ABSTRACT
Artemisia rutifolia (Asteraceae) had been used in traditional medicines for the treatment of different ailments. In the current study, an effort was made to explore the phenolic composition, antioxidant, and antimicrobial activities of different solvent extracts obtained from A. rutifolia leaves. The reverse-phase high-performance liquid chromatographic (RP-HPLC) analysis revealed the higher extent of polyphenolic compounds (i.e., gallic acid, caffeic acid, chlorogenic acid, syringic acid, sinapic acid, p-coumaric acid, m-coumaric acid, ferulic acid, vanillic acid, myricetin, and quercetin) in methanol extract. Methanol extract consistently showed the highest total phenolic contents (98 ± 2 µg GAE/mg of plant extract), total flavonoid contents (28 ± 0.0 µg QE/mg of plant extract), antimicrobial activity, free radical (DPPH) scavenging (IC50 = 39 µg/mL) activity, and reducing power (18.3 ± 0.2 mg GAE/g of plant extract) followed by those of chloroform and hexane extracts, respectively. The current study concluded that extracts of A. rutifolia are novel natural source of antioxidative and antimicrobial agents for the treatment of oxidative stress-related disorders and microbial infections.
Introduction
The earth is filled with an overwhelming plant biodiversity, and efforts have been made to categorizing them based on their size, forms, habitat, structure, anatomy, and biochemical and molecular features to interpret the relationships among the plants.[Citation1–Citation4] Biochemical attributes of the plants are likely related to wide array of bioactive compounds in them.[Citation5,Citation6,Citation7] Among the different classes of bioactive compounds, phenolics are extensively studied for their antioxidative and antimicrobial attributes.[Citation8,Citation9] Pharmacological aspects of phenolics had inspired the scientists to explore novel natural sources of phenolic compounds for wellness of mankind. In this context, the current study was undertaken to valorize medicinal plant (Artemisia rutifolia) from Pakistani flora, in order to locate novel natural therapeutic agents.
Artemisisarutifolia (A. rutifolia) is endemic to Pakistan. This shrub of vernacular name “Afsanteen” may reach the height of 20 to 80 cm.[Citation10] It is traditionally used for the treatment of fever, asthma, abdominal pain, inflammation, cancer, and other ailments.[Citation11] It was examined that essential oil from A. rutifolia was found rich in germacranolide, thujone, guaianolide, and eudesmanolidesesquiterpenoids.[Citation12] Although attention has been paid to explore pharmacological activities of plants of Asteraceae,[Citation13,Citation14] there was complete gap of knowledge about phytochemical and pharmacological aspects of A. rutifolia. Therefore, in the current study, for the first time, an effort was made to explore phenolic profile as well as antioxidant and antimicrobial activities of different extracts from A. rutifolia leaves.
Materials and methods
Chemicals and reagents
Gallic acid (GA),2,2′-diphenyl-1-picrylhydrazyl (DPPH), ascorbic acid, quercetin, all other reference compounds, and cell culture reagents were purchased from Sigma-Aldrich (Saint Louis, MO, USA). Sodium carbonate, Folin-Ciocalteu’s phenol reagent, ferric chloride, aluminum chloride, potato dextrose agar, and nutrient agar were provided by Applichem, GmbH, Darmstadt, Germany. Potassium ferricyanide and agar powder were purchased from DAEJUNG Chemicals and metals, Korea. Methanol, chloroform, and hexane used in the current study were obtained from Merck KGaA (Darmstadt, Germany). All other chemicals used were of analytical grade.
Plant material
The leaves of A. rutifolia were collected from Chitral, Province Khyber-Pakhtunkhwa, Pakistan, and identified by Dr. Adeel Mahmood.
Preparation of extract
Shade-dried leaves of A. rutifolia were powdered with food processor (Gaba National, GN-811). The powdered material was passed through strainer (0.50 mm). Extraction was carried out as described previously. Briefly, 10 g of powdered leaf material was extracted with 100 mL of solvents (methanol, chloroform, and hexane) of varying polarity on an orbital digital shaker (Burell Scientific, USA) at 350 rpm for 12 hrs. The extracts were filtered with the help of Whatman No. 1 filter paper. Filtrates were evaporated in vacuum-drying oven (Memmert, Germany) to constant weight. Temperature of vacuum-drying oven was adjusted to 65°C, 62°C, and 70°C for methanol, chloroform, and hexane, respectively. Dried extracts were scratched with the sterilized spatula. Extracts were transferred in extract vials and preserved in a refrigerator at −4°C for further use.
Determination of total phenolic contents
Total phenolic contents of A. rutifolia leaf extracts (methanol, chloroform, and hexane) were estimated using Folin-Ciocalteu’s reagent method as reported previously.[Citation15–Citation16] Briefly, one mL of each extract solution at appropriate dilution was mixed with 500 µL of Folin-Ciocalteu’s reagent and 7.5 mL of double-deionized water. Then, one mL of 5% Na2CO3 (W/V) solution was added. The reaction mixture was incubated at room temperature for 90 minutes. Absorbance was measured at 760 nm by using UV–vis spectrophotometer (Lambda EZ 201, Perkin Elmer, USA). Amount of total phenolic contents was calculated using gallic acid standard curve (5 µg/mL to 90 µg/mL) and the content of phenolics expressed as µg gallic acid equivalents (GAEs) per mg of extract.
