ABSTRACT
The aim of the study was to determine the effects of adding of coriander extracts to turkey meatballs. Meat samples were stored in modified atmosphere at 4°C ± 1°C for 9 days. The addition of coriander extract at 500 ppm level delayed the process of lipid oxidation for the period of 6 days of storage and growth inhibition of aerobic microorganisms for the period of 9 days of storage. The usage of a dose of 500 ppm as well as a dose of 200 ppm had no significant effect on the sensory features, but had an impact on colour parameters of the meatballs. In the groups with coriander extract, volatile terpenes were identified.
Introduction
Currently, in the food industry synthetic antioxidants, such as butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA), tertiary butyl hydroquinone, and propyl gallate (PG), have been widely used in meat and poultry products to reduce lipid peroxidation and consequently reduce the negative impact of primary and secondary oxidation components on food quality and stability.[Citation1,Citation2] Although synthetic antioxidants can quickly inhibit lipid oxidation, they may have carcinogenic potential.[Citation3,Citation4] Therefore, the recent trend is to substitute synthetic antioxidants by natural compounds, mainly using antioxidants from plant extracts because of concerns about negative consumer perception and the long-term safety of synthetic antioxidants.[Citation5] Natural antioxidants extracted from plants can be used as an alternative to the synthetic antioxidants because of their equivalence or greater effect on inhibition of lipid oxidation and haem pigment (nitrosohemachrome) protection.[Citation6] The most common antioxidants used in the chilled and frozen meat products are herbs and spices. Spices can be added to the meat in fresh or dried form or in aqueous or alcoholic extracts. The addition of rosemary, thyme, sage, oregano, pepper, black pepper, turmeric, basil, and dill to meat products may also improve their sensory characteristics and extend shelf life.[Citation7] The antioxidant properties of spices are mainly evident due to phenolic compounds, such as phenolic acids, phenolic diterpenes, and flavonoids.[Citation8] Antioxidants prevent the formation of reactive oxygen form, the binding of free radicals, quenching of the singlet oxygen as well as chelate the transition of metal ions such as iron, and demonstrate anticancer, anti-arteriosclerotic, and anti-inflammatory properties.[Citation4,Citation9]
Along with the antioxidant properties, herbs and spices contribute to the colour enhancement of meat, not only by inhibiting degradation of haem pigment and malondialdehyde (MDA), but also by delaying processes of metmyoglobin formation and decreasing processes of protein oxidation. On the other hand, the addition of extracts can change the sensory attributes, especially colour of meat, within a range not acceptable to the consumers.[Citation10] This is particularly relevant to poultry meat, which is prone to colour, aroma, and other sensory attributes changes. Due to the positive effect on oxidation as well as the negative effect on the sensory acceptability of meat, the addition of natural extract should be well-balanced.
Many of works have already been done on plant products as natural antioxidants in meat and meat products. These antioxidants have been extracted from different plant parts, such as leaves, roots, fruits, seeds, and barks.[Citation11] There are a lot of publications concerning the antioxidant effects of Labiatae family herb extracts, especially that of sage, rosemary, thyme, and oregano.[Citation7,Citation12] However, less attention has been given to the use of coriander (Coriandrum sativum L.) as a source of natural antioxidants added to refrigerated packed meat,[Citation9] in spite of the fact that antioxidant effects of coriander extracts on the lipid oxidation in pork meat were higher than those of sage, rosemary, and thyme extracts. Coriander is an annual plant from the Apiaceae (Umbelliferae) family and may be a promising source of natural antioxidants, mainly because many plants from this family have a high number of polyphenols in their composition.[Citation13] Furthermore, they also constitute antibacterial, antiviral, antifungal, anti-inflammatory, antidiarrheal, and antiulcer pharmacologicals. Coriandrum sativum is a good source of polar secondary plant metabolites, i.e. polyphenols are known as compounds reducing the oxidizing processes of lipids degradation.[Citation14] It has also been shown that coriander seed oil has strong antimicrobial properties against Gram-negative Campylobacter jejuni, which is a common pathogen in food products.[Citation15] According to Dimić et al.[Citation16] coriander extract also has inhibitory activity towards five moulds. It completely inhibited Aspergillus parasiticus, Cladosporium cladosporioides, Eurotium herbariorum, Penicillium chrysogenum, and Aspergillus carbonarius. The aim of this study was to determine the effects of adding different levels of aqueous solution of coriander extracts (Coriandrum sativum L.) on the quality of turkey meatballs packed in a modified atmosphere (80% O2, 20% CO2) and stored under refrigerated conditions (4°C ± 1°C) over a period of 9 days.
