ABSTRACT
The oxidative stability and compositional characteristics of the pumpkin seed oil (PSO) exposed to microwaves were studied during heating at 170°C. The oxidative indices such as free fatty acid (FFA), peroxide value (PV), p-anisidine value (p-AV), TOTOX, specific extinctions and thiobarbituric acid (TBA) value of oils were significantly increased, and the increments were found to be significantly higher (P < 0.05) in unroasted seed oil as compared to roasted seed oil. The relative contents of polyunsaturated fatty acids (PUFAs) were decreased to 84.7%, and saturated fatty acids (SFAs) were increased to 119.5% in unroasted sample, after 9 h of heating. On the other hand, in 12 min roasted samples, the relative contents of PUFAs were decreased to 97.0%, and SFAs were increased to 102.6% after 9 h of heating. The triacylglycerol species LLL and OLL levels were decreased as a consequence of increased heating time, and the reduction tended to be significantly higher in unroasted samples as compared to roasted ones. The oxidation products formed were also investigated by FTIR. The present results indicated that microwave roasting of pumpkin seeds markedly enhanced the oxidative stability of the oils during heating.
Introduction
The transformation of by-products and wastes generated by agro-food companies is of high importance as only a small portion of plant material is utilized directly for human consumption. As a residue of agro-food industries, pumpkin seeds are potentially good sources of antioxidants, beneficial to human health.[Citation1] PSO is nutritionally valuable and is used as salad or cooking oil in some countries.[Citation2,Citation3] Moreover, PSO can be an excellent source of bioactive molecules and polyunsaturated fatty acids and is used as preservatives and functional ingredients.[Citation4,Citation5] The impacts of different processing methods used to prepare oilseeds for human consumption are of utmost importance. Roasting is a rapid processing method that enhances the oxidative and tocopherol stability, antioxidant attributes, and amounts of saturated fatty acids in oils.[Citation6–Citation9] However, microwave processing of foods is a recent development, which is gaining momentum in household as well as large-scale food applications.[Citation10] Recent studies suggest that through microwave exposure of seeds, greater amount of bioactive compounds may penetrate into the produced oil.[Citation11,Citation12] Wroniak et al.[Citation13] stated that microwave pretreatment of rapeseed significantly increased oxidative stability of oils. Hojjati et al.[Citation14] reported that pistachios could be successfully roasted using microwaves as a fast and economical method that enhanced the total unsaturated fatty acid and phenolic contents.
On the other hand, during heating of oil, thermolytic and oxidative reactions occur with the formation of both volatile and nonvolatile decomposition products.[Citation15] Monitoring the changes in properties of oils during heating is an effective method to assess thermal oxidation changes in the oils. Vaidya and Choe[Citation15] observed roasting of seeds prior to the oil extraction improved the heat stability of the mustard seed oil. Of course, each oil has a characteristic pattern of TAGs, and the properties of a particular oil are determined mainly by the abundance of different TAG molecular species.[Citation16] The changes in stability, composition, and distribution of fatty acids in triacylglycerols of oil, which occur during conventional oven roasting[Citation17–Citation19] and microwave roasting[Citation20,Citation21] of pumpkin seeds (Cucurbita pepo L.), have been well investigated. However, there is very little or no information available on the influence of microwave roasting on oxidative stability and composition of pumpkin (Cucurbita maxima Duch.) seed oils during heating. This study was therefore undertaken to investigate the effect of microwave roasting prior to oil extraction on the oxidative stability, fatty acid composition, and triacylglycerol species of the pumpkin (Cucurbita maxima Duch.) seed oils during thermal oxidation/heating at elevated temperature.
Materials and methods
Materials
Pumpkin seeds (Cucurbita maxima Duch.) were collected in October 2014 directly from the grower located in the Kustia, Bangladesh. The total weight of 50 pumpkin seeds was 8.12 g, and length, width, and thickness of each seed were in average 1.85, 0.95, and 0.31 cm. respectively. The seeds were cleaned, dried in sunlight, and stored in a refrigerator at 4°C in sealed polyethylene bags. All chemicals and solvents used were of analytical grade. Thioburbituric acid was from HiMedia Laboratories (Mumbai, India). Acetic acid and standards were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). All other chemicals and solvents were from Merck (Darmstadt, Germany or Mumbai, India) unless otherwise stated.