Total flavonoid contents
The total flavonoid contents of A. rutifolia plant extracts were determined using aluminum chloride method as described previously.[Citation17] Each extract solutions (0.5 mL) was mixed with 1.5 mL of 95% ethanol (V/V), 0.1 mL of 10% aluminum chloride (m/V), 0.1 mL of 1 mol L–1 potassium acetate, and 2.8 mL of water. The reaction mixture was incubated for 30 minutes at room temperature, and absorbance of the reaction mixture was measured at 415 nm using a spectrophotometer (Shimadzu, Japan). The total flavonoid contents were determined using calibration curve (R2 = 0.99) of quercetin (0.25 to 60 µg/mL). Total flavonoid contents were expressed as µg quercetin equivalents (QEs) per mg of plant extract.
HPLC analysis of phenolic compounds
The hydrolysis of A. rutifolia leaf extracts was performed as described previously.[Citation18] Briefly, the test samples (50 mg) of each extract (methanol, chloroform, and hexane) were dissolved in 24 mL of methanol and were homogenized. About 16 mL of distilled water was added followed by 10 mL of 6M HCl. The mixture was then thermostated for 2 hr at 95°C. The final solution was filtered using 0.45-µm nylon membrane filter (Biotech, Germany) prior to high-performance liquid chromatography (HPLC) analysis.
The separation of plant samples on gradient HPLC (LC-10A, SHIMADZU, JAPAN) was performed using Shim-Pack CLC-ODS (C118), 25cm × 4.6 mm, 5 µm column. The chromatographic separation was carried out using mobile-phase gradient: A (H2O: acetic acid-94:6, pH = 2.27) and B (acetonitrile 100%). The gradient used was 15% solvent B (0–15 min), 45% solvent B (15–30 min), and 100% solvent B (35–45 min) with 1 mL/min flow rate. The UV–visible detector (λ max 280 nm) was used for separation of phenolic acids and flavonoids. The identification of phenolic acids and flavonoid was established by comparing the retention time and UV–visible spectra of the peaks with those previously obtained by injection of standards. The quantification was performed using calibration curves. The limit of detection (LOD) and limit of quantification (LOQ) were calculated based on the standard deviation of the response and slope using the calibration curves data.
Free radical scavenging activity (DPPH assay)
Free radical scavenging activity of each plant extract solution was determined by 2,2-diphenyl-1-picrylhydrazyl radical (DPPH) assay as described previously.[Citation19] Briefly, extract solution was added separately to an equal volume of 100 µM DPPH solution in methanol. The reaction mixture was incubated at room temperature for 15 min. Then, the absorbance of the reaction mixture was recorded at 517 nm using a UV–visible spectrophotometer. The percentage scavenging of DPPH radicals was calculated using the following equation:
where Ac is absorbance of control at 30 min, and As is the absorbance of sample solution at 30 min.
Ferric reducing–antioxidant power (FRAP) assay
Reducing powers were estimated using ferric reducing–antioxidant power (FRAP) assay.[Citation20] About 1 mL of each extract solution was combined with 2.5 mL of phosphate buffer (0.2 M; pH = 6.6) and 2.5 mL of potassium ferricyanide (1% W/V). The test tubes were incubated at 50°C for 25 min. Then, 2.5 mL of trichloro-acetic acid solution (10% W/V) was mixed to each reaction mixture. Then, 2.5 mL of each reaction mixture was taken in separate test tubes and diluted with 2.5 mL of distilled water, followed by the addition of 500 µL of ferric chloride (0.1% W/V) solution. The test tubes were incubated for 30 minutes at room temperature. The absorbance of each reaction mixture was measured at 700 nm using UV–vis spectrophotometer. Total antioxidant activity was calculated using gallic acid calibration curve (5 µg/mL to 90 µg/mL), and total antioxidant contents were expressed as mg gallic acid equivalent/g of plant extract.
Antimicrobial activity
Disc diffusion assay: The agar disc diffusion assay was used to determine the antimicrobial activity of A. rutifolia leaf extracts.[Citation21] Briefly, the sterile nutrient agar (100 mL) was inoculated with 100 µL suspension of each tested bacteria, and sterile potato dextrose agar was individually inoculated with 100 µL of each tested fungi. The inoculated nutrient agar and potato dextrose agar were then poured into sterilized petri plates individually. Different dilutions (3.5, 7, 14, and 28 mg/mL) of each extract were made with biological-grade dimethylsulfoxide (DMSO).Sterile filter discs impregnated with 50 µL of diluted plant extract solution were placed in inoculated petri plates with the help of sterile forceps. The plates were then incubated at 37°C for 24 hr and at 27°C for 48 hr for maximum bacterial and fungal growth, respectively. Antibacterial and antifungal activities were evaluated by measuring diameter (millimeter) of inhibition zones with the help of zone reader.
