ABSTRACT
Spectroscopic and molecular docking techniques were used to study the interaction of bis(indolyl)methane (BIM), poorly soluble in aqueous solutions, with bovine α-casein, as an efficient drug carrier. The results of Fourier transform infrared and circular dichroism spectroscopy demonstrated that α-casein interacts with BIM molecule mainly via hydrophobic and hydrophilic interactions, resulting changes in the secondary structure of α-casein. The fluorescence quenching measurements at 293–310 K revealed that the quenching mechanism was static, and the binding site of BIM to α-casein was singular. Thermodynamic analysis, along with the negative value of and positive value of
, indicates that the hydrophobic interaction, hydrogen bonds, and van der Waals forces played major roles in the complex formation process. The distance between donor and acceptor, obtained according to fluorescence resonance energy transfer and docking studies, indicated that BIM induced a change from a hydrophobic environment around the Trp-193 of α-casein to an aqueous or hydrophilic environment.
Introduction
Different routes of drug administration possess various levels of advantages and drawbacks. For example, oral and nasal deliveries result in high levels of drug in the blood with comparably poor release profiles; aerosol preparations are complex and problematic with regard to the load of drug. The lack of targeting, with possible damage to healthy cells, is one of the drawbacks of transdermal delivery. In order to overcome the delivery challenges, “targeted drug delivery” has been developed in the last few decades[Citation1].
Indole derivatives have been focused for their prominent role in various commercial applications for centuries. Bis(indolyl)methane (BIM), one of the key derivatives of indole, exhibits a wide range of important biological activities[Citation2]. In animal models, BIM also has shown efficacy in treatments for a range of carcinomas, including prostate,[Citation3] breast,[Citation4] pancreatic,[Citation5] bladder,[Citation6] renal cell,[Citation7] lung,[Citation8] and colon cancers[Citation9,Citation10]. BIM exhibits antimicrobial, antifungal, and antibacterial activities[Citation11,Citation12]. The oxidized form of BIM is utilized as a dye[Citation13] and as a colorimetric sensor[Citation14]. The major problem with BIM is its extremely low solubility in aqueous solution and poor biocompatibility.
Milk proteins may form structures useful for delivery of some bioactive compounds. These proteins have surface activity, resulting in their easy adsorption at interfaces. Therefore, they have been considered as a medium for delivering certain bioactive molecules that normally have low bioaccessibility[Citation15,Citation16]. Milk proteins are classified into two well-defined groups: casein and whey or serum protein[Citation17,Citation18]. In contrast to the whey proteins, caseins exist in particles containing many thousands of individual protein molecules, known as the casein micelles[Citation15]. Casein micelles contain four main phosphoproteins: (38%),
(10%),
(40%), and
(12%) casein, with molecular weights between 19 and 25 kDa. They contain a large number of proline residues, making them rheomorphic structures. The content of α-helix or β-sheet structures is low in caseins due to high levels of proline[Citation17,Citation19]. All the caseins also possess a high content (35–45%) of polar amino acids (Val, Leu, Ile, Phe, Tyr, and Pro) and therefore, low solubility in aqueous systems may be expected. However, the high content of phosphate groups, low level of sulfur-containing amino acids, and high carbohydrate content influence the polar amino acids. One of the structural differences between
and
is that while
contains no cysteine,
contains two cysteines, which normally exist as intermolecular disulfide bonds; that is, under non-reducing conditions,
–casein exists as a disulfide-linked dimer. The number of phosphate groups in the
–casein is 8, occasionally 9, and in
–casein it may be 10, 11, 12, or 13[Citation17].
In recent years, researches have shown that casein can be used to overcome the limitations related to the low solubility of polyphenol,[Citation20,Citation21] resveratrol,[Citation22,Citation23] curcumin,[Citation24,Citation25] vitamin D2 and A,[Citation26,Citation27] and lipids[Citation28] in water. In this work, the interaction between BIM with bovine milk α-casein was studied using Fourier transform infrared (FTIR), circular dichroism (CD), and fluorescence spectroscopy. The relative interaction strength and binding mode were measured by fluorescence spectroscopy. Some conformational changes of protein can be revealed by synchronous spectra. Besides, the interaction of BIM and α-casein was investigated using molecular docking technique.
