ABSTRACT
In this research, dextran nanofibres were produced as novel carriers for entrapment of vitamin E. Morphological studies indicated that developed fine fibres were in the nano range without spherical beads. Thermal behaviour, chemical structure, and crystalline structure of vitamin E-loaded nanofibres were analysed by differential scanning calorimetry, Fourier transform infrared spectroscopy, and X-ray diffraction which showed that the vitamin was well incorporated within the amorphous structure of fibres without chemical interactions. Nanofibres were used for fortification of cheese. Sensory analysis of the fortified product was performed which showed better acceptability and texture of cheese containing nanofibres in comparison to blank and direct fortified samples. Nanofibres showed the capability of holding water and thus increasing cheese firmness. The release of vitamin E in gastrointestinal media showed that about 14% of vitamin could be released in gastric media and about 30% released in intestinal media. Finally, the results revealed that electrospinning can be used for production of dextran ultrathin fibres to entrap hydrophobic compounds with high potential for design of novel functional foods.
Introduction
Production and consumption of functional food is a subject of interest of both food factories and consumers. Vitamin E is a natural fat-soluble antioxidant which shows health benefits such as reducing cardiovascular disease, diabetes, and cancer.[Citation1–Citation3] However, its utilization for food fortification is limited due to its instability against heat and oxygen and low water solubility. Therefore, delivery systems should be designed to protect functional bioactives from undesirable processing, storage, and consumption conditions.[Citation4–Citation6] Delivery vehicles should be biocompatible, biodegradable, and should not have inverse effect on stability, texture, flavour, or appearance of food or beverage. Some researchers have worked on encapsulation of vitamin E for food[Citation7,Citation8] or pharmaceutical applications.[Citation9]
Nanofibres are defined as fibres with diameter in a nanometre range. They showed different applications in food technology such as filtration, enhancement of mechanical properties of films, and delivery of bioactives. Nanofibres are also preferred because they can be easily and controllably manufactured from natural polymers such that their functional and physical features are controlled and/or manipulated during their production.[Citation10] This technique has been previously used for encapsulation of food bioactives[Citation11] or probiotic bacteria.[Citation12]
Dextran is a bacterial polysaccharide that consists of R-l, 6 linked d-glucopyranose residues with some R-1, 2-, R-1, 3-, or R-1, 4 linked side chains. The most important property that makes dextran very versatile is that it is soluble in both water and some organic solvents. Some efforts have been made to produce nanofibres from dextran for tissue engineering[Citation13] and wound dressing.[Citation14] However, there is not any publication on encapsulation of food bioactives by dextran nanofibres. Due to the instability of vitamin E and superior features of nanofibres, the objectives of this study were to encapsulate vitamin E using dextran nanofibres, investigate the physicochemical properties of produced delivery system, and evaluate the potential application of vitamin-loaded nanofibres for cheese fortification.
Materials and methods
Materials
Dextran (produced by Leuconostoc mesenteroides, molecular mass of 70000) and tocopheryl acetate were supplied from Sigma Chemical Company (Missouri, USA) and DSM Nutritional Products Ltd Company (Heerlen, The Netherlands), respectively. Ethanol was purchased from Merck (Darmstadt, Germany). All other chemical compounds were of analytical grade.
Methods
Preparation of vitamin-loaded dextran nanofibres
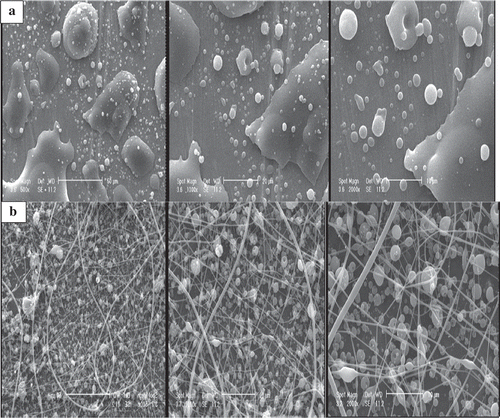
Initially the spinnability of solutions with dextran concentrations of 0.1875 and 0.375 g/ml was investigated. Despite systematic variations in the applied voltage (9–15 kV), flow rate (0.5–1.5 ml/h), and tip-to-target distance (5–20 cm), no fibres were obtained () except the sample with 0.375 g/ml dextran under a voltage of 14 kV, flow rate of 1 ml/h, and distance of 15 cm which was mainly composed of spherical beads (). Dextran solutions were prepared by dissolving 1 and 1.25 g dextran in 1 ml of 80:20 v/v water:ethanol solution and thoroughly mixed overnight.[Citation15] Vitamin-loaded solutions were prepared by dissolving 1 mg vitamin E in each 2 ml of the biopolymer solution. For preparing vitamin-loaded nanofibres, the solutions were delivered into insulin syringe (with an inner diameter of 0.318 mm) placed in a programmable syringe pump (Vita Teb Co., Iran). The flow rate of the pump was adjusted to 1 ml/h. In this research two voltage levels of 13 and 15 kV were applied by a positive high-voltage supply (Vita Teb Co., Iran). The distance between needle tip and collector was set to 15 cm.