Estimation of minimum inhibitory concentration (MIC) values
A broth microdillution method was used to assess minimum inhibitory concentration (MIC).[Citation22] Briefly, a volume of 100 µL of each extract solution (28 mg/mL) and positive controls (Rifampicin and Terbinafine) was pipetted into the first row of each 96-well microtitre plate. To all the following wells, for bacteria, 50 µL of nutrient broth (NB) and for fungi, 50 µL of Sabouraud dextrose broth (SDB) were added onto individual microtiter plates. Then, twofold serial dilutions were done using a multichannel micropipette so that each well carried 50 μL of plant extract solution in a serially decreasing concentration. Thereafter, 10 μL inoculum of each tested microbial strain was added to each well followed by the addition of 10 μL of resazurin (indicator for the assessment of microbial growth). Dimethyl sulfoxide (DMSO) in NB and SDB was used as negative control, while NB containing Rifampicin and SDB containing Terbinafine were used as positive control for bacterial and fungal strains, respectively. The plates were incubated at 37°C for 24 hr and at 27°C for 48 hr for bacteria and fungi, respectively. The change in color of indicator (resazurin) was dependent on microbial growth. The lowest concentration of color indicator (resazurin) changes from purple to pink was defined as minimum inhibitory concentration (MIC).
Statistical analysis
The experimental results were expressed as mean ± SD (standard deviation) of three independent experiments. Comparison among different solvent extracts of same plant leaves and with positive controls was carried out using the analysis of variance (ANOVA), followed by the Tukey’s test. P values less than 0.05 were regarded as significant.
Results and discussion
Total phenolic and total flavonoid contents of A. rutifolia leaf extracts
Phenolic compounds play leading role against wide range of degenerative diseases. They are extensively studied for their antioxidant, anticarcinogenic, and antimicrobial attributes.[Citation23] We evaluated, in this study, the total phenolic and total flavonoid contents of A. rutifolia leaf extracts. The results are shown in . The amount of total phenolic contents in three different extracts ranged from 30 ± 1.9 µg GAE/mg of plant extract to 98 ± 2.2 µg GAE/mg of plant extract. Among the different understudy solvent extracts, methanol extract showed the highest extent of total phenolic contents (98 ± 2.2 µg GAE/mg of plant extract). Chloroform extract (53 ± 1.2 µg GAE/mg of plant extract) of A. rutifolia leaves exhibited significantly higher (P < 0.05) total phenolic contents than hexane extract (30 ± 1.9 µg GAE/mg of plant extract).
Figure 1. Polyphenolic compounds in different solvent extracts of A. rutifolia leaves. (A) Total phenolic compounds expressed as microgram gallic acid equivalent per milligram of plant extract. (B) Total flavonoid compounds expressed as microgram quercetin equivalent per milligram of plant extract.
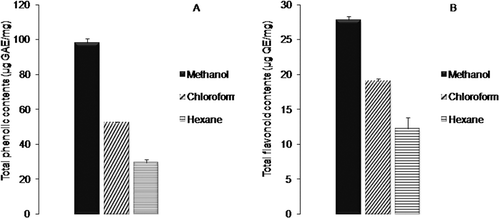
In A. rutifolia leaves, the total flavonoid contents were in range of 12 ± 1.6 µg QE/mg of plant extract to 28 ± 1.0 µg QE/mg of plant extract. The highest level of total flavonoid contents was recorded in methanol extract (28 ± 1.0 µg QE/mg of plant extract) followed by chloroform and hexane extracts, respectively. The current study revealed the methanol extract as potent source of polyphenolic (total phenolic and total flavonoid) compounds. These results are consistent with previous findings where the maximum amount of total phenolic and total flavonoid contents was examined for methanol extract, while the minimum amounts were noted for hexane extract.[Citation24]
High-performance liquid chromatography (HPLC) analysis of A. rutifolia leaf extracts
In view of growing interest in therapeutic potential of plant phenolics,[Citation8] the current study was conducted to qualify and quantify individual polyphenolic (phenolic acids and flavonoids) compounds using HPLC technique. All the polyphenolic compounds (phenolic acids and flavonoids) were identified by comparing with retention times (tR) and UV spectra of standards that were run under similar experimental conditions. Calibration curves of each compound were used to calculate the quantitative data for respective phenolic compound. The analytical characteristics such as regression equation, correlation coefficient, and the values of LOD and LOQ are shown in . The developed HPLC analysis was used for simultaneous separation of (gallic acid, caffeic acid, chlorogenic acid, syringic acid, sinapic acid, p-coumaric acid, m-coumaric acid, ferulic acid, and vanillic acid) and flavonoids (quercetin, and myricetin) within thirty minutes, and results are consigned in ; . The results showed that the amount of phenolic acids varied from 0.1 ± 0.2 (µg/g of dry weight of plant) to 17 ± 0.1 (µg/g of dry weight of plant) in three different solvent extracts obtained from A. rutifolia leaves. Gallic acid was the dominant phenolic acid detected in methanol (17 ± 0.1 µg/g of dry weight of plant) and chloroform (9 ± 0.1 µg/g of dry weight of plant) extracts. The major phenolic acid identified from hexane extract of A. rutifolia was p-coumaric acid (5 ± 0.0 µg/g of dry weight of plant).