Materials and methods
Bovine α-casein (70%, Sigma-Aldrich, Germany) was dissolved in pH 7.0 phosphate buffer containing 80 mM NaCl, 5.55 mM Na2HPO4, and 3.15 mM NaH2PO4 with an ionic strength of 0.1 M. The protein concentration after dialysis was determined from the absorbance at 280 nm using a UV–visible spectrophotometer and adjusted to the required concentration according to an extinction coefficient of 1.09 ml mg–1 cm–1[Citation29].
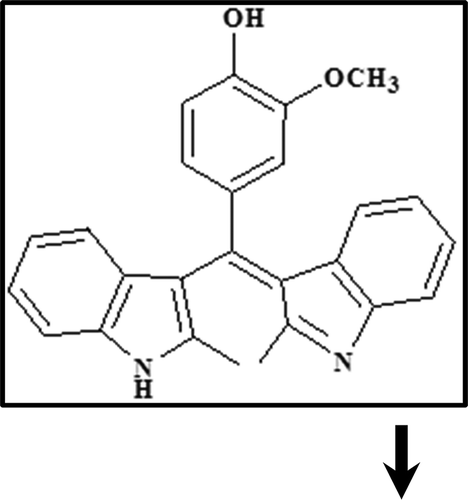
BIM () was synthesized and purified by the method described previously[Citation30]. As BIM was insoluble in water, a stock solution (1 mM) of BIM in acetonitrile was first prepared and then diluted with buffer solution so that the acetonitrile content in the final solution did not exceed 6%. All solutions were prepared using double distilled water and used freshly following their preparation.
FTIR and CD spectroscopy measurements
In order to conduct FTIR measurements, a solution of BIM was added stepwise to the protein solution (0.25 mM) with constant stirring to ensure homogeneity of solution and to reach the target BIM concentrations of 0.125, 0.25, and 0.5 mM. The solutions were then freeze-dried in a Christ Alpha 1-2 LD freeze dryer at –40°C and 0.15 atm pressure for 4 h, the vacuum was broken at the end of the freeze-drying cycle. The pellets were prepared by mixing the freeze-dried sample with KBr and compacted under vacuum for 3 min. FTIR measurements were carried out at room temperature by an ALPHA FTIR spectrometer (Bruker optics) using KBr pellet in the range of 4000–400 cm−Citation1. CD spectra of α-casein and its BIM complex were obtained using JASCO spectropolarimeter model j-810. For measurements in the far-UV region (195–245 nm), a quartz cell with a path length of 0.1 cm was used in nitrogen atmosphere. Protein concentration was kept constant (8.3 μM) and BIM concentration varied (8.30, 16.6, and 33.20 μM). The protein secondary structure was calculated using CDNN software, which indicates the different assignments of secondary structures.
Fluorescence spectroscopy
Emission spectra were recorded by a Cary eclipse spectrofluorimeter equipped with a thermostat, which controls the temperature of the cell compartment with a precision of ±0.1°C. For preparation of BIM–α-casein complex, a 1 mM stock solution of BIM in acetonitrile was prepared. α-Casein was dissolved in sodium phosphate buffered saline (PBS), pH 7.0. The entrapment of each BIM in casein nanoparticle at different BIM to α-casein molar ratios was performed by adding different volumes of BIM solution in acetonitrile to the α-casein solution in PBS with continuous stirring. The fluorescence excitation for α-casein was performed at [Citation31].
Determination of synchronous fluorescence spectra
In order to verify the binding sites of BIM to α-casein molecules, the synchronous fluorescence spectra of α-casein solutions along with the increase in BIM concentrations were investigated in wavelength ranges from 245 to 350 nm for and
.