Morphological study
The morphology of particles was examined using a scanning electron microscope (SEM) (Quanta 200, FEI). Samples were mounted on aluminium stubs using double-sided carbon tape and sputter-coated with gold under an argon atmosphere.[Citation16]
X-ray diffraction analysis
X-ray diffraction (XRD) analyses were carried out in order to characterize crystalline products. XRD patterns of samples were obtained using power X-ray diffractometer (Philips, Xpert-Pro, The Netherlands) with Cu Ka1 radiation generated at 30 mA and 50 kV. The samples were run over the most informative range from 3° to 90° of 2θ.[Citation14]
Fourier transform infrared analysis
Certification of the encapsulation of vitamin E in the nanofibre and formation of any possible interactions between vitamin and fibres was performed by Fourier transform infrared (FTIR) spectroscopy. Infrared spectra were scanned by an FTIR spectroscopy (WQF-510; Bomem, Canada) and recorded in the wave range of 400–4000 cm−1 at a resolution of 4 cm−1. Sample powder was mixed with KBr with sample to KBr ratio of 1:100.[Citation17]
Differential scanning calorimetry analysis
Investigation of thermal behaviour of nanofibres is important to evaluate their stability under different temperatures. Thermal analysis of the fibres was studied by differential scanning calorimetry (DSC) (TA instruments, model DSC 204). Approximately 5 mg of particles were loaded in standard aluminium pans. The analysis was carried out at a temperature heating rate of 5°C/min and temperature range of 20–350°C.
Release study
Percentage of surface vitamin was determined by rapid shaking of nanofibre in chloroform for 2 min. Encapsulation efficiency was determined by HPLC method at a wavelength of 290 nm. Vitamin E release profiles from the nanofibres were studied in gastric (pH:1.2) and intestinal (pH:7.4) simulated solutions by applying dialysis bag method at 37°C. Nanofibre solution was sealed into the dialysis bag (Sigma, Canada) with a 12 kDa cut-off. The bag was then placed in a 48 ml gastric buffer for 2 h. It was subsequently subjected to 48 ml intestinal buffer for 6 h. At specified intervals, the amount of released vitamin was determined by HPLC method at the wavelength of 290 nm. To study the release kinetic of vitamin E in the gastric and intestinal media, the release data were kinetically modelled by zero-order, first-order and Higuchi models (Eqs. 1-3)[Citation18]:
where C is the vitamin release percentage at time t and k is the kinetic constant.
Sensory analysis
Fortified cheese was produced to study the potential application of produced vitamin E-loaded nanofibres. Three cheese samples were produced as blank, direct fortified vitamin E, and vitamin E-loaded nanofibre. The recommended dietary allowance for vitamin E is 10 mg α-tocopherol equivalents (TE) per day for men and 8 mg TE/day for women.[Citation19] Vitamin containing cheeses were enriched under which their vitamin addition be 20% of daily need in each serving sample (100 g cheese). Vitamin E or nanofibres were added to the cheese matrix and manually mixed with the salt. Cheese making was performed according to the procedure reported by Elsamani et al.[Citation20] Each of the 25 panelists (older than 18 years) tested a sample of each cheese and recorded their responses on a 7-point hedonic scale (1, dislikes; 7, likes very much) for flavour, after taste, texture (firmness), mouth-feel, colour (yellowness), homogeneity, and total acceptance.[Citation21] Data were subjected to analysis of variance and means were compared using LSD test.