Table 1. High-performance liquid chromatography (HPLC) study of methanol, chloroform, and hexane extracts of A. rutifolia leaves for identification of polyphenolic compounds.
Table 2. High-performance liquid chromatography (HPLC) study of methanol, chloroform, and hexane extracts of A. rutifolia leaves for identification of polyphenolic compounds.
Figure 2. HPLC phenolic profile of (A) methanol, (B) chloroform, and (C) hexane extracts of A. rutifolia leaves. Peaks: (1) myricetin, (2) quercetin, (3) gallic acid, (4) caffeic acid, (5) chlorogenic acid, (6) syringic acid, (7) p-coumaric acid, (8) vanillic acid, (9) m-coumaric acid, (10) ferulic acid, and (11) sinapic acid.
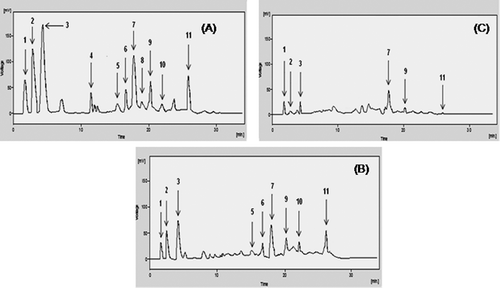
Flavonoids had also been noticed in significant amount from A. rutifolia leaf extracts. Quercetin was the main flavonoid identified from A. rutifolia methanol (14 ± 0.1 µg/g of dry weight of plant) and chloroform extracts (7 ± 0.2 µg/g of dry weight of plant). Hexane extract of A. rutifolia contained 3 and 1.3 µg/g of dry weight of plant of myricetin and quercetin, respectively. Higher extent of phenolic compounds in methanol extract might be owing to identical chemical nature of extractant (methanol) and compounds to be extracted (phenolics). These results are in accordance with the current study,[Citation25] which revealed that polar solvents had greater potency to extract phenolic compounds than nonpolar extractants.
Antioxidant activity of A. rutifolia leaf extracts
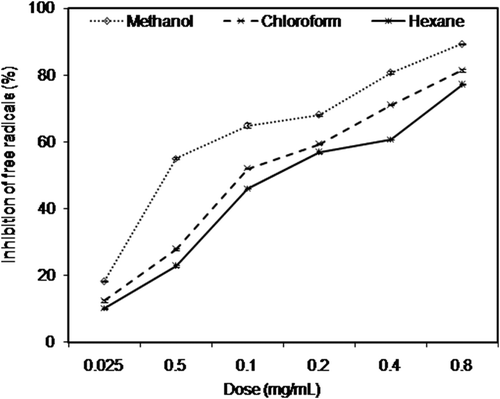
Two classical assays named by free radical (DPPH) scavenging assay and ferric reducing–antioxidant power (FRAP) assay were used to assess antioxidant potential of A. rutifolia leaf extracts. Free radical (DPPH) scavenging activity of A. rutifolia leaf extracts was investigated in dose-dependent mode (0.025 to 0.8 mg/mL) as shown in . DPPH inhibition (%) of three extracts ranged from 8 ± 0.9 % to 76 ± 1.4%. The antioxidant activity of three extracts was evaluated by estimation of extract concentration (IC50) required to scavenge 50% of 2,2-diphenyl-1-picryl hydrazil (DPPH) radicals. shows the results of free radical (DPPH) scavenging activity of different A. rutifolia extracts, expressed in terms of IC50 values and compared with synthetic antioxidant compounds (ascorbic acid and gallic acid). It is evident from the results () that methanol extract had the highest free radical (DPPH) scavenging activity with the lowest IC50 value of 39 µg/mL. Hexane extract obtained from A. rutifolia leaves presented the highest IC50 value (173 µg/mL) and thus the lowest antioxidant potency. Overall, the order of antioxidant activity of three extracts and two synthetic antioxidants was as follows: gallic acid (IC50 = 20 µg/mL) > ascorbic acid (IC50 = 32 µg/mL) > methanol (IC50 = 39 µg/mL) > chloroform (IC50 = 85 µg/mL) > hexane (IC50 = 173 µg/mL). It was examined that solvents (methanol, chloroform, and hexane) used for extraction had strong influence on antioxidant efficacy of A. rutifolia leaves. Our results are consistent with previous studies which narrated the strong influence of extracting solvents on antioxidant activity.[Citation26,Citation27]
Table 3. Ferric reducing antioxidant power (FRAP) and free radical (DPPH) scavenging activity of A. rutifolia leaf extracts.
