ABSTRACT
Camel bone was demineralized through HCl acidulation process at different concentrations (0.0%, 1.5%, 3.0%, and 6.0%) over 1–5 days. The level of demineralization was acid concentration and soaking time dependent. Highest demineralization (62.0%) was recorded in bone sample treated with 6.0% dilute acid for 5 days. Energy dispersive X-ray spectroscopy (EDX) elemental analysis revealed reduction in Ca and increase in N and H, while O remains unaffected. Particulate characteristics by scanning electron microscope showed an increased surface roughness of bone after demineralization. Fourier transform infrared (FT-IR) analysis of ossein depicted the presence of functional group similar to that of bone protein (collagen). Statistical optimization by central composite design (CCD) revealed a significant quadratic model for optimum values of extraction temperature, pH, and extraction time. The highest gelatin yield from camel bone was 23.66% at optimum extraction condition (71.87°C, pH 5.26, and 2.58 h) and the bloom was 205.74 g. Camel bone is suitable for production of gelatin with good potentials in food and nonfood applications.
Introduction
Gelatin is an important protein produced through partial hydrolysis of collagen from animal parts and by-products such as cartilages, bones, tendons, and hides.[Citation1,Citation2] The ability of gelatin to form a thermo-reversible gel at normal body temperature and high water content makes it an exceptional food ingredient. A good-quality gelatin is translucent, brittle, colorless (sometimes slightly yellow), bland in taste, and odorless. Gelatin has been found useful as stabilizer and filler in dairy products and other food industries.[Citation3,Citation4]
Recently, the global gelatin production was estimated to be net over 300,000 metric tons: 46% was from pigskin, 29.4% from bovine hides, 23.1% from bones, and 1.5% from other parts.[Citation5] Although camels have been recognized as source of meat and milk,[Citation6] utilization of camel’s bones for gelatin production has not been fully explored. Bone gelatin is primarily used for pharmaceutical purposes because of its high level of purity. Prior to extraction of gelatin from bone samples, the bone must be demineralized using dilute acid solution to remove inherent calcium composition that laces the bone matrix. Structurally, the function of calcium salt deposited in the organic matrix of bone is to maintain the bone integrity by holding the scaffolds and crosslinks that improves bone strength and rigidity.[Citation7,Citation8] Removal of calcium salt from bone through acidulation with dilute acid is an essential step toward successful gelatin production since result of acid treatment produces bone protein (collagen) which contained water-soluble gelatin. Extraction temperature, pH, and extraction period are common operating parameters influencing gelatin production. The effect of processing conditions on the properties of gelatin from fish bone with pretreatment of hydrochloric acid has been reported.[Citation9] Adoption of varied level of processing parameters and raw material sources often dictates the consistency in functionality of gelatin or collagen.
Recently, there has been increased interest in effective use of underutilized resources and industrial wastes to reduce production cost and environmental hazards. Utilization of animal bone for gelatin production offers opportunities including waste to wealth and waste minimization. Gelatins from bovine and porcine are becoming less acceptable because of (i) increasing allergen cases, and (ii) tradition and religious believes. This has called for production of gelatin from other sources. Therefore, camel bone gelatin can be a good alternative source of gelatin. To the best of our knowledge, there is less effort in identifying demineralization processing conditions suitable for camel bone. Even developed demineralization processing conditions for cattle bone are kept as company secret, and conflicting values are reported by other practitioners. Therefore, the focus of the present investigation covered the effect of processing conditions on characteristics of ossein and gelatin from camel bone.
Methodology
Preparation of camel bone
Bones of camel were obtained from sacrificed healthy animals; muscles, periosteum, and adherent soft tissues were removed from the specimen and bone marrow was discarded. All raw materials were sourced from our research collaborators from King Saud University, Kingdom of Saudi Arabia.