Results and discussion
Electrospinning of vitamin E-loaded dextran solutions
In this work dextran nanofibres were formed for encapsulation of vitamin E. As mentioned earlier under low concentrations (0.1875 and 0.375 g/ml) no appropriate fibres were formed. However, for higher polymer concentrations deposition of particles and beads and formation of continuous fibres were observed.[Citation15] Vitamin E-loaded dextran solutions were spun with concentrations 1 and 1.25 g/ml extruded at 1 ml/h under voltages of 13 and 15 kV and distance of 15 cm from collector. These results are consistent with those reported by Deitzel et al. (2001) which indicated that the electrospun membrane of polyethylene oxide (PEO) was a mixture of fibres and beads when the solution viscosity was low.[Citation22] The fibres are produced when the product of solution intrinsic viscosity (η) and biopolymer concentration (c), known as the Berry’s number,Becr, is greater than a certain critical value (Be=[η]c) which is the characteristic property of each biopolymer.[Citation23] Below this critical value the jets of polymer solution are unstable and expected to break up and form droplets. However, for high viscosity solutions, the jets are broken up and riven into filaments to the collecting plate and form fibres.[Citation24]
Morphology of vitamin-loaded dextran nanofibres
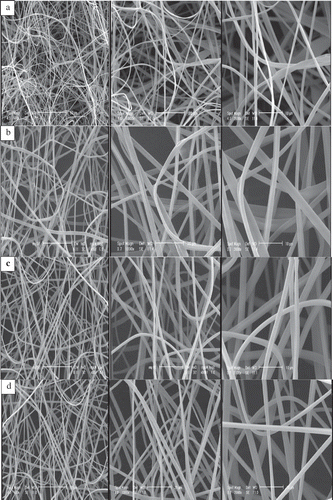
demonstrates SEM images of the electrospun dextran fibres. The SEM images of the vitamin-loaded fibres did not show the presence of beads, vitamin crystals, or other types of the aggregates over the surface of the fibres which implies that vitamin E was well incorporated within the electrospun fibres. Similar results were reported for poly(vinyl alcohol) nanofibre loaded with fish oil under low oil concentrations. While fibres produced from solutions with a fish oil load higher than 1.5% (w/w) showed spindle-like enlargements interspersed along the fibres.[Citation25] The average diameter of dextran fibres was approximately 400–1000 nm. Higher concentration of dextran at higher applied voltage led to thinner and more fine fibres (400 nm) (). The applied voltage should be sufficient to overcome the surface tension at the tip of the Taylor cone, in order to initiate the ejection of the charged jet.[Citation26] Using of higher voltage to a certain point, initially leads to the formation of fibres of smaller diameter, then thicker fibres are formed. Thus there is an ideal applied voltage that results in a minimum fibre diameter depending on the polymer and interacting parameters.[Citation27] Under a fixed applied voltage, higher amounts of polymer in the solution led to thinner and more uniform fibres. Changing the polymer concentration can vary the solution viscosity. Moreover, the surface tension coefficient depends on the polymer and solvent.[Citation24] It should be noted that there is also an optimum concentration under which beads or nanoparticles might be formed and for higher concentrations the solution is not spinnable.[Citation27] Similar diameter sizes were obtained for polyurethane–dextran[Citation28] and whey protein[Citation29] nanofibres.
XRD analysis
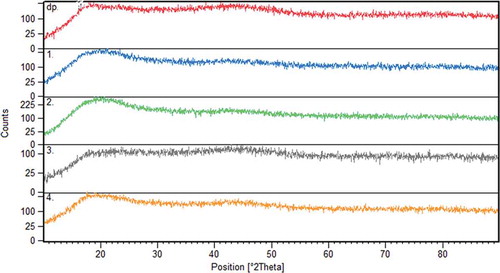
Vitamin E-loaded dextran nanofibres and pure dextran powder were subjected to XRD analysis in order to further illustrate the physical structure and distribution of vitamin E in the nanofibres (). The crystal structure depends on characteristics of the polymer (molecular weight),[Citation30] process parameters (applied voltage and flow rate), and the solvent medium (evaporation rate and polymer–solvent interaction).[Citation30] Dextran powder had typical broad peaks around 2θ of 20° and 45°, since it has an amorphous structure. The electrospun dextran fibres were amorphous as denoted by the absence of a characteristic diffraction peak and have the same peaks proving that loading the vitamin did not change the nature of the dextran nanofibres. It could be due to low weight proportion of vitamin to dextran. It also indicated that there was a good compatibility between dextran and vitamin E.[Citation14]
FTIR analysis
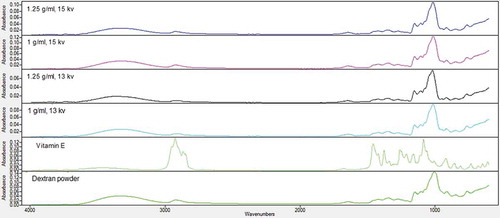
FTIR spectra were analysed to confirm the composition of the fibres. reveals the infrared spectra of pure dextran powder, pure vitamin E, and vitamin-loaded dextran fibres over the wavenumber range from 400 to 4000 cm−1. Several characteristic bands of dextran are observed, e.g. C-H bending at 760 and 846 cm–1, C-O-C stretching band at 1005 cm−1, C–O stretching at 1274 cm–1, CH3 bending at 1435 cm–1, CH stretching at 2922 cm–1, CH3 stretching at 3089 cm–1, and OH stretching at 3335 cm–1. FTIR spectrum of vitamin E showed characteristic C=O stretching vibration at around 900–1077 cm−1, C-O formation at 1205 cm−1, C=C formation at 1683 cm−1, and C-H alkanes group at 2925 cm−1. Absorption band of vitamin E at 3400–3650 cm−1 is attributed to the terminal hydroxyl group and peak at 1050–1250 cm−1 is due to the C–O stretching.