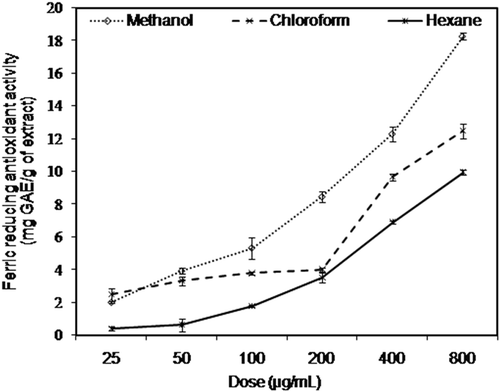
Ferric reducing–antioxidant power (FRAP) assay is an appropriate method to estimate reducing capacities of plant extracts.[Citation29] Antioxidant potential of A. rutifolia leaf extracts was also assessed by their ability to reduce certain precursors of oxidation. depicts concentration-dependent (25 to 800 µg/mL) reducing potential of three extracts ranging from 0.4 ± 0.1 mg GAE/g of plant extract to 18 ± 0.2 mg GAE/g of plant extract. The results () showed significantly (P < 0.05) higher ferric reducing capacities of methanol extract as compared to chloroform and hexane extracts. An earlier study demonstrated that reducing power of plants is an indicator of their antioxidant potential.[Citation29]
Electron or hydrogen atom-donating abilities of polyphenolic (phenolics and flavonoids) compounds were considered as major contributor of structure–antioxidant activity relationship. Redox property of phenolics and flavonoids is root cause of different antioxidative mechanisms such as adsorption and neutralization of free radicals, chelation of metal ions, termination of autoxidative chain reactions, decomposition of peroxides, and quenching of singlet or triplet oxygen.[Citation30]
Phenolics possess at least one 6-carbon (aromatic) ring bearing one or more hydroxyl groups.[Citation31] Antioxidant activity of phenolic compounds is mainly due to their tendency to release electrons. This reducing ability of phenolic compounds is correlated with high nucleophilic character of aromatic ring.[Citation32] Flavonoids are characterized by 15-carbon flavan structure with varying degree of free hydroxyl groups.[Citation33] Antioxidant potential of flavonoids mainly resides in their ability to scavenge free radicals that depend on number as well as location of hydroxyl groups on the flavonoid skeleton. In the current study, higher antioxidative potential of methanol extract might be consequence of higher extent of reductones (polyphenolic compounds) in it, which could react with free radicals to stabilize and terminate radical chain reaction, as well as to reduce Fe+3 ions of ferricyanide complex to ferrous form.[Citation34]
Antimicrobial activity of A. rutifolia leaf extracts
The antimicrobial activity of methanol, chloroform, and hexane extracts obtained from A. rutifolia leaves was tested by disc diffusion and minimum inhibitory concentration assays against a panel of eight microorganisms (Escherichia coli, Pasteurellamultocida, Bacillus subtilis, Staphylococcus aureus, Aspergillus flavus, Aspergillus niger, Fusarium solani, and Rhizoctoniasolani). The data were expressed as diameter of inhibition zone (mm) and minimum inhibitory concentration (MIC) values. The results are shown in and .
Table 4. Antimicrobial activity of A. rutifolia leaf extracts estimated by disc diffusion assay (mm).
Table 5. Minimum inhibitory concentration (MIC) values (mg/mL) of A. rutifolia leaf extracts and positive controls (µg/mL) against microbial strains.
Different extracts of A. rutifolia exhibited appreciable antimicrobial activity against all the tested microorganisms. Variable inhibition zones (IZ) and MIC values (mg/mL) were noticed in three extracts of A. rutifolia leaves. It was examined that diameter of inhibition zones against all microorganisms increased with increase in concentration of A. rutifolia leaf extracts from 3.5 to 28 mg/mL. Overall, the methanol extract from A. rutifolia leaves was strongly bactericidal and fungicidal than that of other two extracts (chloroform and hexane) with larger inhibition zones (8.0 to 25.7 mm) and smaller MIC values (3.5 to 7 mg/mL). Among the different bacterial strains, methanol extract of A. rutifolia exhibited the largest inhibition zone (19.3 mm) against B. subtilis, while the smallest against E. coli (13.3 mm). In contrast to our findings, previous studies reported comparatively smaller inhibition zones against B. subtilis and E. coli by methanol extracts of different Artemisia species such as A. abrotum, A. pallens, A. nilagirica, A. annua, A. absinthum, and A. santonicum.[Citation35,Citation36,Citation37,Citation38] However, some other research groups examined larger inhibition zones (32 mm) against B. subtilis and E. coli (14 mm) by methanolic extracts of Artimisia campestris and Artimisia annua, respectively.[Citation39,Citation40] Phenolic acids and flavonoids such as gallic acid, ferulic acid, chlorogenic acid, syringic acid, caffeic acid, p-coumaric acid, quercetin, and myricetin were reported to be responsible for potent antimicrobial activity against wide range of microorganisms (e.g., E. coli, P. aeruginosa, S. aureus, B. subtilis, S. pneumonia, S. typhimurium, and G. boninense) by different mechanisms including substrate deprivation, binding with surface-exposed adhesins or polypeptides in microbial cell, making complex with microbial cell wall, inhibiting membrane bound enzymes, and by disrupting the microbial cell membrane.[Citation41–Citation43] Consequently, the excellent antimicrobial potential of methanol extract might be attributed to high percentage of phenolic compounds in it as shown in . Moreover, the synergistic effect of different chemical compounds in methanol extract might be a major contributor of its antimicrobial activity.[Citation44]