Materials and methods
Materials and meat sample preparation
Fresh poultry meat from turkey breast of normal pH values (5.5–5.8) was obtained from a farm situated in the north-east part of Poland by the company Indykpol S.A. The meat was transported to the laboratory in ice boxes, under chilled conditions (4°C ± 1°C) on the 3rd day after slaughter. Natural powder extract of coriander seeds (Coriandrum sativum L.) was purchased from the company Firmenish (Geneva, Switzerland). The meat was ground (mincer – model PI-22-TU-T EDESA, Czosnow, Poland), using an 8-mm plate and divided randomly into three portions. Each portion was mixed with NaCl (0.5%). The treatments were: 200 ppm of coriander extracts (G1 group), 500 ppm of coriander extracts (G2 group), and a control group established by adding only salt (C group). Next, meatballs consisting of 20 ± 1 g of meat were formed. All meatballs in pairs were packed in a modified atmosphere (80% O2 and 20% CO2). Oxygen-permeable polyvinyl chloride film (Cryovac, Fresh Food Packaging) was used in packaging. All samples were kept in the refrigerator (4°C ± 1°C) and analysed on the 1st, 3rd, 6th, and 9th days of storage.
Evaluation of natural extract
The total phenolic acid estimation was carried out according to the Arnov method.[Citation17] The absorbance was measured by UV-Visible Spectrophotometer Hitachi U-2900 (Japan) at 490 nm. The total phenolic acid content was expressed as caffeic acid equivalent. The total flavonoid content in the investigated extracts was determined spectrophotometrically according to Polish Pharmacopoeia,[Citation18] using a method based on the formation of complex flavonoid-aluminium with the maximum absorptivity at 430 nm. The flavonoids content was expressed as a log of quercetin equivalents per gram of dried extract (de), by using a standard graph. All measurements were carried out in five repetitions. Free radical scavenging effects of the extracts on 2,2-diphenyl-1-picrylhidrazyl (DPPH) were estimated according to the method of Chen and Ho.[Citation19] Absorbance of the resulting solutions was measured spectrophotometrically by UV-Visible Spectrophotometer Hitachi U-2900 (Japan) at 517 nm.
Evaluation of meat samples
pH and chemical composition
The pH value was measured using the potentiometric method with a handheld pH meter (Model 205, Testo AG, Lenzkirch, Germany). The pH meter was calibrated using two buffer solutions (pH = 4.0 and pH = 7.0). The measurements were performed in triplicate. Near infrared spectroscopic method (NIR) analysis was performed using an NIRFlex Solids N-500 spectrophotometer (Büchi Labortechnik Ltd, Germany) to examine the chemical composition of samples. Results were expressed as percentage (protein, fat, water, connective tissue, and ash). Samples (100 g) were homogenized (Büchi B-400 homogenizer), placed into a Petri plate, and scanned in a triplicate. The measurements were performed in triplicate.
Measurement of the colour
The measurement of the colour of turkey was performed in the CIE L*a*b* system (L*– lightness, a* – colour axis ranging from greenness (−a*) to redness (+a*), b* – colour axis ranging from blueness (−b*) to yellowness (+b*)) using a Minolta chromameter (CR-400, Konica Minolta Inc., Tokyo, Japan). The measuring head with a diameter of 8 mm, a D65 illuminant, and a standard 2° observer was used. The chromameter was calibrated using a white standard plate (L* = 98.45, a* = −0.10, b* = −0.13). The evaluation of colour parameters was carried out by measuring 10 different places on the each sample. ∆E was calculated by the following equation:
∆E indicates the degree of overall colour change in comparison to colour values of the control group. Colour parameters were measured on the 1st, 3rd, 6th, and 9th days of storage.
Measurement of lipid oxidation (thiobarbituric acid reactive substances)
Secondary lipid oxidation products from raw meat were determined according to the method described by Robles-Martinez et al.[Citation20] with modification in Brodowska et al.[Citation21] Briefly, ground meat (2.5 g) was mixed and homogenized with 25 ml of trichloroacetic acid solution and 1.25 ml of antioxidant (0.5% PG and ethylenediaminetetraacetic acid in ethyl alcohol/water 1:1) for 30 s at 1200 rpm (WT 500 homogenizer, Wiggenhauser, Germany). After being centrifuged for 10 min at 8000 rpm (centrifuge MPW-251, MPW Med. Instruments, Warsaw, Poland), 5 ml of 2-thiobarbituric acid (0.02 mM/l) was added to 5 ml of supernatant. Then samples were heated in a water bath (90°C) for 40 min to develop the pink colour. Subsequently, samples were cooled in an ice bath. The absorbance was measured at 532 nm, against a blank, using a UV-VIS spectrophotometer (Shimazu UV-1800, Tokyo, Japan). A calibration curve was evaluated with 1.1.3.3-tetramethoxypropane. The results were expressed as mg of MDA/kg meat. The measurements were performed in triplicate.