Molecular docking
The α-casein structure was obtained according to the SWISS-MODEL; protein model was prepared based on the target-template alignment using Promod-II. The coordinates, which are conserved between the target and the template, are then copied from the template to the model. Insertions and deletions are remodeled using a fragment library. Side chains are rebuilt and the geometry of the resulting model is regularized using a force field[Citation32]. Docking experiments were carried out to visualize the binding site of BIM to α-casein. All the docking calculations were performed using Autodock 4.2 Tools[Citation33,Citation34]. The macromolecule was kept rigid, while all the torsional bonds of ligands were set free to rotate. Geometry optimization was carried out with grid resolution of 0.375 Å and grids spacing of 80 Å × 80 Å × 80 Å. For the BIM ligand, 150 separate docking calculations were performed using the Lamarckian genetic algorithm (LGA) method. The structure with lowest free energy of binding in a highly populated cluster was chosen as the optimal docking pose. The ligand structure optimizing calculation was carried out at the 6–31 G** level by employing the Becke three-parameters Lee-Yang-Parr (B3LYP) hybrid density functional theory using the quantum chemistry software Gaussian 03.
Results and discussion
Structural changes revealed by FTIR and CD analysis
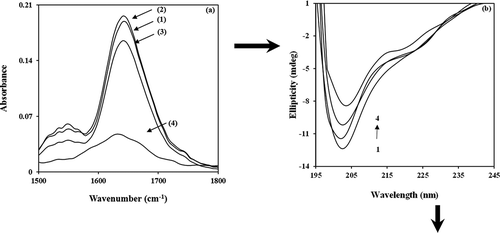
The BIM interaction with α-casein was analyzed by FTIR spectroscopy. The wave number and intensity of α-casein were changed (; α-casein (1)) for C=O stretching (amide I band) at 1642 cm–Citation1 and C–N stretching participant with N–H bending modes (amide II band) at 1548 cm–Citation1, due to hydrophilic contacts between BIM and α-casein[Citation21]. The results of BIM interaction with α-casein, at low BIM concentration, 0.125 mM (; α-casein–0.125 mM BIM (2)), showed a partial increase in intensity. A spectral shift was witnessed for the protein amide I band at 1642 cm–Citation1 and amide II band at 1550 cm–Citation1. As BIM concentration increased to 0.25 mM (; α-casein–0.25 mM BIM (3)), a decrease in intensity was observed for amide I and II bands at 1642 and 1550 cm–1. On the other hand, when the BIM concentration reached 0.5 mM (; α-casein–0.5 mM BIM (4)) two variations were observed for amide I and II bands at 1632 and 1533 cm–1. When the BIM concentration was further increased, the intensity was decreased, suggesting an altering of the secondary structure of the protein with increasing BIM concentrations.
Figure 1. (a) FTIR spectra of (1) free α-casein 0.25 mM, (2) α-casein–0.125 mM BIM, (3) α-casein–0.25 mM BIM, and (4) α-casein–0.5 mM BIM; and (b) far-UV CD spectra of (1) free α-casein 8.3 µM, (2) α-casein–8.3 µM BIM, (3) α-casein–16.6 µM BIM, and (4) α-casein–33.2 µM BIM.
The hydrophobic contacts in the BIM–α-casein complex were investigated in the 2800–3000 cm–Citation1 range of FTIR spectra via CH2 anti-symmetric and symmetric stretching vibrations. Upon the increase of 0.125 mM BIM to α-casein, CH2 vibrations at 2876, 2929, and 2996 cm–Citation1 were shifted to 2880, 2933, and 2966 cm–Citation1, respectively. Protein anti-symmetric and symmetric CH2 stretching vibration shifts suggest the presence of hydrophobic interactions between BIM and α-casein[Citation35]. Based on the results obtained presently, it was demonstrated that α-casein can be used to overcome the limitations related to the low solubility and bioefficacy of BIM.