All the characteristic peaks of dextran and vitamin E were detectable in nanofibres’ spectra, although they showed overlap. However, due to low weight ratio of vitamin, its characteristic bands did not have much effect on spectra of nanofibres in comparison to dextran. The band of hydrogen bond was observed at 3470 cm−1 and the C-OH and C-O stretching modes were detected at 1470 and 1460 cm−1, respectively. The weak bands in the calculated spectrum at 1660 and 1570 cm−1 theoretically correspond to symmetric and asymmetric aromatic ring stretches, respectively.[Citation14,Citation17,Citation31]
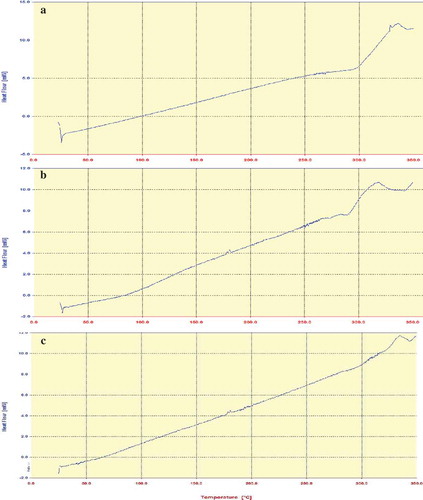
DSC analysis
Based on the SEM results, it is observed that treatment fabricated from the 1.25 g/ml dextran solution under a voltage of 15 kV had the best size; therefore this sample was selected for DSC analysis. DSC thermograms at the range of 20°C to 350°C are presented in . The thermogram of tocopheryl acetate exhibited the presence of an endothermic event around 340°C which was a characteristic of the thermal behaviour of this material.[Citation32] Dextran showed a wide endothermic peak at 320°C followed by an exothermic peak. Due to low weight proportion of vitamin to dextran, the characteristic peak of vitamin E in thermogram of vitamin E-loaded nanofibre was weak but yet detectable while its peak started from lower temperatures which was due to its overlap with dextran peak. It could be stated that no particular chemical interaction between vitamin E and dextran occurred which means that the vitamin was well dispersed in the fibre matrix and the crystallinity of the polymer was not affected by vitamin E incorporation. Aforementioned XRD data also show that the crystalline structure of dextran remained intact; FTIR results agreed with DSC data that not any adverse reactions were formed between dextran and vitamin.
Release study
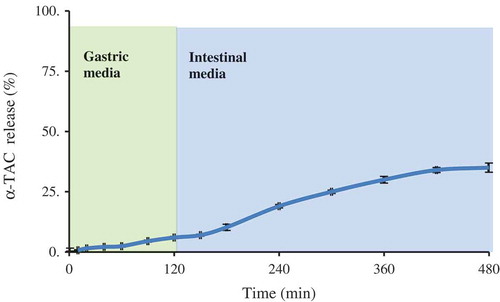
Encapsulation efficiency of vitamin E-loaded nanofibre was 98.3%. It could be due to high solubility of vitamin in dextran. The release profile of vitamin E from nanofibres is depicted in . The release percentages were about 6% and 35% in the gastric and intestine media, respectively. It is indicated that produced fibre can postpone vitamin release in acidic gastric media. Vitamin release from produced nanofibres was kinetically studied using zero-order, first-order, and Higuchi models. shows model parameters and their corresponding coefficients of determination. As can be observed, for gastric, intestine, and whole release media zero-order model showed the best fitness. It is indicated that release of vitamin is not concentration dependent and just varies with release time.