Acid demineralization of camel bone
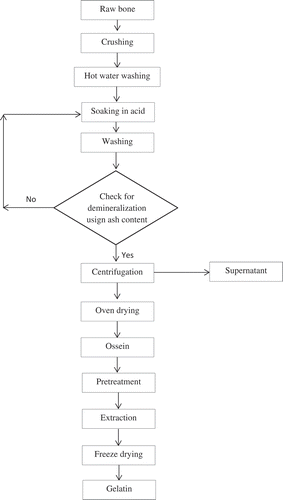
Demineralization of bone was conducted according to flow chart given in . Dried bones were cleaned by washing with tap water and draining afterwards. Bone samples were then crushed into particles of size <2 cm. Adhering lipid was removed by washing the bone thrice with hot water at 80°C. Demineralization process was carried out by soaking defatted crushed bone in 0.0%, 1.5%, 3.0%, and 6.0% of HCl solutions for different duration between 1 and 5 days at room temperature. Acid solution was changed every day. Demineralized bone (ossein) samples were centrifuged, washed with distilled water to remove any attached salt, and then dried at 50°C for 24 h.
Extraction
A mass of 10 g of demineralized camel bones (osseins) was pretreated by soaking in 100 ml of 6% HCl for 3 days at room temperature. The acid was thoroughly removed by rinsing the samples three times with tap water and one time with distilled water. Distilled water was added to the pretreated osseins (ratio of 1:3 – weight to volume), and gelatin was extracted under different conditions using response surface methodology, implemented in Design expert software, version 6.0.8. Gelatin solution was filtered through Whatman No. 1 filter paper (Whatman, Maidenstone, UK). Gelatin solution was then freeze dried (Labconco Corporation, Kansas City, MO, USA) and ground. Gelatin extraction yield was calculated as grams of dry gelatin per 100 g of initial untreated bones.
Determination of ash content
Ash content was determined using gravimetric method as described in AACC method (1999). About 2 g of samples were subjected to incinerated regimes: 350°C for 1 h, 450°C for 1 h, and 590°C for 12 h.
Gel strength
The bloom strength of camel bone and skin gelatin was determined according to the standard method.[Citation10] Samples were weighed into bloom jar (⊘ = 2.3 cm, height = 3.6 cm) and dissolved in distilled water to a final concentration of 6.67% w/v. The bloom strength was determined using a texture analyzer (Stable Micro Systems, Surrey, UK) with a 30 kg load cell, equipped with 1.27 cm diameter flat-faced cylindrical plunger. The maximum force (in grams) taken when the plunger had completely depressed the gelatin gel’s surface was recorded.
Fourier transform infrared (FT-IR) spectrometer analysis
Samples for the FT-IR analysis were prepared by grinding finely and pressing to form pellet disc. Approximately 1.5 mg of sample was grinded with 9 g of KBr and pressed to form pellet disc with 10 mm diameter. The disc was placed in the sample compartment before the spectra was obtained. FT-IR spectra were acquired with the FT-IR spectrometer (Bruker Tensor 27). Spectra were collected in the wave number region of 400–4000 cm–Citation1, with a spectral resolution of 4 cm–Citation1. Background was subtracted, and the peaks were identified and labeled using OPUS software program.
Morphological analysis and elemental analysis
Morphology of raw bone, osseins (prepared in day 2 and day 5), was analyzed using scanning electron microscopy (SEM). Dried ossein samples were mounted on stubs using double-sided carbon tape, and sputter coated with gold for 20 s using an Emitech K575X Sputter Coating Unit, to prevent surface charging by the electron beam. The samples were then examined using a FEI Quanta 3D FEG Dual Beam (FEI Ltd, Hillsboro, USA) SEM with an attached EDAX ED APOLLO XV Silicon Drift Detector with a 5–10 kV accelerating voltage.
Elemental analysis of bone and ossein was measured simultaneously during morphological analysis with the aid of energy dispersive X-ray spectroscopy (EDX). EDX detected and documented elements of interest; the gold elemental peak from each spectrum was recorded to ascertain the effect of treatment on the ossein, though the gold was added during sputter coating.