Volatile organic compounds profile analysis
Analysis was performed with an electronic nose Heracles II Analyzer (Alpha M.O.S., France) based on the ultra-fast gas chromatography with headspace and with autosampler HS 100. The system was equipped with the trap to pre-concentrations of light volatiles like the Solid Phase Microextraction (SPME), two different polarity columns DB-5 and DB-1701 (10 m × 0.18 mm × 0.4 µm), and two flame ionization detectors (FIDs). The main volatile compounds were investigated using their Kovats retention indexes calculated on the basis of retention indexes and retention times of alkanes blend standard C6-C16 (Restek) analysed under the same conditions as the experiment. Compounds were recognized by the AroChemBase library, which was systematically updated with retention indexes based on the standards. All tested samples (2.5 g each) were put into 20 ml vials. The vials were closed with a cap with a silicon/teflon septum. Vials with tested samples were incubated in a temperature of 55°C for 10 min. The injection volume was 3500 µl with a speed of 125 µl/s. Analyses were divided and simultaneously analysed in two columns; cross identifications were then possible. Temperature conditions of the analysis: trapping 40°C, temperature programme 40°C by 5 s, increase 4°C/s, isotherm 270°C by 30 s, FID1/FID2 270°C, injector 200°C. Samples were analysed in three repetitions on 1st, 3rd, 6th, and 9th days of storage.
Microbiological analysis
The analysis was performed by Sanitary–Epidemiological Station in Warsaw according to PN-EN ISO 4833-1 recommendations.[Citation22] An amount of 1 ml of the appropriate 10-fold dilution was placed in two plates of L-agar with 0.1% glucose. The total number of aerobic microorganisms was expressed as CFU (colony-forming unit) per gram of turkey meat from at least three experiments.
Sensory analysis
The sensory characteristics of roasted meatballs were assessed by a trained panel of 10 members using quantitative-descriptive analysis method (QDA) [Citation23] for different attributes. Panelists were trained and had participated in sensory evaluation for many years. The panelists assessed the samples using a 7-point scale. The sensory scores ranged from 0 (the lowest intensity) to 7 (the highest intensity). Panelists were given apple juice with water (1:5) to rinse their mouth between samples. Seven sensory traits of cooked turkey meatballs, such as off-odour, herbal odour, tenderness, juiciness, salty taste, herbal taste, and overall acceptability, were assessed.
Sensory evaluation was conducted in a laboratory environment. This was required in order to obtain accurate, repeatable, and reliable results. The roasting process of turkey breast meatballs was conducted in a convection-steam oven (model CPE 110, Küppersbuch, Großkuchentechnik, Galsenkirchen, Germany) at a constant temperature of 180°C for a period of 20 min. The coded samples were served to panelists at approximately 60°C on trays in accordance with the ISO-6658.[Citation24]
Statistical analysis
All experiments were carried out in triplicate (except for colour, which was carried out in 10 replicates) and average values with standard deviation were reported. The normality of data distribution was tested using the Shapiro–Wilk test. The statistical differences were determined by one-way ANOVA and Tukey’s post hoc test. The significance level was determined as α = 0.05. All analyses were performed using Statistica Software version 8.0 (StatSoft, Tulsa, Oklahoma, USA).
Results and discussion
Antioxidant potential of coriander extract
In the study, the presence of phytochemicals in dry extracts from seeds is essential. The content of phenolic compounds in total stood at 38.24 mg of gallic acid (GAE)/100 g dry weight (dw) extract, phenolic acids 9.72 mg GAE/100 g dw extract, flavonoids 18.01 mg GAE/100 g dw extract, and the amount of tannins was equal to 0.58%. The potent radical scavenging activity (DPPH) was 19.31%. Following the conducted analysis of secondary metabolites contents, it is possible to conclude that the antioxidant activity of dry extracts is in fact modified by the number of flavonoid compounds. This was also confirmed by other authors.[Citation25,Citation26] According to Jia et al.[Citation27], in coriander, DPPH radical scavenging activity was strongly correlated with the content of caffeic acids. The spice extract samples that had a high antioxidant capacity have shown a tendency for high phenolic content. The same situation occurred with the total of antioxidant capacity. However, some previous researchers reported that spices from the Apiacae family have a strong antioxidant effect, but they did not compare with spices from other families.[Citation28] According to Shan et al.,[Citation29] Apiacae spices extracts contained more flavonoids than Labiatae spices; however, their total antioxidant capacity and total phenolic content were significantly lower than those of the Labiatae. It was due to the fact that the spice extracts in the Apiacae were found to be lacking rosmarinic acid with very powerful antioxidant activity, which was present in significant amounts in Labiatae spices. Coriander extract also contained other substances, e.g. a very high concentration of protocatechuic acid and caffeic acid, responsible for a high antioxidant potential.[Citation29]
Chemical analysis, pH, basic chemical composition, and thiobarbituric acid reactive substances
There were no statistically significant differences in pH values between the analysed samples (). However, some changes were stated during the storage time. In all the analysed samples, pH values increased during the storage and statistically significant differences were noted for coriander 200 ppm on the third day of storage. Our results are supported by the research of Selani et al.,[Citation30] who also found no changes in the pH values of raw meat containing antioxidant substances such as BHT and sodium erythorbate as well as antioxidant substances derived from grape seed and peel extract.