Roasting and oil extraction
Pumpkin seeds were arranged in a single layer in Pyrex petri dishes (12 cm diameter) and then placed on the turntable plate of the microwave oven (MS3042G, LG, China). After covering the dishes, the contents were then roasted at a frequency of 2450 MHz (capable of generating 510 W power) for 4, 8, and 12 min based on trial results. After roasting, the seeds were allowed to cool at room temperature and thoroughly mixed prior to crushing and stored at 4°C in sealed plastic bags. Afterwards, the seeds were reduced to powder and moisture contents were determined by AOAC official method 930.15.[Citation22] The Soxhlet method (no. 963.15) as described by AOAC was used to extract oil with n-hexane for 8 h.[Citation22] After extraction, the oil in hexane mixture was filtered followed by evaporation of the solvent in vacuo at 45°C. The resulting oil was weighed and stored into capped glass bottles at a temperature below ̶ 16°C for further studies.
Thermal oxidation of samples
The roasted or unroasted PSOs (70 g) were put into 100 mL beakers and placed in an electric oven at 170°C in order to accelerate the lipid oxidation and thermal degradation. Oil samples were collected at intervals of 0, 3, 6, and 9 h for analyses.
Oxidative indices
American Oil Chemists’ Society official methods (AOCS)[Citation23] were used for determining FFA content (method Ca 5a-40), PV (method Cd 8-53), and TBA value (method Cd 19-90). Specific extinctions (method p2.15) at 233 and 269 nm (ECitation1%233 and ECitation1%269) and p-AV (method p2.4) of the samples were determined by means of a spectrophotometer (T 60, PG Instruments, Leicestershire, UK) according to the PORIM test methods.[Citation24]
Colour development
As an index of colour development,[Citation25] the absorbance at 420 nm of 5.0% (w/v) solutions of oils in chloroform was determined with a spectrophotometer (T 60U, PG Instruments, Leicestershire, UK).
Fatty acid composition (FAC)
FAC was determined by rapid method of AOCS[Citation23] Cd 14c-94 using gas chromatography (7890A, Agilent Technologies, Santa Clara, USA) furnished with a SP-2340 (Supelco Inc., Bellefonte, PA, USA) fused silica capillary column (60 m x 0.25 mm i.d x 0.20 μm film thickness) and a flame ionization detector. Fatty acids of the oil samples were transesterified to their corresponding methyl esters following PORIM[Citation24] test method p3.4 prior to analysis by gas chromatography. The carrier gas was nitrogen at a pressure of 70 psi. The oven temperature was set as follows: initial temperature of 100°C and programmed to increase to 250°C at 10°C/min. The detector and injector temperatures were both maintained at 250°C. Methyl esters were quantified by comparing the retention times and peak area of the unknowns with known FAME standards (Supelco, Bellefonte, PA).
Triacylglycerol (TAG) composition
The TAG composition of the oil was analyzed according to IUPAC[Citation26] standard method 2.325 by high-performance liquid chromatography (HPLC). TAGs were analysed by injecting the sample (10% w/v oil in acetone) through a Rheodyne valve into a 0.2 μL loop of HPLC system (Waters 2695, Waters Corporation, Milford, USA) equipped with refractive index detector. The column (50 mm long, 2 mm i.d) was packed with 5 μm Chromolith RP18e (Merck, Darmstadt, Germany) and kept in an oven at 20°C. The mobile phase was acetone: acetonitrile (65:35, v/v) at a flow rate of 0.7 mL/min. Identification of the TAGs was performed by comparing with the standards (Sigma-Aldrich Co., St. Louis, MO, USA).
FT-IR spectroscopy
FT-IR spectra were recorded on a spectrometer (IRAffinity- 1S, Shimadzu Corporation, Kyoto, Japan) equipped with a high-sensitivity pyroelectric detector (deuterated L-alanine doped triglycine sulphate) and connected to software of the lab solutions DBIR operating system. Samples were applied to a sodium chloride cell, and spectra were run between 850 cm–Citation1 and 4000 cm–Citation1 with a 15 scans and 4 cm−Citation1 resolution. The spectra were corrected against the background spectrum of air. The spectra were recorded as absorbance values at each data point.
Statistical analysis
All data were expressed as the mean and standard deviation (SD) and were subjected to one way analysis of variance (ANOVA). Mean values were compared at P<0.05 significant level by Duncan’s multiple range test using IBM SPSS 22 statistics.
Results and discussion
Moisture content of fresh pumpkin seed was 5.5% and decreased to 4.0, 3.9, and 3.2% with increase of roasting times 4, 8, and 12 min, respectively. The yields of crude oils from roasted seeds were in the range of 38.3% to 39.7% (dry basis), same as to the value 39.0% reported by Francesco et al.[Citation4] Roasted samples had significantly higher (p < 0.05) oil yields than the unroasted one (37.4%). Obtained results concurred with previously published results by some authors[Citation6] who reported that the oil content increased as a result of seed roasting process.