Results and discussion
Demineralization of camel bone
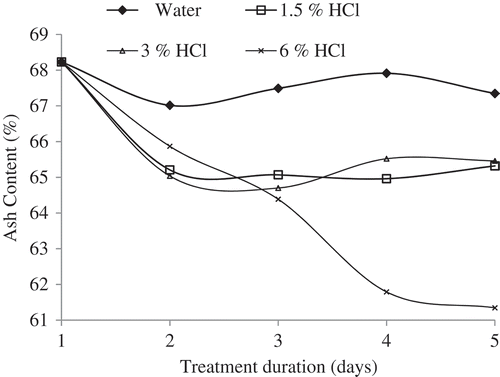
Bone gelatin production depends on efficient removal of calcium from the apatite through acid demineralization. Demineralization process is a critical process that removes as many inorganic chemical components in form of hydroxyapatite from the bone matrix and releases the organic collagen (ossein). Since ash content measures the amount of inorganic minerals in bones, it was therefore adopted as a measure of demineralization process. Bone demineralization depends on concentration of acid and soaking period. Change in ash content of camel bone soaked in different concentrations (0%, 1.5%, 3%, and 6%) of HCl was investigated over a 5-day period. Except for control (sample soaked in distilled water), there was reduction in ash content of all demineralized samples (). Initial rate of ash reduction was rapid for the first day in all demineralized samples, possibly due to acute surface washing of mineral ions. This observation agrees with previous findings that bone demineralization was rapid in day 1 as a result of calcium removal from the peripheral part of the bone.[Citation11]
However, in other treatment, a downward trend was recorded for ash content as concentration of HCl increased from 1.5% to 6%. From day 2 onward, the ash content reduction of by 1.5% and 3% HCl remained largely unchanged. This could be due to insufficient hydrolysis process of the acid in penetrating deeper layers of the bone crosslinks. The concentration of fresh HCl has been reported to influence efficient removal of calcium and other elements during demineralization.[Citation12] Similarly, result showed that increased concentration of HCl actually removed bone elements beyond the surface which was first attacked. The present result was corroborated by observation of other workers that opined increased HCl concentration and elevated treatment period could result in release of ossein.[Citation13] A sharp continuous reduction in ash content was observed for 6.0% HCl up to day 4 and then stabilized afterward. This observation was consistent with other investigations which postulated that calcium removal depends on HCl concentration and duration of treatment.[Citation14] Therefore, in order to produce ossein with adequate demineralization from camel bone, 6.0% HCl, 5 or 6 days, and room temperature form the appropriate conditions.
Effect of demineralization on chemical structures of camel bone
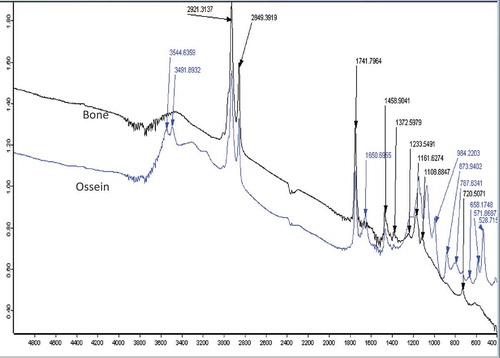
FT-IR was used to determine structural changes resulting from demineralization of camel bone. shows FT-IR spectra of the control and the demineralized sample. There were variations in the peaks and their intensities between the two spectra. Higher peak intensity was observed in demineralized sample compared with the control. This is possibly due to increase in vibration of the functional groups after demineralization. Spectra peaks that are higher than 1500 cm−Citation1 are assigned to vibrations from the amide groups from ossein.[Citation15,Citation16] The intense peak at 3544 cm−Citation1 corresponds to OH− vibrations, while carbonate replacement in the resulting apatite originates a peak at 873 cm−Citation1 and a double band in the 1372–1459 cm−Citation1 signified CH2 wag and bend.[Citation14,Citation17] Higher spectra intensities at 787, 873, and 984 cm−Citation1 were assigned for skeletal stretch, CO32– bending, and symmetrical PO43– stretching of ossein, respectively.[Citation18] Comparatively, the appearance of new peaks corresponding to collagen showed that demineralization impacted the camel bone and released huge calcium, thus ease vibration of molecules, especially proteins, finger print from the original bone.