CD spectroscopy was used to analyze conformation changes in α-casein during interactions with BIM. CD spectra were measured in the range of 195–245 nm; further investigation of α-casein conformational changes in the presence of BIM is shown in . The results indicate that the presence of BIM in the range of 8.3–33.2 μM induces changes in the secondary structure of α-casein. The featured spectrum with increase of BIM, twin negative peaks at ~202 nm, and a shoulder at ~222 nm suggested that the decrease in unordered structures of α-casein is accompanied by an increase in the α-helix content ( and )[Citation36]. The CD data show that α-casein can be used as carrier, because the addition of BIM introduces more stability to the secondary structure of the protein.
Table 1. Secondary structure of free α-casein (CD spectra) and their BIM complexes at pH 7 calculated by CDNN software.
Fluorescence measurements
In order to describe the binding interactions of α-casein with BIM, the intrinsic fluorescence of the protein was measured in the presence of various amounts of BIM. The intrinsic fluorescence of α-casein may change and therefore the intensity of intrinsic fluorescence of protein can be decreased by addition of the quencher (BIM). The fluorescence emission spectra of α-casein upon the addition of BIM are shown in . Since α-casein has two tryptophan residues located in a primarily hydrophobic domain of the α-casein micelle, they can provide valuable information about the interaction and formation of the BIM–α-casein complex. It can be seen that BIM has quenched the fluorescence of α-casein without any shift in the wavelength of emission peak maximum. This may be due to an insignificant dielectric change in the local environment of tryptophan residue[Citation26]. Both static and dynamic processes can be described by the well-known Stern–Volmer equation as follows[Citation37,Citation38]:
Figure 2. Fluorescence quenching of (a) in PBS of pH 7, at 25°C by BIM concentration: 0, 0.33, 2, 4, 6, 8, 10, 15, 20, 30, and 40 μM, inset: the variation of Fo/F versus [BIM]; and (b) Stern–Volmer plots of the interaction between BIM and α-casein at 20°C (▲), 25°C (∆), 30°C (●), and 37°C (○).
![Figure 2. Fluorescence quenching of (a) in PBS of pH 7, at 25°C by BIM concentration: 0, 0.33, 2, 4, 6, 8, 10, 15, 20, 30, and 40 μM, inset: the variation of Fo/F versus [BIM]; and (b) Stern–Volmer plots of the interaction between BIM and α-casein at 20°C (▲), 25°C (∆), 30°C (●), and 37°C (○).](/cms/asset/5c0d24b4-e928-4d08-8aa5-147c4b85ca23/ljfp_a_1247857_f0002_b.gif)
Figure 3. (a) Modified Stern–Volmer plots, (b) double logarithm plots of the interaction between BIM and α-casein at 20°C (▲), 25°C (∆), 30°C (●), and 37°C (○).
where and
are fluorescence intensities in the presence and absence of quencher,
is the concentration of quencher,
is Stern–Volmer constant,
is the bimolecular quenching rate constant, and
is the lifetime of the fluorophore in the absence of quencher; 4.53 ns for α-casein[Citation35]. The plot of
versus
for α-casein is presented in the inset of . The plot in lower concentrations of BIM (up to 20 μM) is linear; for its higher concentrations (above 20 μM) it becomes curved. This result suggests that the type of quenching of α-casein in low concentrations of BIM follows single mechanism kinetics, whereas for higher concentrations of BIM, there is combined quenching, that is, both static and dynamic quenching[Citation39]. In order to differentiate static from dynamic quenching, the temperature effect on the quenching constant
was examined. The plots of
versus
at four temperatures are shown in .
values were obtained from the slopes of the plots. The results indicate the occurrence of a static quenching mechanism in formation of the BIM–α-casein complex at lower concentrations of
. This conclusion comes from the fact that the quenching constant
decreases as the temperature increases from 293 to 310 K (up to 20 μM) ( and )[Citation40]. The
between 293 and 310 K is shown in .
Table 2. Quenching constants ( and
), number of substantive binding sites
, and binding constant (
) for interaction of BIM with α-casein micelle obtained from the fluorescence measurements in phosphate buffer of pH 7 at different temperatures.