Oxidative indices
As shown in , FFA levels of oils were significantly increased (P < 0.05) as a consequence of increasing thermal oxidation time. The increase in FFA content for unroasted sample was significantly higher (P < 0.05) than that for the roasted samples. At the end of 9 h heating, the amount of FFA was found to be highest (11.7%) in unroasted sample with the lowest (9.0%) in 12 min roasted sample. From , it was found that accumulation of primary oxidation products as measured by PV was faster in unroasted sample compared to the roasted samples during the thermal oxidation. Vaidya, and Eun[Citation27] also reported the lower increment of PV in roasted walnut oil than in unroasted sample during accelerated storage. There was an initial sharp increase in the PV for all the samples from 0 to 6 h, and after that the rate slowed down. This reduction could be explained by the formation of secondary oxidation products from very unstable primary oxidation products. As reported elsewhere, the PV decreases as oxidation proceeds due to rapid decomposition of hydroperoxides.[Citation28]
Figure 1. Changes of chemical characteristics of unroasted (MW-0) and roasted (MW-4, roasted at 4 min; MW-8, roasted at 8 min; and MW-12, roasted at 12 min) PSOs during heating at 170°C. (a) FFA, (b) peroxide value, (c) p- Anisidine value, (d) TOTOX value, and (e) TBA value. Each value is the mean ± standard deviation of triplicate determinations. Values in each heating grouping with different letters on bar are significantly different (p < 0.05).
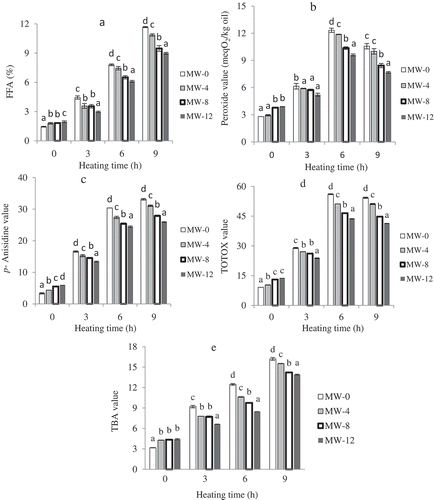
The PV and p-AV are commonly used to estimate degree of oxidative deterioration in heated oils. The seed roasting significantly (p < 0.05) reduced p-AV in PSOs as compared to unroasted samples (Fig. 1c). Cai et al.[Citation29] reported similar results after 12 days of oxidation of pine nut oil. The p-AVs from lowest to highest were observed in samples roasted at 12, 8, 4, and 0 min after 9 h heating. From the present study, oils produced from roasted seeds had an extended shelf-life, probably through the generation of Maillard browning reaction products (MRPs). Various mechanisms involved in the antioxidant activity of MRPs include radical chain-breaking activity,[Citation30] scavenging of reactive oxygen species, decomposing hydroperoxides and metal chelation, thus retarding the formation of primary and secondary oxidation products;[Citation31] thereby extending the shelf life of oils. p-AV is often used in the industry in conjunction with PV to calculate the so-called total oxidation or TOTOX value given as: TOTOX = 2PV + p-AV.[Citation32] As shown in Fig. 1d, significant differences (p < 0.05) in TOTOX values in PSOs were observed during thermal oxidation. The TOTOX values, from lowest to highest, were displayed in samples roasted at 12, 8, 4, and 0 min. The obtained results clearly demonstrated greater oxidative stability of PSOs prepared with increased roasting time. As shown in , the changes in TBA values of roasted PSOs were found to be significantly lower (P < 0.05) compared to unroasted seed oil. This indicates that the oils from unroasted seeds were more susceptible to oxidation at high temperature than roasted ones. Moreover, a sharp increase in TBA values was noted for unroasted oils at 3 and 6 h heating followed by a decrease; this may be due to volatilisation of secondary oxidation products or their break down.