Energy-dispersive X-ray spectra of mineral analyses of camel bone
EDX spectroscopy is used for rapid detection and measurement (qualitative and quantitative) of the energy permits elemental analysis of a mineral dense sample. EDX spectroscopy was used to analyze the elemental composition of camel bone and ossein (bone treated with 6% HCl for 6 days). The result shows presence C, N, O, Au, and Ca (). The presence of C and N in the bone matrix has been reported in HCl-demineralized bone matrix.[Citation19] Presence of Au is due to coating of sample with gold prior to analysis in line with previous report.[Citation20] There was a great reduction in the peak height of Ca after demineralization with 6.0% HCl. The percentage of Ca reduced was from 7.25% to 0.40% during the conversion of bone to ossein. This evinced demineralization of Ca during the conversion of bone to ossein. Reduction in the spectra peak of Ca has been reported for demineralized human femur. The bulk of Ca removal usually contained hydroxyapatite which is direct result of HCl reaction with surface and sub-surface Ca content of bone. It is believed that reduction in Ca content through diffusion HCl through the bone crosslinks orchestrates emergence of ossein.[Citation19] Other elements increased during ossein production from camel bone except for oxygen. Previous reports have shown that surface of bone contains CaCO3 and Ca2(PO4)2 both for sheltering adhering nitrogenous and carbonaceous materials.[Citation21,Citation22] Bone demineralization led to removal of oxygen-containing anions (CO32– to CO2 and PO43–) because they are converted to soluble compounds thus suggesting the observed reduction of oxygen ().
Table 1. Elemental composition of camel bone and ossein.
Morphology of camel bone and ossein
Scanning electron microscope was used to obtain morphological characteristic of camel bone and ossein. shows morphological changes of camel bone due to demineralization. The initial smooth and intact surface of camel bone was lost during to demineralization. After 2 days, the resulting matter surface becomes rough which increases after 5 days of demineralization. Ca salt, hydroxyapatite, inherent in bone structures is responsible for strengthening of the matrix and crosslinks.[Citation23] Hence, removal of Ca salts during HCl demineralization led to disorganized bone scaffolds. Surface roughness dominated with observed crevices and apertures might be due to loss in Ca salt.
Gelatin extraction from ossein (demineralized camel bone)
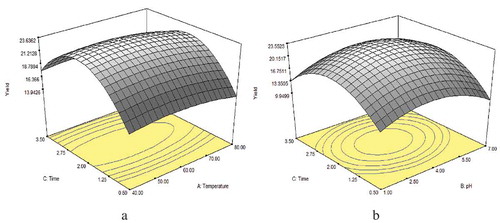
In order to obtain high gelatin yield, three processing conditions (temperature, pH, and time) were investigated using center composite design (CCD) in random surface methodology (RSM). shows that the yield of gelatin range from 8.54% to 25.33% for bone. Significant (P < 0.05) effects were observed at quadratic level of time and pH for gelatin extraction from ossein. The effect of extraction time might be due to the additional time required to uncoil the crosslink in collagen prior to gelatin formation.
Table 2. Optimization result: yield of gelatin from camel skin and bone.
The estimated model coefficients were calculated by regression analysis on the yield of gelatin from camel bone. Statistical analysis revealed that the model was significant (P < 0.05), suggesting that the model is suitable for prediction of gelatin yields from bone (Eq. (1)):
where A represents temperature, B represents pH, and C represents extraction time.
The optimum regions in graphical format ( and ) of the response of the optimization study are easy to visualize. The figures show that optimum regions were achieved at certain points within the range of extraction conditions. The analysis of variance (ANOVA) result of the gelatin yield showed that extraction parameters had profound effects on the yield. The model describing the effects of investigated parameters was highly significant (P ˂ 0.05). Extraction temperature, pH, and extraction time linearly significantly contributed to the optimum gelatin yield. The ANOVA also revealed a positive interaction between temperature and time as their interaction significantly affected the optimum gelatin yield obtainable from the camel bone. All extraction factors exhibited optimum region toward the center points with obvious oval shape at their respective contour lines. Further statistical analysis gave optimum conditions () for high gelatin yield (23.66%) from camel ossein. Validation of the optimized process gave gelatin yield of 21.33% which is very close to theoretical yield.
