200.
- Lee, S.U.; Lee, J.H.; Choi, S.H.; Lee, J.S.; Ohnisi- Kameyama, M.; Kozukue, N.; Levin, C.E.; Friedman, M. Flavonoid Content in Fresh, Home-Processed, and Light- Exposed Onions and in Dehydrated Commercial Onion Products. Journal of Agriculture and Food Chemistry 2008, 56, 8541–8548.
- Chope, G.A.; Terry, L.A.; White, P.J. Effect of Controlled Atmosphere Storage on Abscisic Acid Concentration and Other Biochemical Attributes of Onion Bulbs. Postharvest Biology and Technology 2006, 39, 233–242.
- Zeilenski, H. Contribution of Low Molecular Weight Antioxidants to the Antioxidant Screen of Germinated Soybean Seeds. Plant Foods for Human Nutrition 2003, 58, 1–20.
- Sowokinos, J.R. Stress Induced Alteration in Carbohydrate Metabolism. In The Molecular and Cellular Biol. Potato; Vayda, M.E., Park, W.D., Eds.; CAB International: Wallingford, UK, 1990; 137–158.
- Khalil, A.W.; Zebb, A.; Mahmood, F.; Tariq, S.; Khattak, A.B.; Shaha, H. Comparison of Sprout Quality Characteristics of Desi and Kabuli Type Chickpea Cultivars (Cicer arietinum L.). LWT-Food Science & Technology 2007, 40, 937–945.
- Khatoon, N.; Prakash, J. Nutrient Retention in Microwave Cooked Germinated Legumes. Food Chemistry 2006, 97, 115–121.
- Paredes-lopez, O.; Mora-escobedo, R. Germination of Amaranth Seeds: Effects on Nutrient Composition and Color. Journal of Food Science 1989, 54, 761–762.
- Agrahar-Murugkar, D.; Kotwaliwale, N.; Kumar, M.; Gupta, C. Effect of Sprouting on Rheological Properties of Soy-Butter. LWT - Food Science and Technology 2013, 54, 95–100.
- Yan, S.; Wu, X.; Dahlberg, J.; Bean, S.R.; MacRitchie, F.; Wilson, J.D.; Wang, D. Properties of Field-Sprouted Sorghum and Its Performance in Ethanol Production. Journal of Cereal Science 2010, 51, 374–380.
- Murugkar, D.A. Effect of Sprouting of Soybean on the Chemical Composition and Quality of Soymilk and Tofu. Journal of Food Science and Technology 2014, 51, 915–921.
- Hara, T.; Sasaki, T.; Tetsuka, T.; Ikoma, H.; Kohyama, K. Effects of Sprouting on Texture of Cooked Buckwheat (Fagopyrum esculentum Moench) Noodles. Plant Production Science 2009, 12, 492–496.
- Sharma, K.; Assefa, A.D.; Kim, S.; Ko, E.Y.; Park, S.W. Change in Chemical Composition of Onion (Allium cepa L. cv. Sunpower) During Post-Storage Under Ambient Conditions. New Zealand Journal of Crop and Horticultural Science 2014, 42, 87–98.
- Tian, S.; Nakamura, K.; Kayahara, H. Analysis of Phenolic Compounds in White Rice, Brown Rice, and Germinated Brown Rice. Journal of Agricultural and Food Chemistry 2004, 52, 4808–4813.
- Maillard, M.N.; Soum, M.H.; Boivin, P.; Berset, C. Antioxidant Activity of Barley and Malt: Relationship with Phenolic Content. LWT - Food Science and Technology 1996, 29, 238–244.
- Mol, J.; Jenkins, G.; Schafer, E.; Weiss, D. Signal Perception, Transduction, and Gene Expression Involved in Anthocyanin Biosynthesis. Critical Reviews in Plant Sciences 1996, 15, 525–557.
- Randhir, R.; Kwon, Y.N.; Shetty, K. Effect of Thermal Processing on Phenolics, Antioxidant Activity and Health-Relevant Functionality of Select Grain Sprouts and Seedlings. Innovative Food Science and Emerging Technologies 2007, 9, 355–364.
- Vale, A.P.; Cidade, H.; Pinto, M.; Beatriz, M.; Oliveira, M.B. Effect of Sprouting and Light Cycle on Antioxidant Activity of Brassica oleracea Varieties. Food Chemistry 2014, 165, 379–387.
- Chavan, J.K.; Kadam, S.S. Nutritional Improvement of Cereals by Sprouting. Critical Reviews in Food Science and Nutrition 1989, 28, 401–437.
- Pajak, P.; Socha, R.; Galkowska, D.; Roznowski, J.; Fortuna, T. Phenolic Profile and Antioxidant Activity in Selected Seeds and Sprouts. Food Chemistry 2014, 143, 300–306.
- Duenas, M.; Martinez-Villaluenga, C.; Limon, R.I.; Penas, E.; Frias. J. Effect of Germination and Elicitation on Phenolic Composition and Bioactivity of Kidney Beans. Food Research International 2015, 70, 55–63.
- Albishi, T.; John, J.A.; Al-Khalifa, A.S.; Shahidi, F. Antioxidative Phenolic Constituents of Skins of Onion Varieties and their Activities. Journal of Functional Foods, 2013, 5, 1191–1203.