Table 1. The mean and standard deviation of pH of turkey samples enhanced with different antioxidant solutions depending on the storage time.
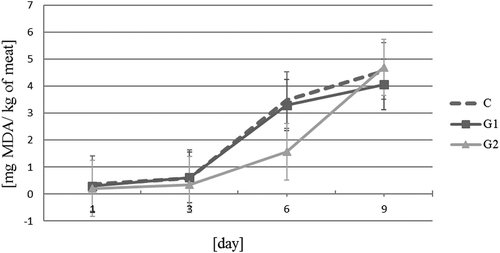
No significant changes in basic composition during 9 days of storage were observed. On the 1st day of storage the percentage content of the basic chemical composition of turkey meat was as follows: moisture – 77.03 ± 0.21; fat – 1.98 ± 0.49; protein – 19.03 ± 0.23; ash – 1.61 ± 0.03, and connective tissue – 1.46 ± 0.45. Thiobarbituric acid reactive substances (TBARS) values of all samples increased during the storage (p < 0.05). represents the effect of the addition of spice extracts to the raw turkey meat on the lipid oxidation in the period of 9 days of storage. Between groups there was no difference for certain days except of day 6. A significant effect of the addition of 500 ppm coriander (G2) to turkey meat on the decrease in the level of TBARS was stated only on the 6th day. The effect of coriander on lipid oxidation of meat can be the result of the initial antioxidant potential of coriander extracts, resulting from the presence of phenolic, flavonoids, or the absence of other substances, e.g., rosmarinic acid. Tanabe et al.[Citation9] demonstrated that a higher addition of coriander liquid extracts, (0.002 and 0.005 ml in 0.2 ml pork homogenate) containing oleoresins, to homogenized pork meat was effective in inhibiting lipid oxidation and reducing TBARS values. Darughe et al. (2012) studied the antioxidant effects of the coriander essential oils (CEO) in cake and found that the antioxidant effect of CEO might also be due to the presence of terpenoid components (camphor, limonene, α-pinene, and geraniol). CEO at a percentage of 0.05, 0.10, and 0.15 are very effective in preventing the formation of primary and secondary oxidation products. It was found that at the proportion of 0.02%, the effect of coriander oils was almost equal to synthetic BHA.[Citation5] According to Bhat et al.,[Citation31] coriander is a good source of polyphenols and phytochemicals with a high antioxidant activity. The antioxidant potential of phenolic compounds is associated with the hydroxyl group linked to the aromatic ring, which is capable of donating hydrogen atoms with electrons and neutralizing free radicals. This mechanism may decrease oxidation stress and blocks lipid oxidation.[Citation32]
Effect of coriander extract on instrumental colour value
The changes in the instrumental colour values of turkey meatballs during the storage are shown in . No significant differences in the case of L*colour parameters were observed between treatments on the 1st and 3rd days of storage. The value of L* parameter on the 9th day of storage was statistically significantly higher than the value of L* parameters on the 1st, 3rd, and 6th days of storage. An increase in ∆E values was observed in the case of both G1 and G2 groups. The highest value of ∆E was observed on the 9th day of storage in the case of both groups. Cruz-Romero et al.[Citation33] classified overall colour changes into seven groups: a ∆E value between 0 and 0.2 means imperceptible colour change; 0.2 and 0.5 a very small difference; 0.5 and 1.5 a small difference; 1.5 and 3.0 a slight significant change; 3.0 and 6.0 a significant change; 6.0 and 12.0 a very significant change; and >12 a great change. According to this classification, after 9 days of storage, G1 and G2 groups were characterized as samples with significant colour changes. The observer can perceive two different colours. The pink colour of meat during the storage in vacuum packaging is very stable, whereas it can be oxidized on the surface when stored aerobically; however, pink colour may remain in the centre of the meat.[Citation34] On the 1st day of storage, parameter a* in C group is not statistically significantly different from parameter a* in G1 group, but parameter a* in C and G1 groups was statistically different from parameter a* in G2 group. On 6th and 9th days of storage, parameter a* in C, G1, and G2 groups was not statistically significantly different. In G2 group on the 9th day of storage, parameter a* was statistically significantly different from parameter a* from the rest of the days of storage. Karpińska-Tymoszczyk[Citation35] found that antioxidant additives stabilized the red colour of turkey meatballs, but samples with a mixture of natural and synthetic antioxidants showed higher a* values than samples with rosemary extract. Devathal et al.[Citation36] reported a reduction in the L* value and an increase in the a* values due to the supplementation of natural antioxidants to chicken patties.