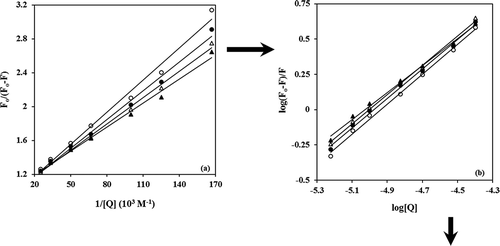
The modified Stern–Volmer plot is useful when studying a protein that has tryptophans in different environments (buried environment or surface environment) or fluorescence is heterogeneous and residues do not have an identical accessibility to the quencher. Therefore, the quenching data were analyzed according to the modified Stern–Volmer Eq. (2):
where and
are the same parameters as in the Stern–Volmer equation and
is the effective quenching constant for the accessible fluorophores, which is analogous to associative binding constants for the quencher–acceptor system, and
is the fraction of accessible fluorescence. The plots of
versus
, represent the slope
and the intercept
of the straight line[Citation41,Citation42]. Thus, the ratio of the ordinate and the slopes gives
. The values of
for α-casein in the presence of the BIM at 293, 298, 303, and 310 K were found to be 1.00, 1.04, 1.06, and 1.08, respectively. The plots of
versus
are shown in . The obtained values of
() show that increasing the temperature from 293 to 310 K decreases the binding constant. For static quenching, the number of sites
can be obtained from following equation, if there are independent binding sites to a set of equivalent sites on macromolecules[Citation43,Citation44]:
The plots of versus
are presented in , where
is the slope of straight line. The number of BIM molecules bond per α-casein (
) from 293 to 310 K are shown in and . This figure illustrates that BIM binds to α-casein with 1:1 stoichiometry. The binding studies were carried out in the temperature range of 293–310 K. Thermodynamic parameters of the interaction between BIM with α-casein were calculated and analyzed. Equilibrium features of the BIM–α-casein complex can be conveniently characterized by three thermodynamic parameters, that is, standard molar Gibbs free energy changes
the standard molar enthalpy changes (
) and the standard molar entropy changes (
.
can be calculated from the standard equilibrium constant
and using the familiar relationship (4):
where is analogous to the associative binding constants at the corresponding temperature
, and
and
refer to the gas constant and the absolute temperature, respectively. The van’t Hoff equation gives a linear plot of
versus
, if the heat capacity change for the reaction is essentially zero[Citation44]:
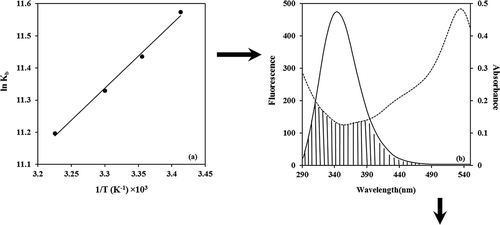
From a plot of versus
(),
can be calculated from the slope of the straight line and
was computed by Eq. (6):
Figure 4. (a) van’t Hoff plot for the interaction of BIM and α-casein. (b) Spectral overlaps of UV–vis absorption (dashed lines) of BIM with the fluorescence emission (solid lines) of α-casein in PBS buffer of pH 7, and
.
The thermodynamic parameters for the interaction between α-casein and BIM were calculated and are shown in . The negative signs of at four temperatures indicate the spontaneity of the binding processes between α-casein and BIM. It is also observed from that binding of BIM to α-casein is an exothermic process; however, the value of entropy change is positive. It is obvious that the process is driven by enthalpy change and entropy change. Under these circumstances, electrostatic interaction cannot be allowed, because BIM is not able to be ionized in these conditions. These results suggest that the hydrophobic interaction, hydrogen bond, and van der Waals forces play the major role in the formation of BIM–α-casein complex[Citation45]. These results also suggest that BIM stabilizes the secondary structure of α-casein. This is consistent with CD measurements discussed earlier, when an increase in the BIM concentration enhanced the α-helix of α-casein and reduced the random coil of its structure.
Table 3. Thermodynamic parameters for interaction of BIM with α-casein micelle obtained from the fluorescence measurements in phosphate buffer of pH 7 at different temperatures.