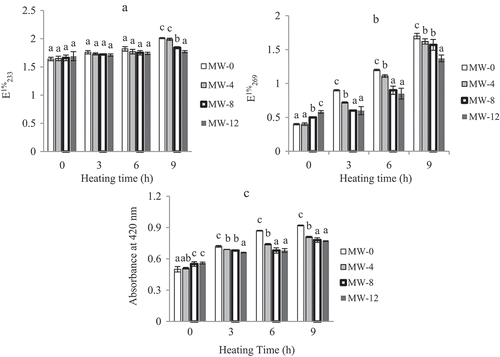
As shown in and , ultraviolet absorptions at 233 nm (ECitation1%233) were increased insignificantly (P < 0.05), whereas at 269 nm (ECitation1%269), these were increased significantly (P < 0.05) for all the samples throughout the heating periods. The levels of conjugated dienes and trienes at the end of 9 h heating were however highest in unroasted samples, with lowest level found in 12 min roasted samples. These lower levels are the indications of good oxidative stability of roasted samples compared to unroasted ones.
Figure 2. Changes of specific extinctions at 232 (a) and 268 nm (b), and in colour (absorbance at 420 nm) (c), of unroasted (MW-0) and roasted (MW-4, roasted at 4 min; MW-8, roasted at 8 min; and MW-12, roasted at 12 min) pumpkin seed oils during heating at 170°C. Each value is the mean ± standard deviation of triplicate determinations. Values in each heating grouping with different letters on bar are significantly different (p < 0.05).
Colour development
Figure 2c presents the absorbance values of PSOs from raw and roasted seeds as indices for colour development in the products. The absorbance values ranged from 0.50 to 0.92 at 420 nm were increased significantly (P < 0.05) during thermal treatment, and these increments were found to be higher in the unroasted samples. The increase in colour of oils with increasing roasting and thermal temperature was due to the formation of MRPs at elevated temperatures. Thus, the present results lend support to earlier finding, which indicated an increase in the colour of oils with increasing roasting temperature of cashew nut.[Citation33]
Fatty acid composition
As typified in , the major fatty acids in fresh PSO were linoleic (37.9%), oleic (33.9%), palmitic (18.9%), and stearic (7.7%). The average joined content of these four fatty acids was 97.8%, being slightly lower than that in published results 98.8%.[Citation34] Other fatty acids, such as C12:0, C14:0, C16:1, C18:3, C20:0, C20:1, C22:0, and C22:1,were also present in concentrations less than 1.0%. The fatty acid composition suffered slight changes upon microwave roasting. During roasting, the percentage of linoleic acid tended to decrease slightly, whereas the percentage of palmitic and stearic acids increased slightly. This trend was probably due to PUFA degradation and was in good agreement with that reported by Henna & Tan[Citation35] and Vaidya & Choe.[Citation36] The contents of total SFA, MUFA, and PUFA in the studied oils were 27.1, 34.2, and 38.6%, respectively, for unroasted and 29.1, 33.3, and 37.5%, respectively, for 12 min roasted samples before thermal treatment (). In unroasted samples, relative contents of PUFA decreased rapidly to 84.7%, while of SFA increased rapidly to 119.5% after 9 h of heating. There was a slight decrease of relative contents of PUFA in 4, 8 and 12 min roasted samples to 93.3, 94.6 and 97.0%, respectively, at the end of 9 h heating. On the other hand, relative contents of SFA in the samples roasted at 4, 8 and 12 min increased slightly to 109.6, 105.0 and 102.6, respectively, at the same time. These results were consistent with previous reports showing that contents of PUFA decreased rapidly, whereas contents of SFA increased during lipid peroxidation.[Citation36,Citation37] Moreover, the ratio of polyunsaturated to saturated fatty acids (P/S) of all samples decreased with oxidation time, which enabled to evaluate the oil oxidation.[Citation38] The highest decreased amount was observed for the unroasted sample (0.42 unit) followed by 4 min (0.20 unit) and 8 min (0.13 unit) with the least in 12 min (0.07 unit) roasted samples. These results indicated the oxidation proceeded more slowly in the roasted samples than in the unroasted ones during thermal treatment.
Table 1. Fatty acid composition (%) of unroasted and roasted pumpkin seed oils before thermal oxidation.
Table 2. Saturated, monounsaturated, and polyunsaturated fatty acid composition of unroasted and roasted pumpkin seed oils during thermal oxidation.
Triacylglycerol (TAG) composition
As shown in , dominant TAG (P, palmitic; S, stearic; O, oleic; L, linoleic) species consisted of POL (23.5%), OOL (15.8%), OLL (14.0%), POO (11.3%), and PLL-MOL (9.7%), with small amounts of LLL, PLP, OOO, and POP and minor amounts (<1.5%) of SOO, POS, and SOS. These results are almost similar with the findings of other researchers.[Citation20] The TAGs species LLL, OLL, and PLL-MOL levels of oil samples were decreased as a consequence of increasing heating time, and the reduction tended to be significantly higher (P < 0.05) in the unroasted samples compared to roasted samples (, , and 3c). These results are in accordance with the results obtained in FAC analysis by GC.