The antimicrobial activity of chloroform and hexane extracts was also considerable with inhibition zones in the range of 7.4 to 22.6 mm, and MIC values ranged from 3.5 to 14 mg/mL. We observed greater resistance of gram-negative bacteria (Escherichia coli and Pasteurella multocida) to three extracts as compared to gram-positive bacterial strains (Bacillus subtilis and Staphylococcus aureus). This might be attributed to the presence of lipopolysaccharides in outer membrane of gram-negative bacteria that shield the penetration of antimicrobial agents, while gram-positive bacteria lack any such membrane complexity and often prove trouble-free target for antimicrobial agents.[Citation45] The findings of the current study are in accord with antimicrobial activity of essential oil from Asteraceae.[Citation46] Antimicrobial activity of A. rutifolia leaves was scarce in literature. To the best of our knowledge, this study is the first report on antimicrobial potential of A. rutifolia leaves.
Conclusions
Concluding the results, our study explored the new findings in the field of drug discovery from natural flora. Although the methanol extract of A. rutifolia leaves presented sensibly higher polyphenol levels and appreciable biological activities than other two extracts, overall the three extracts presented relatively potent antioxidant and antimicrobial potential that could explain their promise for prevention or treatment of diseases. The profiling of polyphenols by HPLC has allowed identifying some of the medicinally important phenolic acids and flavonoids. The knowledge of phenolic composition in A. rutifolia leaves will help to explore their potential as source of natural antioxidants for food and pharmaceutical industries. Moreover, the comparison of phenolic composition among solvents of variable polarity will help to optimize the solvent for extraction of phenolic compounds. The considerable antioxidant and antimicrobial activities of this medicinal plant predict its future role in public health.
Acknowledgments
The authors are also thankful to Higher Education Commission, Pakistan, for financial assistance to carry out the work.
References
- Mishra, P.K.; Ram, R.B.; Kumar, N. Genetic Variability, Heritability, and Genetic Advance in Strawberry (Fragaria ananassa Duch.). Turkish Journal of Agriculture and Forestry 2015, 39, 451–458.
- Nemli, S.; Kianoosh, T.; Tanyolac, M.B. Genetic Diversity and Population Structure of Common Bean (Phaseolus vulgaris L.) Accessions Through Retrotransposon-Based Interprimer Binding Sites (iPBSs) Markers. Turkish Journal of Agriculture Forestry 2015, 39, 940–948.
- Tsou, C.; Li, L.; Vijayan, K. The Intra-familial Relationships of Pentaphylacaceae as Revealed by DNA Sequence Analysis. Biochemical Genetics 2016, 54, 270–282.
- Ipek, A.; Yilmaz, K.; Sıkıcı, P.; Tangu, N.A.; Oz, A.T.; Bayraktar, M.; Ipek, M.; Gulen, H. SNP discovery by GBS in Olive and the Construction of a High-Density Genetic Linkage Map. Biochemical Genetics 2016, 54, 313–325.
- Ashraf, A.; Sarfraz, R.A.; Mahmood, A.; Din, M.U. Chemical Composition and In vitro Antioxidant and Antitumor Activities of Eucalyptus camaldulensis Dehn. leaves. Industrial Crops and Products 2015, 74, 241–248.
- Ashraf, A.; Sarfraz, R.A.; Anwar, F.; Shahid, S.A.; Alkharfy, K.M. Chemical Composition and Biological Activities of Leaves of Ziziphus mauritiana L. native to Pakistan. Pakistan Journal of Botany 2015, 47, 367–376
- Tahir, H.U.; Sarfraz, R.A.; Ashraf, A.; Adil, S. Chemical Composition and Anti-diabetic Activity of Essential Oils Obtained From Two spices (Syzygium aromaticum and Cuminum cyminum). International Journal of Food Properties 2016, 19, 2156–2164.
- Irshad, M.; Ahmad, I.; Mehdi, S.J.; Goel, H.C.; Rizvi, M.M.A. Antioxidant Capacity and Phenolic Content of the Aqueous Extract of Commonly Consumed Curcurbits. International Journal of Food Properties 2011, 17, 179–186.
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in human Health and Disease. Oxidative Medicine and Cellular Longevity 2009, 5, 270–278.
- Ali, H.; Qaiser, M. The Ethnobotany of Chitral Valley, Pakistan with Particular Reference to Medicinal Plants. Pakistan Journal of Botany 2009, 4, 2009–2041.