Gel strength of camel bone gelatin
Gel strength, a measure of firmness, is an important mechanical property of gelatin that is often used for quality assessment. It measures the amount of force required to push a plunger through a depression of 4 mm into the matured gelatin. The value of the force (in grams) is referred to as bloom number. Based on the optimum extraction conditions in , the gel strength of optimized camel bone gelatin reached 205.74 ± 2.6 g. The result obtained for camel bone gelatin falls within that range of commercial gelatin obtained from mammals. The gelling strength of commercial gelatins ranges from 100 to 300 g, but gelatins with bloom values of 250–260 are most desired.[Citation24] Gelatin used in food usually runs from 125 to 250 bloom; the unflavored gelatin sold in supermarkets is at the higher end of this range. The highest grade in commerce is around 300 bloom, which is used in food applications and to make special effects makeup to create prostheses, such as fake wounds. Gelatins with low bloom numbers are used to clear cloudiness in beverages.[Citation25]
Table 3. Gelatin yield and optimum conditions for camel skin and bone gelatin extraction.
Conclusion
This investigation offered adequate insight into chemical and structural changes caused by dilute acid during conversion of camel bone to ossein and then to gelatin. Acidic treatment reduced elemental calcium within 5 days to yield ossein with collagen-like characteristics. The Ca removal process was engineered by re-organization and morphological modification through removal of hydroxyapatite. FT-IR analysis revealed presence of nitrogenous organic residue in the final product. We equally conducted studies on conversion of camel-bone ossein to gelatin. Gelatin was successfully extracted from camel bone and skin under tolerable conditions. Application of CCD-RSM assisted in optimizing the extraction conditions (temperature, pH, and extraction time) and the extracted gelatins were characterized. Further work will be carried out to explore food and non-food applications of camel gelatin.
Funding
The project was funded by the National Plan for Science, Technology, and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia (award number 12-AGR2487-02).
Additional information
Funding
References
- Zhao, Y.; Li, Z.; Yang, W.; Xue, C.; Wang, Y.; Dong, J.; Xue, Y. Modification of Gelatine with Galla chinensis Extract, a Natural Crosslinker. International Journal of Food Properties 2016, 19, 731–744.
- Shahiri Tabarestani, H.; Sedaghat, N.; Jahanshahi, M.; Motamedzadegan, A.; Mohebbi, M. Physicochemical and Rheological Properties of White-Cheek Shark (Carcharhinus dussumieri) Skin Gelatin. International Journal of Food Properties 2015, 19(12), 2788–2804.
- Gómez-Guillén, M.; Turnay, J.; Fernández-Dıaz, M.; Ulmo, N.; Lizarbe, M.; Montero, P. Structural and Physical Properties of Gelatin Extracted from Different Marine Species: A Comparative Study. Food Hydrocolloids 2002, 16, 25–34.
- Liu, H.; Lin, Y.; Guo, S. Structural Characteristics of Tilapia (Oreochromis mossambicus) Bone Gelatin: Effects of Different Liming Methods. International Journal of Food Properties 2015, 18, 2360–2373.
- Gómez-Guillén, M.; Pérez-Mateos, M.; Gómez-Estaca, J.; López-Caballero, E.; Giménez, B.; Montero, P. Fish Gelatin: A Renewable Material for Developing Active Biodegradable Films. Trends Food Science and Technology 2009, 20, 3–16.
- Abouheil, M.; Basmaeil, S.; Bakkar, M. A Standard Method for Jointing Camel Carcasses with Reference to the Effect of Slaughter Age on Carcass Characteristics in Najdi Camels. 3. Partition and Distribution of Carcass Fat. Animal Science 1991, 4, 219–225.
- Muyonga, J.; Cole, C.; Duodu, K. Fourier Transform Infrared (FTIR) Spectroscopic Study of Acid Soluble Collagen and Gelatin from Skins and Bones of Young and Adult Nile Perch (Lates niloticus). Food Chemistry 2004, 86, 325–332.
- Liu, H.; Han, J.; Guo, S. Characteristics of the Gelatin Extracted from Channel Catfish (Ictalurus Punctatus) Head Bones. LWT-Food Science and Technology 2009, 42, 540–544.