Figure 3. Changes of triacylglycerol composition of unroasted (MW-0) and roasted (MW-4, roasted at 4 min; MW-8, roasted at 8 min; and MW-12, roasted at 12 min) PSOs during heating. (a) LLL, (b) OLL, (c) PLL-MOL, (d) OOL, (e) POL, (f) PLP, (g) OOO, (h) POO, (i) POP. Each value is the mean ± standard deviation of triplicate determinations. Values in each heating grouping with different letters on bar are significantly different (p < 0.05).
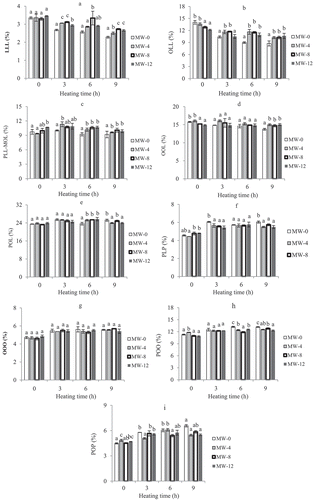
No significant change in OOL level was noticed in all samples at the end of 3 and 6 h heating; a significantly greater loss (P < 0.05) was observed in unroasted samples after 9 h heating (Fig. 3d). These results depend on differences in the amounts of TAGs composed of oleic and linoleic acids. There was a slight increase in percentage of POL, PLP, OOO, POO, or POP during heating, and these increments tended to be lower in the roasted oils (–3i). The reason is these TAGs are predominantly composed of saturated or monounsaturated fatty acids. These results are in accordance with those reported by earlier researchers.[Citation20] Good agreement between the fatty acid and TAG composition was also found in this work.
Evaluation by FT-IR
FTIR analysis provides a rapid means of evaluating the oxidative state of an oil or of monitoring changes in oil undergoing thermal stress.[Citation39] illustrated the most important spectral changes under oxidative conditions produced in roasted (0, 4, and 12 min) PSOs. The assignments of functional groups responsible for IR absorption peak are as follows: 3008 (C–H stretching vibration of the cis-double bond); 2927 and 2854 (symmetric and asymmetric stretching vibration of CH2); 1745 (C=O stretching vibrations); 1465 (bending vibrations of the CH2 and CH3); 1377 (bending vibrations of CH2 groups); 1261, 1161, 1099, and 1016 (C–O stretching vibration).[Citation40,Citation41] Spectral changes appearing at the 3050–2800 and 1745 cm−Citation1 region, after heating at elevated temperatures, aid the oxidation process monitoring.[Citation42]
Figure 4. Changes of FT-IR spectra of PSOs extracted from unroasted and roasted pumpkin seeds during heating. a) 0 h heating b) 3 h heating c) 6 h heating d) 9 h heating.
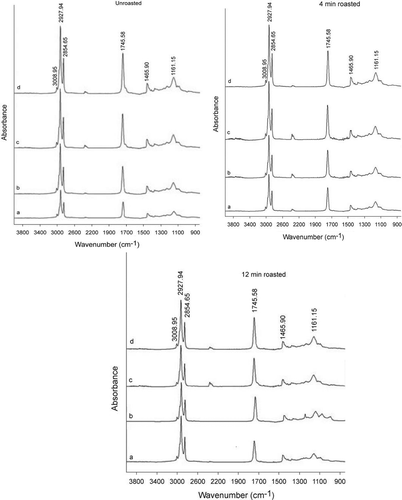
The intensity (absorbances) of the cis-double bond near 3008 cm−Citation1 (shoulder) remained almost unaltered or suffers a very slow shifting towards smaller values during oxidative stress. A similar trend was followed by Liang et al.[Citation43] for walnut oils during heating. The bands at 2927 cm−Citation1 and 2854 cm−Citation1 increased their absorbance due to surrounding chemical changes as a consequence of the oxidation process. This fact can be related to the advanced state of oxidation of samples.[Citation44] There was another major absorbance near 1745 cm−Citation1 associated with the appearance of saturated aldehyde functional groups,[Citation45] tended to increase with the oxidative treatment. The higher the intensity at 1745 cm−Citation1, the more carbonylic compounds present. The intensity of the observed band at near 1465 tended to increase with the oxidative treatment. A similar result was achieved by Valdés et al.[Citation44] when monitoring the oxidative stability in almonds by ATR-FTIR. A very weak band near 1161 cm−Citation1 was proved to be closely related to the proportion in the sample of saturated acyl groups.[Citation41] The intensity of this band suffered similar changes during the oxidation process and its intensity was increased. A similar trend was found for the band 1163 cm−Citation1 by Liang et al.[Citation43] in walnut oils during heating. The results of this study showed that the absorbance value was influenced by the roasting and heating conditions employed. During heating, the absorbance values () of all peaks increased (except at 3008 cm−Citation1) in all oils. These increments were found to be higher in the unroasted oil, indicating the oxidation proceeded more slowly in the roasted samples compared to unroasted ones.