- Hussain, I.; Bano, A.; Ullah, F. Traditional Drug Therapies from Various Medicinal Plants of Central Karakoram National Park, Gilgit-Baltistan, Pakistan. Pakistan Journal of Botany 2011, 43, 79–84.
- Sharopov, F.S.; Setzer, W.N. Thujone Rich Essential Oils of Artemisia rutifolia Stephan Ex Spreng Growing Wild in Tajikistan. Journal of Essential Oil Bearing Plants 2011, 2, 136–139.
- Amir, I.; Martino, D.L.; Marandino, A.; Lamia, H.; Mohsen, H.; Sacndolera, E.; Feo, D.V.; Mancini, E. Chemical Composition and Biological Activities of the Essential Oil from Artemisia herba-alba Growing Wild in Tunisia. Natural Product Communication 2013, 8, 407–410.
- Khlifi, D.; Sghaier, R.M.; Amouri, S.; Laouini, D.; Hamdi, M.; Bouajila, J. Composition and Anti-oxidant, Anti-cancer and Anti-inflammatory Activities of Artemisia herba-alba, Rutachalpensis L. and Peganum harmala L. Food and Chemical Toxicology 2013, 55, 202–208.
- Jagadish, L.K.; Krishnan, V.V.; Shenbhagaraman, R.; Kaiyarasan, V. Comparative Study on the Antioxidant, Anticancer and Antimicrobial Property of Agaricus bisporus (J.E. Lange) Imbach Before and after Boiling. African Journal of Biotechnology 2009, 8, 654–661.
- Slinkard, K.; Singleton, V.L. Total Phenol Analysis: Automation and Comparison with Manual Methods. American Journal of Enology and Viticulture 1997, 28, 49–55.
- Kosalec, I.; Bakmaz, M.; Pepeljnjak, S.; Knezevic, S.V. Quantitative Analysis of the Flavonoids in Raw Proplis from Northern Croatia. Acta Pharmaceutica 2004, 54, 65–72.
- Pak-Dek, M.S.; Osman, A.; Sahib, G.N.; Saari, N. Effects of Extraction Techniques on Phenolic Components and Antioxidant Activity of Mengkudu (Morinda citrifolia L.) Leaf Extracts. Journal of Medicinal Plant Research 2011, 20, 5050–5057.
- Roy, P.; Amdekar, S.; Kumar, A.; Singh, V. Preliminary Study of the Antioxidant Properties of Flowers and Roots of Pyrostegiavenusta (Ker Gawl) Miers. BMC Complementary and Alternative Medicine 2011, 23, 11–69.
- Chan, E.W.C.; Lim, Y.Y.; Omar, M. Antioxidant and Antibacterial Activity of Leaves of Etlingera Species (Zingerberaceae) in Peninsular Malaysia. Food Chemistry 2007, 104, 1586–1593.
- NCCLS (National Committee for Clinical Laboratory Standards). Performance Standards for Antimicrobial Disc Susceptibility Test (6th ed.) Approved Standard 1997, M2-A6: Wayne, PA.
- NCCLS (National Committee for Clinical Laboratory Standards). Performance standards for Antimicrobial Disc Susceptibility Test (6th ed.) Approved Standard 1999, M2-A6: Wayne, PA.
- Msaada, K.; Tammar, S.; Salem, N.; Bachrouch, O.; Sriti, J.; Hammami, M.; Selmi, S.; Azaiez, S.; Hadj-Brahim, A.; Sane, K.A.; Limam, F.; Marzouk, B. Chemical Composition and Antioxidant Activities of Tunisian Thymus Capitatus L. methanolic extract. International Journal of Food Properties 2016, 19, 1381–1390.
- Yumrutas, O.; Saygideger, S.D. Determination of Antioxidant and Antimutagenic Activities of Phlomisar-meniacaand Menthapulegium. Journal of Applied Pharmaceutical Science 2012, 1, 36–40.
- Barchan, A.; Bakkali, M.; Arakrak, A.; Pagan, R.; Laglaoui, A. The Effects of Solvents Polarity on the Phenolic Contents and Antioxidant Activity of Three Mentha Species Extracts. International Journal of Current Microbiology and Applied Sciences 2014, 3, 1–14.
- Zhao, H.; Dong, J.; Lu, J.; Chen, J.; Li, Y.; Shan, L.; Lin, Y.; Fan, W.; Gu, G. Effects of Extraction Solvent Mixtures on Antioxidant Activity Evaluation and Their Extraction Capacity and Selectivity for Free Phenolic Compounds in Barley (Hordeum vulgare L.). Journal of Agriculture and Food Chemistry 2006, 54, 7277–7286.
- Ashraf, A.; Sarfraz, R.A.; Rashid, M.A.; Shahid, M. Antioxidant, antimicrobial, antitumor, and cytotoxic activties of an important medicinal plant (Euphorbia royleana) from Pakistan. Journal of Food and Drug Analysis 2015, 23, 109–115.