- Taheri, A.; Abedian Kenari, A.M.; Gildberg, A.; Behnam, S. Extraction and Physicochemical Characterization of Greater Lizardfish (Saurida tumbil) Skin and Bone Gelatin. Journal of Food Science 2009, 74, 160–165.
- Boran, G.; Regenstein, J.M. Fish Gelatin. Advances in Food and Nutrition Research 2010, 60, 119–143.
- Novitskaya, E.; Castro-Ceseña, A.; Po-Yu, C.; Vasquez, J.; Urbaniak, R.; Lee, S.; Hirata, G.; McKittrick, J. Investigations into Demineralized Cortical Bone. Materials Research Society Symposium Proceedings 2011, 1301, 1–8.
- El-Bassyouni, G.T.; Guirguis, O.W.; Abdel-Fattah, W.I. Morphological and Macrostructural Studies of Dog Cranial Bone Demineralized with Different Acids. Current Applied Physics 2013, 13, 864–874.
- Horneman, D.A.; Ottens, M.; Hoorneman, M.; Van Der Wielen, L.A.; Tesson, M. Reaction and Diffusion during Demineralization of Animal Bone. AIChE Journal 2004, 50, 2682–2690.
- Margarida, F.S.C.; Gabriela, M.; João, F.; Fernando, J.; Helena, F. Influence of Hydrochloric Acid Concentration on the Demineralization of Cortical Bone. Chemical Engineering Research and Design 2011, 89, 116–124.
- Wang, L.; An, X.; Yang, F.; Xin, Z.; Zhao, L.; Hu, Q. Isolation and Characterisation of Collagens from the Skin, Scale and Bone of Deep-Sea Redfish (Sebastes mentella). Food Chemistry 2008, 108, 616–623.
- Figueiredo, M.; Martins, A.; Gamelas, J. Characterization of Bone and Bone-Based Graft Materials using FTIR Spectroscopy; INTECH: Rijeka, 2012.
- Chang, M.C.; Tanaka, J. FT-IR Study for Hydroxyapatitecollagen Nanocomposite Cross-linked by Glutaraldehyde. Biomaterials 2002, 23, 4811–4818.
- Wang, L.; An, X.; Yang, F.; Xin, Z.; Zhao, L.; Hu, Q. Isolation and Characterisation of Collagens from the Skin, Scale and Bone of Deep-Sea Redfish (Sebastes mentella). Food Chemistry 2008, 108, 616–623.
- Power, R.C.; Salazar-García, D.C.; Wittig, R.M.; Henry, A.G. Assessing Use and Suitability of Scanning Electron Microscopy in the Analysis of Micro Remains in Dental Calculus. Journal of Archaeological Science 2014, 49, 160–169.
- Charlier, P.; Huynh-Charlier, I.; Munoz, O.; Billard, M.; Brun, L.; De La Grandmaison, G.L. The Microscopic (optical and SEM) Examination of Dental Calculus Deposits (DCD). Potential Interest in Forensic Anthropology of a Bio-Archaeological Method. Legal Medicine 2010, 12, 163–171.
- Bogert, L.J.; Hastings, A.B. The Calcium Salts of Bone. Journal of Biological Chemistry 1931, 94, 473–481.
- Salazar-García, D.C.; Richards, M.P.; Nehlich, O.; Henry, A.G. Dental Calculus is not Equivalent to Bone Collagen for Isotope Analysis: A Comparison between Carbon and Nitrogen Stable Isotope Analysis of Bulk Dental Calculus, Bone and Dentine Collagen from Same Individuals from the Medieval Site of El Raval (Alicante, Spain). Journal of Archaeological Science 2014, 47, 70–77.
- Khalil, M.S.; Beheri, H.H.; Fattah, W.I.A. Structural and Electrical Properties of Zirconia/Hydroxyapatite Porous Composites. Ceramics International 2002, 28, 451–458.
- Monsur, H.A.; Jaswir, I.; Salleh, H.M.; Alkahtani, H.A. Effects of Pretreatment on Properties of Gelatin from Perch (Lates niloticus) Skin. International Journal of Food Properties 2014, 17, 1224–1236.
- Schrieber, R.; Gareis, H. Gelatine Handbook: Theory and Industrial Practice. John Wiley & Sons: Germany, 2007.