Table 2. The mean and standard deviation of colour components of turkey samples enhanced with different antioxidant solutions depending on the storage time.
Effect of coriander extract on volatile organic compounds
Twelve main compounds were identified of a volatile organic compound (VOC) profile; 3 compounds were recognized as alcohols, 2 as aldehydes, 2 as ketones, and 5 as terpenes. The results of VOCs profile analysis are represented in . In the present study, alcohols, aldehydes, and ketones were found in the control group (group C). Ahn et al.[Citation37] identified five compounds in raw turkey (control group): 2 alcohols (ethanol and 3-methyl-1-butanol), 2 aldehydes (propanal and 2-methylpentanal), and 1 ketone (cyclohexanone). Ten volatile compounds were present in the examined groups G1 and G2. The majority of compounds were typical for coriander, namely terpenes: α-pinene, β-pinene, α-terpinene, myrcene, and linalool.[Citation38] Terpenes were identified in coriander seeds and leaves by Shahwar et al.[Citation39] and Yildiz.[Citation40] Some of the compounds observed in the control group were also found in the groups containing coriander extract. Ethanol and cyclohexanone, which were found in the control group, were not present in groups G1 and G2. Alcohols are characteristic products of microorganism activity in meat; therefore, the absence of ethanol and cyclohexanone in samples containing coriander extract could indicate that microbiological growth was inhibited.[Citation41] The percentages of volatile compounds for each of the three groups, C, G1, and G2, are shown in . It was observed that the percentage participation of propanal (aldehyde) in volatile compounds of profile G2 was constant. Aldehydes are representative as a product of auto-oxidation of unsaturated fatty acid process and microbial activity.[Citation42]
Table 3. Volatile compounds of turkey samples.
Visualization of the results of variance analysis was presented on a scores plot (). Samples are represented in a two-dimensional plane with reference to selected components: PC1 and PC2.
Figure 2. PCA analysis (score plot for main variation) of volatile profile for turkey samples C, G1, and G2 stored for 1, 3, 6 and 9 days. C: control samples with addition of 0.5% NaCl. G1: samples with addition of 0.5% NaCl and 200 ppm of coriander extract. G2: samples with addition of 0.5% NaCl and 500 ppm of coriander extract.
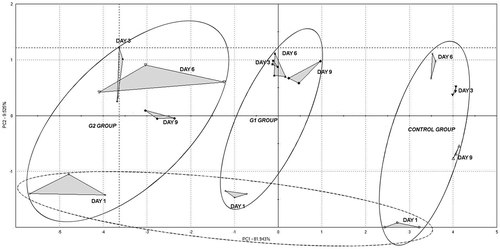
During the first day of storage, the scent profile was different from any other day, regardless of the type of studied group. However, during the 3rd, 6th, and 9th days, the scent profile for particular groups was similar. represents the classification of scent profiles with regard to the experimental group they belonged to. Those groups, irrespective of the storage date, appeared on the separate parts of the score plot.