- Szollosi, R.; Varga, I.S. Total Antioxidant Power in Some Species of Labiatae (Adaptation of FRAP method). Acta Biologica Szegediensis 2002, 46, 125–127.
- Priyanka, C.; Kadam, D.A.; Kadam, A.S.; Ghule, Y.A.; Aparadh, V.T. Free Radical Scavenging (DPPH) and Ferric Reducing Ability (FRAP) of Some Gymnosperm Species. International Journal of Research in Botany 2013, 2, 34–36.
- Abdelwahab, S.I.; Abdul, A.B.; Elhassan, M.M.; Mohan, S.; AlZubairi, A.S.; Mariod, A.A. Antimicrobial and Free Radical Scavenging Activities of Dichloromethane Extract of Goniothalamus umbrosus. International Journal of Tropical Medicine 2009, 4, 32–36.
- Michalak, A. Phenolic Compounds and Their Antioxidant Activity in Plants Growing Under Heavy Metal Stress. Polish Journal of Environmental Studies 2006, 15, 523–53
- Munin, A.; Levy, F.E. Encapsulation of Natural Polyphenolic Compounds; a Review. Pharmaceutics 2011, 3, 793–829.
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: an Overview. The Scientific World Journal 2013, 2013, 16.
- Gulcin, Ilhami.; Huyut, Z.; Elmastas, M.; Aboul-Enein, H.Y. Radical Scavenging and Antioxidant Activity of Tannic Acid. Arabian Journal of Chemistry 2010, 3, 43–53
- Suresh, J.; Vasavi, R.A.; Rajan, D.; Ihsanullah, M.; Khan, M.N. Antimicrobial Activity of Artemisia abrotanum and Artemisia Pallens. International Journal of Pharmacognosy and Phytochemical Research 2010, 3, 18–21.
- Sengul, M.; Ercisli, S.; Yildiz, H.; Gungor, N.; Kavaz, A.; Cetin, B. Antioxidant, Antimicrobial Activity and Total Phenolic Content within the Aerial Parts of Artemisia absinthum, Artemisia santonicum and Saponaria officinalis. Iranian Journal of Pharmaceutical Research 2011, 10, 49–56.
- Tajeshmiri, A.; Issapour, F.; Moslem, M.N.; Lakeh, M.T.; Kolavani, M.H. In vitro Antimicrobial Activity of Artemisia annua Leaf Extracts Against Pathogenic Bacteria. Advanced Studies in Biology 2014, 6, 93–97.
- Ahameethunisa, A.R.; Hopper, W. Antibacterial Activity of Artemisia nilagirica Leaf Extracts Against Clinical and Phytopathogenic Bacteria. BMC Complementary and Alternative Medicine 2010, 10, 6.
- Massiha, A.; Pahlaviani, M.M.K.; Issazadeh, K.; Bidarigh, S.; Zarrabi, S. Antibacterial Activity of Essential and Plant Extracts of Artemisia (Artemisia annua L.) in vitro. Zahedan Journal of Research in Medical Sciences 2013, 15, 14–18.
- Naili, M.B.; Alghazeer, R.O.; Saleh, N.A.; Al-Najjar, A.Y. Evaluation of Antibacterial and Antioxidant Activities of Artemisia campestris (Asteraceae) and Ziziphus lotus (Rhamnacea). Arabian Journal of Chemistry 2010, 3, 79–84.
- Lou, Z.; Wang, H.; Zhu, S.; Ma, C.; Wang, Z. Antibacterial Activity and Mechanism of Action of Chlorogenic Acid. Journal of Food Science 2011, 76, 398–403.
- Stojkovic, D.; Petrovic, J.; Sokovic, M.; Glamoclija, J.; Kukic-Markovic, J.; Petrovic, S. In situ Antioxidant and Antimicrobial Activities of Naturally Occurring Caffeic Acid, p-Coumaric Acid and Rutin, Using Food Systems. Journal of the Science of Food and Agriculture 2013, 93, 3205–3208.
- Griep, M.A.; Blood, S.; Larson, M.A.; Koepsell, S.A.; Hinrichs, S.H. Myricetin Inhibits Escheria Coli DnaB Helicase but not Promise. Bioorganic and Medicinal Chemistry 2007, 15, 7203–7208.
- Mothana RA, Al-Sadi MS, Al-Yahya MA, Al-Rehaily AJ, Khaled JM. GC and GC/MS Analysis of Essential Oil Composition of the Endemic Soqotraen Leucasvirgata Balf.f. and Its Antimicrobial and Antioxidant Activities. International Journal of Molecular Sciences 2013, 14, 23129–23139.
- Wang X, Quinn PJ. Endotoxins: Lipopolysaccharides of Gram-Negative Bacteria. Sub-Cell. Biochemistry 2010, 53, 3–25.
- Rashid S, Rather MA, Shah WA, Bhat BA. Chemical Composition, Antimicrobial, Cytotoxic and Antioxidant Activities of the Essential Oil of Artemisia indica Willd. Food Chemistry 2013, 138, 693–700.