Energy transfer
In order to measure the distance between a ligand and macromolecules, fluorescence resonance energy transfer (FRET) is a reliable method. The Fӧrster distance is specified by the spectral overlap of the donor (α-casein) emission with the acceptor (BIM) absorption. Upon association, FRET will exhibit a decrease in the intensity of donor quenching, which can be used to calculate the distance between the donor and the acceptor[Citation37]. The energy transfer is then used for evaluation of the binding distance (r) between the BIM and two tryptophan residues in the α-casein. The efficiency of energy transfer (E) is calculated by Eq. (7):
where and
are the same parameters in the Stern–Volmer equation, r is the distance between the donor (α-casein) and acceptor (BIM), and
is the Fӧrster critical distance (nm) when the efficiency
is 50%, which can be calculated by the following equation[Citation37]:
In the case of milk proteins, is a factor to describe the relative orientation of the transition dipoles of the donor and acceptor,
is average refractive index of the medium (average) in the region of wavelength where spectral overlap is significant,
is the fluorescence quantum yield of the donor, and
is overlap integral,
expresses the extent of overlap between the normalized fluorescence emission spectrum of donor and the acceptor absorption spectrum. It is noteworthy to indicate that J is given by Eq. (9)[Citation45,Citation46]:
where is the fluorescence intensity of the fluorescent donor at
, and
is the molar absorption coefficient of the acceptor at a specific wavelength
. shows the spectral overlap of fluorescence emission of solution and absorption spectrum of the BIM solution.
Eqs. (7)–(9) made it possible to calculate that ,
,
. The distance between the donor and acceptor
is less than 7 nm, indicating that the mechanism of energy transfer for quenching is non-radiative[Citation33,Citation34]. The short distance values suggested a strong interaction between BIM and tryptophan residues in β-casein.
Synchronous fluorescence study
The synchronous fluorescence spectra provide significant information about the microenvironment near the chromophore molecules. In this study, synchronous fluorescence spectroscopy was used to get some information about structural changes and binding sites of BIM when it interacts with α-casein molecule. It has been reported that synchronized fluorescence spectrum is a valuable way for distinguishing the tryptophan (Trp) and tyrosine (Tyr)
residues in protein[Citation47].
and show synchronous fluorescence spectroscopy of α-casein with and
nm, respectively. In , for
nm, a significant decrease in synchronous fluorescence intensities with a blue shift to 4.0 nm in their emission wavelength maximum (290–286 nm) was observed. It is suggested that the Tyr residues of α-casein might have moved into nonpolar hydrophobic cavities, resulting in the observed increase of hydrophobicity around the Tyr residues. According to , for
, when the concentration of BIM increases, a significant decrease is resulted in synchronous fluorescence intensities with a red shift to 5.0 nm in their emission wavelength maximum (282–287 nm). These results show that the hydrophobicity around the Trp residue of α-casein decreased, while the polarity is shifted to a higher value after binding with BIM, for which the Trp residues might be exposed to an aqueous or hydrophilic environment[Citation47,Citation48].
Docking study
The aim of this study was to use experimental data and computational docking studies for a better understanding of the interaction between BIM with α-casein (). demonstrates that BIM penetration into the structure of α-casein has a major role in the binding interaction of BIM–α-casein complex. In , it is shown that BIM–α-casein is surrounded by Asn-2, His-6, Ala-81, Asn-83, Glu-84, Ile-85, Gln-87, Phe-88, and Leu-153, with an average binding distance between donor and acceptor atoms from 1.6 to 3.2 Å; and a free binding energy of –40.0 kJ mol–Citation1. The total number of hydrogen binding for BIM–α-casein is three. demonstrates that hydrogen bonds form between BIM and Ala-81, Glu-84, and Gln-87 of α-casein. A BIM molecule is located in the vicinity of hydrophobic and hydrophilic residues, resulting in van der Waals contacts and hydrogen binding. As noted previously, hydrophobic interaction, hydrogen bond, and van der Waals forces play major roles in the binding interaction between BIM and α-casein. From the docking study, the observed free binding energy
for BIM–α-casein was –40.0 kJ mol–1, which is more comparable to our experimentally determined free binding energy of
–28.33 kJ mol–1. The difference between experimental and theoretical results might be attributed to the absence of a solvent and the rigidity of receptor in docking study[Citation49]. These results demonstrate that α-casein is a useful vehicle for the solubilization and stabilization of BIM in aqueous solutions.