Microbiological analysis
To verify the effect of coriander extract on the growth of aerobic bacteria in turkey meat during the storage, the number of aerobic bacteria was calculated. Our data demonstrate that 500 ppm of coriander extract in turkey meat inhibits the growth of aerobic microorganisms, whereas a concentration of 200 ppm of the extract has no influence on the growth of aerobic bacteria. The antimicrobial effect of the extract (500 ppm) was observed after the 6th day of storage of turkey meat. On the 6th day of storage, in the control meat the total number of aerobic bacteria was 5.2 × 103 CFU/g, whereas in meat from G1 and G2 groups it was 3.2 × 104 and 9.6 × 103 CFU/g, respectively. On the 9th day of storage, in control meat the total number of aerobic bacteria was 1.5 × 105 CFU/g, whereas in meat from G1 and G2 groups it was 2.6 × 105 and 1.6 × 104 CFU/g, respectively. Analyses of others have shown that coriander has antimicrobial activity.[Citation43] Bhat et al.[Citation31] described that fresh leaves of coriander have bactericidal activity against Salmonella choleraesuis spp. Studies on beef and chicken meat evaluated that coriander oil inhibits the growth of Campylobacter jejuni.[Citation44]
Sensory acceptability and preference
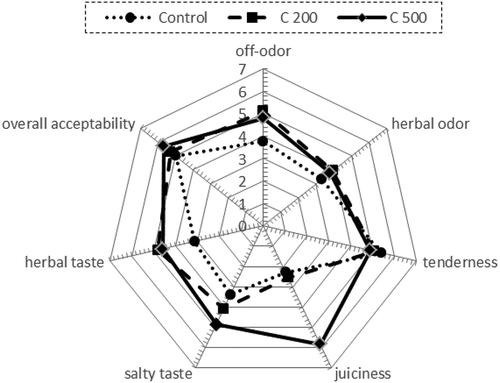
Sensory differences between the analysed samples were not perceived (p > 0.05), apart from juiciness. Turkey meatballs with the addition of 500 ppm coriander extract were characterized with the highest juiciness compared to the control and the sample groups, which contained 200 ppm of this extract (). Therefore, aromatic notes that could be attributed to the addition of coriander extracts caused no negative reactions of samples by panelists. Contini et al.[Citation44] reported that the addition of natural antioxidant extracts has been reported to increase turkey meat tenderness and overall acceptability. Other studies have pointed out that in sensory tests overall acceptability for meat is mostly driven by differences in tenderness and juiciness.[Citation45] Therefore, we can conclude that the use of coriander extracts in turkey meatballs does not cause a deterioration of the sensory quality.
Conclusion
The addition of coriander extract to the turkey meatballs shows a preservative effect in pre-cooked turkey products, stored under refrigeration. Coriander extract at a 500 ppm level was more effective than 200 ppm in inhibiting both lipid oxidation and the increase of microorganisms. Moreover, the addition did not have a negative effect on sensorial properties, indicating that all formulations were equally acceptable. Our results have shown that coriander seed extract has a substantial amount of phenolic compounds, a significant antioxidant effect, and could be a good source of natural antioxidants for commercial application, as a good alternative to synthetic antioxidants.
Funding
We would like to acknowledge the Project BIOFOOD – innovative, functional products of animal origin no. POIG.01.01.02-014-090/09 co–financed by the European Union from the European Regional Development Fund within the Innovative Economy Operational Programme 2007–2013 (made available on equipment).
Additional information
Funding
References
- Biswas, A.K.; Keshri, R.C.; Bisht, G.S. Effect of Enrobing and Antioxidants on Quality Characteristics of Precooked Pork Patties under Chilled and Frozen Storage Conditions. Meat Science 2004, 66, 733–741.
- Jayathilakan, K.; Sharma, G.K.; Radhakrishna K.; Bawa A.S. Antioxidant Potential of Synthetic and Natural Antioxidants and Its Effect on Warmed-Over-Flavour in Different Species of Meat. Food Chemistry 2007, 105, 908–916.
- Zarena, A.S.; Sankar, K.U. A study of Antioxidant Properties from Garcinia mangostana L. Pericarp Extract. Acta Scientiarum Polonorum, Technologia Alimentaria 2009, 8, 1, 23–34.
- Zhang, W.; Xiao, S.; Samaraweera, H.; Lee, E. J.; Ahn, D.U. Improving Functional Value of Meat Products. Meat Science 2010, 86, 15–31.
- Darughe, F.; Barzegar, M.; Sahari, M.A. Antioxidant and Antifungal Activity of Coriander (Coriandrum sativum L.) Essential Oil in Cake. International Food Research Journal 2012, 19, 1253–1260.
- Wójciak, K.M.; Dolatowski, J.; Okoń, A. The Effect of Water Plant Addition on the Oxidative Stability of Meat Products. Acta Scientiarum Polonorum, Technologia Alimentaria 2011, 10, 2, 175–188.
- Velasco, V.; Williams, P. Improving Meat Quality Through Natural Antioxidants. Chilean Journal of Agricultural Research 2011, 71, 313−322.
- Wojdyło, A.; Oszmioński, J.; Czemerys, R. Antioxidant Activity and Phenolic Compounds in 32 Selected Herbs. Food Chemistry 2007, 105, 940–949.
- Tanabe, H.; Yoshida, M.; Tomita, N. Comparison of the Antioxidant Activities of 22 Commonly Used Culinary Herbs and Spices on the Lipid Oxidation of Pork Meat. Animal Science Journal 2002, 73, 389−393.
- Karre, L.; Lopez, K.; Getty, K.J.K. Natural Antioxidants in Meat and Poultry Products. Meat Science 2013, 94, 220–227.