Table 1. Model parameters of vitamin E release from dextran nanofibres.
Sensory analysis
Sensory analysis was conducted for the three cheese samples, i.e. blank cheese, direct vitamin E fortified cheese, and vitamin E-loaded nanofibre fortified cheese. The nanofibre sample that showed smaller diameter was selected for sensory analysis. The results indicated that means of all studied features (except for colour) were significantly different (p < 0.01). Average and standard error of sensory properties are tabulated in . These data indicate that properties of nanofibre-containing cheese sample were all better than direct fortification of vitamin E (except for after taste). Interesting results were obtained for the texture of cheese samples which showed higher firmness of nanofibre-containing sample in comparison to blank and direct fortified cheeses. These results could be attributed to the texturizing effect of produced nanofibres that showed gelling properties. Finally, total acceptance data suggested that the consumer acceptability attributes of cheese containing vitamin E-loaded nanofibres are higher than other samples.
Table 2. Sensory evaluation of blank cheese, direct vitamin E fortified cheese, and vitamin E-loaded nanofibre fortified cheese.
Conclusion
Dextran nanofibres as a delivery system for vitamin E were fabricated by the electrospinning method. The polymer concentration and applied voltages were the main important factors affecting the electrospinnability of the bulk solutions and the morphology of the electrospun nanofibres. Uniform and thin dextran fibres with an average diameter of nanometre-scale (~400 nm) were obtained from 1.25 g/ml of dextran solution under a voltage of 15 kV. The results from SEM, FTIR, XRD, and DSC demonstrated that vitamin E had good compatibility with dextran and was able to distribute uniformly within the polymer fibre matrix. The fibres were in amorphous structure. Sensory analysis showed that produced nanofibres can enhance homogeneity, texture, and acceptance of fortified cheese in comparison to direct vitamin E fortified sample. These findings demonstrated the potential application of nanofibres for entrapment of bioactives and enrichment of food and beverage.
References
- Yang, Y.; McClements, D.J. Encapsulation of Vitamin E in Edible Emulsions Fabricated Using a Natural Surfactant. Food Hydrocolloids 2013, 30(2), 712–720.
- Sylvester, P.W., et al. Tocotrienol Combination Therapy Results in Synergistic Anticancer Response. Frontiers in Bioscience (Landmark edition) 2010, 16, 3183–3195.
- Songsermsakul, P.; Pornphairin, E.; Porasuphatana, S. Comparison of Antioxidant Activity of Grape Seed Extract and Fruits Containing High β-carotene, Vitamin C, and E. International Journal of Food Properties 2013, 16(3), 643–648.
- Fathi, M.; Mozafari, M.; Mohebbi, M. Nanoencapsulation of Food Ingredients Using Lipid Based Delivery Systems. Trends in Food Science & Technology 2012, 23(1), 13–27.
- Fathi, M.; Martin, A.; McClements, D.J. Nanoencapsulation of Food Ingredients Using Carbohydrate Based Delivery Systems. Trends in Food Science & Technology 2014, 39(1), 18–39.
- Dasgupta, N., et al. Fabrication of Food Grade Vitamin E Nanoemulsion by Low Energy Approach, Characterization and Its Application. International Journal of Food Properties 2016, 19(3), 700–708.
- Quintanilla-Carvajal, M.X., et al. Effects of Microfluidisation Process on the Amounts and Distribution of Encapsulated and Non-encapsulated α-Tocopherol Microcapsules Obtained by Spray Drying. Food Research International 2014, 63(Part A), 2–8.
- Tackenberg, M.W., et al. Orange Terpenes, Carvacrol and α-tocopherol Encapsulated in Maltodextrin and Sucrose Matrices via Batch Mixing. Journal of Food Engineering 2014, 135, 44–52.
- Laouini, A., et al. Characterization of Different Vitamin E Carriers Intended for Pulmonary Drug Delivery. International Journal of Pharmaceutics 2014, 471(1–2), 385–390.
- Rezaei, A.; Nasirpour, A.; Fathi, M. Application of Cellulosic Nanofibers in Food Science Using Electrospinning and Its Potential Risk. Comprehensive Reviews in Food Science and Food Safety 2015, 14(3), 269–284.
- Lemma, S.M., et al. Controlled Release of Retinyl Acetate from β-cyclodextrin Functionalized Poly(vinyl alcohol) Electrospun Nanofibers. Journal of Agricultural and Food Chemistry 2015, 63(13), 3481–3488.