Table 3. IR absorbance values of unroasted and roasted pumpkin seed oils during thermal oxidation.
Conclusion
This study reflected the adverse effects of thermal treatment on the oxidative stability and composition of PSOs. Oxidative stability data indicated that the studied oils extracted from roasted seed were the most resistant to the formation of lipid oxidation products. This also revealed that microwave pretreatment might significantly modify the composition of oil extracted from this seed. With high-temperature heating periods, both roasted and unroasted seed oils become oxidized, with a degradation of PUFA and formation of primary and secondary oxidation products. The MRPs possibly formed during roasting, could retard the oxidative deterioration of oils. In conclusion, the observed changes in oxidative indices, FAC, TAGs, and FTIR spectral data at 9 h of oxidative treatment were more evident for unroasted pumpkin seeds indicating a higher extent of oxidation compared to those observed in roasted samples.
References
- Saavedra, M.J.; Aires, A.; Dias, C.; Almeida, J.A.; De Vasconcelos, M.C.; Santos, P.; Rosa, E.A. Evaluation of the Potential of Squash Pumpkin By-products (seeds and shell) as Sources of Antioxidant and Bioactive Compounds. Journal of Food Science and Technology 2015, 52, 1008–1015.
- Maryam, J.; Sayed, A.H.G.; Mehdi, R. The Chemical Composition of the Seeds of Iranian Pumpkin Cultivars and Physicochemical Characteristics of the Oil Extract. European Journal of Lipid Science and Technology 2012, 114, 161–167.
- Wenzl, T.; Prettner, E.; Schweiger, K.; Wagner, F.S. An Improved Method to Discover Adulteration of Styrian Pumpkin Seed Oil. Journal of Biochemical and Biophysical Methods 2002, 53, 193–202.
- Francesco, S.; Maria, C.S.; Marina, P.; Gabriella, F.; Floriana, B.; Maria, G.V. Physico-Chemical Properties and Fatty Acid Composition of Pomegranate, Cherry and Pumpkin Seed Oils. Journal of the Science, Food and Agriculture 2016, 96, 1730–1735.
- Procida, G.; Stancher, B.; Cateni, F.; Zacchigna, M. Chemical Composition and Functional Characterisation of Commercial Pumpkin Seed Oil. Journal of the Science, Food and Agriculture 2013, 93, 1035–1041.
- Jau-Tien, L.; Shih-Chun, L.; Chao-Chin, H.; Yung-Shin, S.; Chia-Ying, H.; Deng-Jye, Y. Effects of Roasting Temperature and Duration on Fatty Acid Composition, Phenolic Composition, Maillard Reaction Degree and Antioxidant Attribute of Almond (Prunusdulcis) Kernel. Food Chemistry 2016, 190, 520–528.
- Bipin, V.; Jong-Bang, E. Effect of Roasting on Oxidative and Tocopherol Stability of Walnut Oil During Storage in the Dark. European Journal of Lipid Science and Technology 2013, 115, 348–355.
- Oracz, J.; Nebesny, E. Antooxidant Properties of Coca Beans (Theobroma cocao L.): Influence of Cultivar and Roasting Conditions. International Journal of Food Properties 2016, 19, 1242–1258.
- Hama, J.R. Comparison of Fatty Acid Profile Changes Between Unroasted and Roasted Brown Sesame (Sesamumindicum L.) Seed Oils. International Journal of Food Properties 2016. doi:10.1080/10942912.2016.1190744.
- Behera, S.; Nagarajan, S.; Rao, L.J.M. Microwave Heating and Conventional Roasting of Cumin Seeds (Cuminum cyminum L.) and Effect on Chemical Composition of Volatiles. Food Chemistry 2004, 87, 25–29.
- Yang, M.; Huang, F.; Liu, Ch.; Zheng, Ch.; Zhou, Q.; Wang, H. Influence of Microwave Treatment of Rapeseed on Minor Components Content and Oxidative Stability of Oil. Food and Bioprocess Technology 2013, 6, 3206–3216.