- Shah M.A.; Don Bosco S.J.; Mir S.A. Plant Extracts as Natural Antioxidants in Meat and Meat Products. Meat Science 2014, 98, 21–33.
- Djenane, D.; Sánchez-Escalante, A.; Beltrán, J.A.; Roncalés, P. Extension of the Shelf Life of Beef Steaks Packaged in a Modified Atmosphere by Treatment with Rosemary and Displayed Under UV-Free Lighting. Meat Science 2003, 64, 417–426.
- Neffati, M.; Sriti, J.; Hamdaoui, G.; Kchouk, M.E.; Marzouk, B. Salinity Impact on Fruit Yield, Essential Oil Composition and Antioxidant Activities of Coriandrum sativum Fruit Extracts. Food Chemistry 2011, 124, 221–225.
- Kaiser, A.; Kammerer, D.R.; Carle, R. Impact of Blanching on Polyphenol Stability and Antioxidant Capacity of Innovative Coriander (Coriandrum sativum L.) Pastes. Food Chemistry 2013, 140, 332–339.
- Rattananachaikunsopon, P.; Phumkhachorn, P. Potential of Coriander (Coriandrum sativum) Oil as a Natural Antimicrobial Compound in Controlling Campylobacter jejuni in Raw Meat. Bioscience, Biotechnology, and Biochemistry 2010, 74, 31–35.
- Dimić, G.; Kocić-Tanackov, S.; Ljiljana Mojović, L.; Pejin, J. Antifungal Activity of Lemon Essential Oil, Coriander and Cinnamon Extracts on Foodborne Molds in Direct Contact and the Vapor Phase. Journal of Food Processing and Preservation 2015, 39, 6, 1778–1787.
- Polish Pharmacopoeia VI. PTFarm, Warsaw, Poland. 2002.
- Polish Pharmacopoeia VII. PTFarm, Warsaw, Poland. 2006.
- Chen, J.H.; Ho, C.T. Antioxidant Activities of Caffeic Acid and Its Related Hydroxycinnaminic Acid Compounds. Journal of Agricultural and Food Chemistry 1997, 45, 2374–2378.
- Robles-Martinez, C.; Cervante, E.; Ke, P.J. Recommended Method for Testing the Objective Rancidity Development in Fish Based on TBARS Formation. Canadian Technical Report of Fisheries and Aquatic Sciences 1982, 1089, 1–3.
- Brodowska, M.; Guzek, D.; Kołota, A.; Głąbska, D.; Górska-Horczyczak, E.; Wojtasik-Kalinowska, I.; Wierzbicka, A. The Effect of Diet on Oxidation and Profile of Volatile Compounds of Semimembranosus after Freezing Storage. Journal of Food and Nutrition Research 2016, 55, 40–47.
- ISO-13299. Sensory Analysis. Methodology. General Guidance for Establishing a Sensory Profile (PN-EN 16801:2016-05), 2016; 54 pp.
- ISO-4833-2. Microbiology of the Food Chain. Horizontal Method for the Enumeration of Microorganisms. Part 2: Colony Count at 30 degrees C by the Surface Plating Technique (PN-EN ISO 4833-1.), 2013; 12 pp.
- ISO-6658. Sensory Analysis. Methodology. General Guidance. (Polska Norma PN-ISO 6658: 1998: Analiza sensoryczna. Metodologia Wytyczne ogólne 1998; 21 pp. (in Polish).
- Wangensteen, H.; Samuelsen, A.B.; Malterud, K.E. Antioxidant Activity in Extracts from Coriander. Food Chemistry 2004, 88, 293–297.
- Kumar, S.R.; Balasubramanian, P.; Govindaraj, P. Krishnaveni, T. Preliminary Studies on Phytochemicals and Antimicrobial Activity of Solvent Extracts of Coriandrum sativum L. Roots (Coriander). Journal of Pharmacognosy and Phytochemistry 2014, 2, 74–78.
- Jia, H.; Ren, H.; Deng, C.; Kato H.; Endo H. Effects of Chinese Parsley (Coriandrum sativum) on Oxidative Stabilities of Diet during Storage as Compared with a Synthetic Antioxidant. International Journal of Food Properties 2012, 15, 6, 1394–1407.
- Singh, G.; Marimuthu, P.; Catalan, C.; de Lampasona, M.P. Chemical, Antioxidant, and Antifungal Activities of Volatile Oil of Black Pepper and Its Acetone Extract. Journal of the Science of Food and Agriculture 2004, 84, 1878−1884.