- Fung, W.-Y.; Yuen, K.-H.; Liong, M.-T. Agrowaste-based Nanofibers as a Probiotic Encapsulant: Fabrication and Characterization. Journal of Agricultural and Food Chemistry 2011, 59(15), 8140–8147.
- Dahlin, R.L.; Kasper, F.K.; Mikos, A.G. Polymeric Nanofibers in Tissue Engineering. Tissue Engineering - Part B: Reviews 2011, 17(5), 349–364.
- Unnithan, A.R., et al. Wound-dressing Materials with Antibacterial Activity from Electrospun Polyurethane–Dextran Nanofiber Mats Containing Ciprofloxacin HCl. Carbohydrate Polymers 2012, 90(4), 1786–1793.
- Jiang, H., et al. Optimization and Characterization of Dextran Membranes Prepared by Electrospinning. Biomacromolecules 2004, 5(2), 326–333.
- Kriel, H.; Sanderson, R.D.; Smit, E. Coaxial Electrospinning of Miscible PLLA-core and PDLLA-shell Solutions and Indirect Visualisation of the Core-shell Fibres Obtained. Fibres & Textiles in Eastern Europe 2012, 20(2), 91.
- Jalaja, K., et al. Modified Dextran Cross-linked Electrospun Gelatin Nanofibres for Biomedical Applications. Carbohydrate Polymers 2014, 114, 467–475.
- Fathi, M., et al. Hesperetin-loaded Solid Lipid Nanoparticles and Nanostructure Lipid Carriers for Food Fortification: Preparation, Characterization, and Modeling. Food and Bioprocess Technology 2012, 6(6), 1464–1475.
- Murphy, S.P.; Subar, A.F.; Block, G. Vitamin E Intakes and Sources in the United States. The American Journal of Clinical Nutrition 1990, 52(2), 361–367.
- Elsamani, M.O., et al. Biochemical, Microbial and Sensory Evaluation of White Soft Cheese Made from Cow and Lupin Milk. LWT - Food Science and Technology 2014, 59(1), 553–559.
- Bermúdez-Aguirre, D.; Barbosa-Cánovas, G.V. Quality of Selected Cheeses Fortified with Vegetable and Animal Sources of Omega-3. LWT-Food Science and Technology 2011, 44(7), 1577–1584.
- Deitzel, J., et al. The Effect of Processing Variables on the Morphology of Electrospun Nanofibers and Textiles. Polymer 2001, 42(1), 261–272.
- Jaworek, A., et al. Nanocomposite Fabric Formation by Electrospinning and Electrospraying Technologies. Journal of Electrostatics 2009, 67, 435–438.
- Fong, H.; Chun, I.; Reneker, D. Beaded Nanofibers Formed during Electrospinning. Polymer 1999, 40(16), 4585–4592.
- García-Moreno, P.J., et al. Encapsulation of Fish Oil in Nanofibers by Emulsion Electrospinning: Physical Characterization and Oxidative Stability. Journal of Food Engineering 2016, 183, 39–49.
- Wongsasulak, S., et al. Electrospinning of Food-grade Nanofibers from Cellulose Acetate and Egg Albumen Blends. Journal of Food Engineering 2010, 98(3), 370–376.
- Castillo, Y.S. Design of Experimentation to Systematically Determine the Interaction between Electrospinning Variables and to Optimize the Fiber Diameter of Electrospun Poly (d, l-lactide-co-glycolide) Scaffolds for Tissue Engineered Constructs. 2012.
- Unnithan, A.R., et al. Wound-dressing Materials with Antibacterial Activity from Electrospun Polyurethane–dextran Nanofiber Mats Containing Ciprofloxacin HCl. Carbohydrate Polymers 2012, 90(4), 1786–1793.
- Sullivan, S.T., et al. Electrospinning and Heat Treatment of Whey Protein Nanofibers. Food Hydrocolloids 2014, 35, 36–50.
- de Oliveira Mori, C.L., et al. Electrospinning of Zein/tannin Bio-nanofibers. Industrial Crops and Products 2014, 52, 298–304.
- Kumar, Y.S., et al. Microgravity Biosynthesized Penicillin Loaded Electrospun Polyurethane–dextran Nanofibrous Mats for Biomedical Applications. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2015, 477, 77–83.
- Almeida, M.M.d., et al. Stability Evaluation of Tocopheryl Acetate and Ascorbyl Tetraisopalmitate in Isolation and Incorporated in Cosmetic Formulations Using Thermal Analysis. Brazilian Journal of Pharmaceutical Sciences 2010, 46(1), 129–134.