- Azadmard-Damirchi, S.; Habibi-Nodeh, F.; Hesari, J.; Nemati, M.; Achachlouei, B.F. Effect of Pretreatment with Microwaves on Oxidative Stability and Nutraceuticals Content of Oil from Rapeseed. Food Chemistry 2010, 121, 1211–1215.
- Wroniak, M.; Rekas, A.; Siger, A.; Janowicz, M. Microwave Pretreatment Effects on the Changes in Seeds Microstructure, Chemical Composition and Oxidative Stability of Rapeseed Oil. LWT - Food Science and Technology 2016, 68, 634–641.
- Hojjati, M.; Noguera-Artiaga, L.; Wojdylo, A.; Carbonell-Barrachina, A.A. Effects of Microwave Roasting on Physicochemical Properties of Pistachios (Pistacia vera L.). Food Science and Biotechnology 2015, 24, 1995–2001.
- Vaidya, B.; Choe, E. Effects of Seed Roasting on Tocopherols, Carotenoids, and Oxidation in Mustard Seed Oil During Heating. Journal of the American Oil Chemists’ Society 2011, 88, 83–90.
- Yoshida, H.; Hirakawa, Y.; Abe, S. Roasting Influences on Molecular Species of Triacylglycerols in Sunflower Seeds (Helianthus annuus L.). Food Research International 2001, 34, 613–619.
- Vujasinovic, V.; Djilas, S.; Dimic, E.; Basic, Z.; Radocaj, O. The Effect of Roasting on the Chemical Composition and Oxidative Stability of Pumpkin Oil. European Journal of Lipid Science and Technology 2012, 114, 568–574.
- Nederal, S.; Skevin, D.; Kraljic, K.; Obranovic, M.; Papesa, S.; Bataljaku, A. Chemical Composition and Oxidative Stability of Roasted and Cold Pressed Pumpkin Seed Oils. Journal of the American Oil Chemists’ Society 2012, 89, 1763–1770.
- Butinar, B.; Bucar-Miklavcic, M.; Mariani, C.; Raspor, P. New Vitamin E Isomers (gamma-Tocomonoenol and Alpha-tocomonoenol) in Seeds, Roasted Seeds and Roasted Seed Oil from the Slovenian Pumpkin Variety ‘Slovenskagolica’. Food Chemistry 2011, 128, 505–512.
- Yoshida, H; Tomiyama, Y.; Hirakawa, Y.; Mizushina, Y. Microwave Roasting Effects on the Oxidative Stability of Oils and Molecular Species of Triacylglycerols in the Kernels of Pumpkin (Cucurbita spp.) Seeds. Journal of Food Composition and Analysis 2006, 19, 330–339.
- Yoshida, H.; Tomiyama, Y.; Kita, S.; Mizushina, Y. Roasting Effects on Fatty Acid Distribution of Triacylglycerols and Phospholipids in the Kernels of Pumpkin (Cucurbita spp.) Seeds. Journal of the Science of Food and Agriculture 2005, 85, 2061–2066.
- AOAC. Official Methods of Analysis of AOAC International; AOAC International: VA, USA, 2005.
- AOCS. Official Methods and Recommended Practices of the American Oil Chemists’ Society, 4th Edn. AOCS press: Champaign, 1987.
- PORIM. PORIM Test Methods. Palm Oil Research Institute of Malaysia: Malaysia, 1995.
- Yoshida, H.; Takagi, S.; Mitsuhashi, S. Tocopherol Distribution and Oxidative Stability of Oils Prepared from the Hypocotyl of Soybeans Roasted in Microwave Oven. Journal of the American Oil Chemists’ Society 1999, 6, 915–920.
- IUPAC. Standard methods for the analysis of oils, fats and derivatives. International Union of Pure and Applied Chemistry, 7th revised and enlarged edn. Blackwell Scientific Publications: Oxford, 1992.
- Vaidya, B.; Eun, J. Effect of Roasting on Oxidative and Tocopherol Stability of Walnut Oil During Storage in the Dark. European Journal of Lipid Science and Technology 2013, 115, 348–355.
- Bester, E.; Butinar, B.; Bucar-Miklavcic, M.; Golob, T. Chemical Changes in Extra Virgin Olive Oils from Slovenian Istra after Thermal Treatment. Food Chemistry 2008, 108, 446–454.
- Cai, L.; Cao, A.; Aisikaer, G.; Ying, T. Influence of Kernel Roasting on Bioactive Components and Oxidative Stability of Pine Nut Oil. European Journal of Lipid Science and Technology 2013, 115, 556–563.