- Shan, B.; Cai, Y.Z.; Corke, H. Antioxidant Capacity of 26 Spice Extracts and Characterization of Their Phenolic Constituents. Journal of Agricultural and Food Chemistry 2005, 53, 7749–7759.
- Selani, M.M.; Contreras-Castillo, C.J.; Shirahigue, L.D.; Gallo, C.R.; Plata-Oviedo, M.; Montes-Villanueva, N.D. Wine industry Residues as Natural Antioxidants in Raw and Cooked Chicken Meat during Frozen Storage. Meat Science 2001, 88, 397–403.
- Bhat, S.; Kaushal, R.; Kaur, M.; Sharma, K. Coriander (Coriandrum sativum L.): Processing Nutritional and Functional Aspects. African Journal of Plant Science 2014, 8, 25–33.
- Velioglu, Y. S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant Activity and Total Phenolics in Selected Fruits, Vegetables and Grain Products. Journal of Agricultural and Food Chemistry 1998, 46, 4113–4117.
- Cruz-Romero, M.; Kelly, A.L.; Kerry, J.P. Effects of High-Pressure and Heat Treatments on Physical and Biochemical Characteristics of Oysters (Crassostrea gigas). Innovative Food Science and Emerging Technologies 2007 8, 30–38.
- Du, M.; Hur, S. J.; Nam, K.C.; Ismail, H.; Ahn, D.U. Volatiles, Colour, and Lipid Oxidation of Broiler Breast Fillets Irradiated before and after Cooking. Poultry Science 2001, 80, 1748–1753.
- Karpińska-Tymoszczyk, M. The Effect of Antioxidants, Packaging Type and Frozen Storage Time on the Quality of Cooked Turkey Meatballs. Food Chemistry 2014, 148, 276–283.
- Devathal, S.K.; Narsaiah, K.; Borah, A. Anti-oxidant Effect of Extracts of Kinnow Rind, Pomegranate Rind and Seed Powders in Cooked Goat Meat Patties. Meat Science 2010, 85, 155–159.
- Ahn, D.U.; Jo, C.; Olson, D.G. Volatile Profiles of Raw and Cooked Turkey Thigh as Affected by Purge Temperature and Holding Time before Purge. Journal of Food Science 1999, 64, 230–233.
- Sriti, J.; Bettaieb, I.; Bachrouch, O.; Talou, T.; Marzouk, B. Chemical Composition and Antioxidant Activity of the Coriander Cake Obtained by Extrusion. Arabian Journal of Chemistry 2014, http://dx.doi.org/10.1016/j.arabjc.2014.11.043 ( in press).
- Shahwar, M.K.; El-Ghorab, A.H.; Anjum, F.M., Butt, M.S.; Hussain, S.; Nadeem, M. Characterization of Coriander (Coriandrum sativum L.) Seeds and Leaves: Volatile and Non Volatile Extracts. International Journal of Food Properties 2012, 15, 4, 736–747.
- Yildiz H. Chemical Composition, Antimicrobial, and Antioxidant Activities of Essential Oil and Ethanol Extract of Coriandrum sativum L. Leaves from Turkey. International Journal of Food Properties 2016, 19, 7, 1593–1603.
- Casaburi, A.; Piombino, P.; Nychas, G. J.; Villani, F.; Ercolini, D. Bacterial Populations and the Volatilome Associated to Meat Spoilage. Food Microbiology 2015, 45, 83–102.
- Leroy, F.; Vasilopoulos, C.; Hemelryck, S.V.; Falony, G.; De Vuyst, L. Volatile Analysis of Spoiled, Artisan-Type, Modified-Atmosphere-Packed Cooked Ham Stored under Different Temperatures. Food Microbiology 2009, 26, 94–102.
- Khan, D.A.; Hassan, F.; Ullah, H.; Karim, S.; Baseer, A.; Abid, M. A.; Ubaidi, M.; Khan, S.A.; Murtaza, G. Antibacterial activity of Phyllantus emblica, Coriandrum sativum, Culinaris medic, Lawsonia alba and Cucumis sativus. Acta Poloniae Pharmaceutica 2013, 70, 855–859.
- Contini, C.; Alvarez, R.; O’Sullivana, M.; Dowling, D.P.; Gargan, S.O.; Monahan, F.J. Effect of an Active Packaging with Citrus Extract on Lipid Oxidation and Sensory Quality of Cooked Turkey Meat. Meat Science 2014, 96, 1171–1176.
- Savel, J.; Branson, R.E.; Cross, H.R.; Stiffer, D.M., Wise, J.; Griffin, D.B.; Smith, G.C. National Consumer Retail Beef Study: Palatability Evaluations of Beef Loin Steaks That Differed in Marbling. Journal of Food Science 1987, 52, 3, 517–519.