- Miranda, L.T.; Rakovski, C.; Were, L.M. Effect of Maillard Reaction Products on Oxidation Products in Ground Chicken Breast. Meat Science 2012, 50, 352–360.
- Kim, H.W; Choi, Y.S.; Choi, J.H.; Kim, H.Y.; Hwang, K.E.; Song, D.H.; Lee, S.Y.; Lee, M.A.; Kim, C.J. Antioxidant Effects of Soy Sauce on Color Stability and Lipid Oxidation of Raw Beef Patties During Cold Storage. Meat Science 2013, 91, 641–646.
- Wan, T.W.; Bahruddin, S.; Boey, P.L. Determination of TOTOX Value in Palm Oleins Using a FI-potentiometric Analyzer. Food Chemistry 2009, 113, 285–290.
- Chandrasekara, N.; Shahidi, F. Oxidative Stability of Cashew Oils from Raw and Roasted Nuts. Journal of the American Oil Chemists’ Society 2011, 88, 1197–1202.
- Nederal, S.; Petrovic, M.; Vincek, D.; Pukec, D.; Skevin, D.; Kraljic, K.; Obranovic, M. Variance of Quality Parameters and Fatty Acid Composition in Pumpkin Seed Oil During Three Crop Seasons. Industrial Crops and Products 2014, 60, 15–21.
- Henna, L.F.S.; Tan, P.P.A Comparative Study of Storage Stability in Virgin Coconut Oil and Extra Virgin Olive Oil Upon Thermal Treatment. International Food Research Journal 2009, 16, 343–354.
- Vaidya, B.; Choe, E. Effects of Seed Roasting on Tocopherols, Carotenoids, and Oxidation in Mustard Seed Oil During Heating. Journal of the American Oil Chemists’ Society 2011, 88, 83–90.
- Vaidya, B.; Choe, E. Stability of Tocopherols and Lutein In Oil Extracted from Roasted or Unroasted Mustard Seeds During the Oil Oxidation in the Dark. Food Science and Biotechnology 2011, 20, 193–199.
- Lee, J.; Kim, M.; Choe, E. Antioxidant Activity of Lignan Compounds Extracted from Roasted Sesame Oil on the Oxidation of Sunflower Oil. Food Science and Biotechnology 2007, 16, 981–987.
- Van de Voort, F.R.; Ismail, A.A.; Sedman, J.; Emo, G. Monitoring the Oxidation of Edible Oils by Fourier Transform Infrared Spectroscopy. Journal of the American Oil Chemists’ Society 1994, 71, 243–253.
- Lerma-Garcia, M.J.; Ramis-Ramos, G.; Herrero-Martinez, J.M.; Simo-Alfonso, E.F. Authentication of Exra Virgin Olive Oils by Fourier Transform Infrared Spectroscopy. Food Chemistry 2010, 118, 78–83.
- Guillen, M.D.; Cabo, N. Characterization of Edible Oils and Lard by Fourier Transform Infrared Spectroscopy. Relationships Between Composition and Frequency of Concrete Bands in the Fingerprint Region. Journal of the American Oil Chemists’ Society 1997, 74, 1281–1286.
- Vlachos, N.; Skopelitis, Y.; Psaroudaki, M.; Konstantinidou, V.; Chatzilazarou, A.; Tegou, E. Applications of Fourier Transform-infrared Spectroscopy to Edible Oils. Analytica Chimica Acta 2006, 573–574, 459–465.
- Liang, P.; Chen, C.; Zhao, S.; Ge, F.; Liu, D.; Liu, B.; Fan, Q.; Han, B.; Xiong, X. Application of Fourier Transform Infrared Spectroscopy for the Oxidation and Peroxide Value Evaluation in Virgin Walnut Oil. Journal of Spectroscopy 2013, 2013, 1–5.
- Valdés, A.; Beltrán, A.; Karabagias, I.; Badeka, A.; Kontominas, M.G.; Garrigós, M.C. Monitoring the Oxidative Stability and Volatiles in Blanched, Roasted and Fried Almonds Under Normal and Accelerated Storage Conditions by DSC, Thermogravimetric Analysis and ATR-FTIR. European Journal of Lipid Science and Technology 2015, 117, 1199–1213.
- Hamilton, R.J. The Chemistry of Rancidity in Foods. In Rancidity in Foods; Allen, J.C.; Hamilton, R.J.; Eds.; Elsevier Applied Science Publishers: London, 1989.