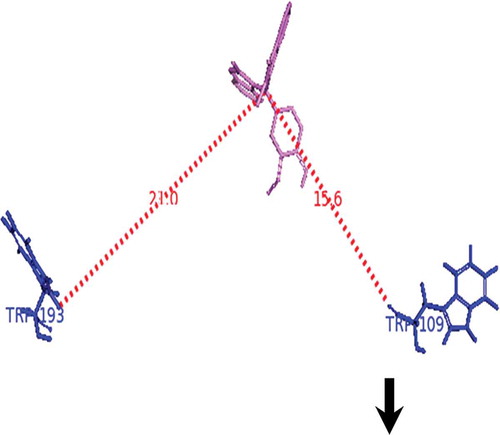
Figure 6. Best conformations for BIM docked to α-casein. The BIM molecule is violet. (a) Whole α-casein with BIM molecule; (b) binding site represented in sticks.
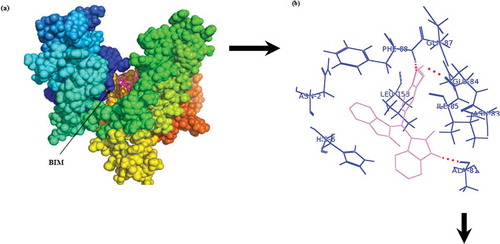
The distance between BIM and Trp-109 and Trp-193 in α-casein was calculated from docking study (). We found the distance between BIM and Trp-109 to be 15.6 Å and that between BIM and Trp-193 to be 21 Å. However, the distance between tryptophan and BIM obtained from FRET calculation in α-casein was found to be 27.8 Å. These results indicate that BIM most possibly causes the hydrophobicity around Trp-193 residue of α-casein. Consequence of these results are more comparable to our synchronous fluorescence study might be exposed Trp residue of α-casein after binding with BIM transferred to an aqueous or hydrophilic environment.
Conclusions
Based on the results of the present study, FTIR spectra shift in wavelength and change in intensity of absorbance indicate that α-casein interacts with BIM molecule mainly via hydrophobic and hydrophilic interactions. This is also accompanied by an increase in the solubility, bioavailability, and antitumor activity of BIM. Besides, FTIR spectra and the CD spectrum indicated that interaction of the BIM with α-casein could lead to conformational changes in α-casein and an increase in its α-helix content and a simultaneous decrease in the unordered structures of α-casein. Fluorescence quenching in the range of 293–310 K showed that BIM can quench the fluorescence intensity of α-casein, and based on measurements of and
, the quenching mechanism is judged as a static process. Further fluorescence studies illustrated that BIM binds to α-casein with a 1:1 stoichiometry. The thermodynamic parameters obtained from fluorescence data showed a negative sign for enthalpy, while the entropy changes were positive. Therefore, hydrophobic interaction, hydrogen bonding, and van der Waals forces play important roles in the binding process. Synchronous fluorescence spectroscopy demonstrated that BIM most probably increases the hydrophobicity around the Trp residue of α-casein; in addition, Fӧrster energy transfer measurement and docking study showed that after addition of BIM, Trp-193 is transferred to an aqueous or hydrophilic environment. The results of docking study demonstrated that the interaction between BIM and α-casein is predominantly in the form of hydrogen bonds and hydrophobic contacts with the hydrophobic residues of α-casein. These results prove that α-casein displays a very good binding and entrapment capacity for this type of hydrophobic anticancer drug. Thus, it may serve as a useful vehicle for the solubilization and stabilization of the drug in aqueous preparations for oral delivery as an antitumor agent.
Acknowledgement
We gratefully acknowledge the Research Council of University of Guilan for supporting this work.
References
- Gribble, G.W. Heterocyclic Scaffolds II: Reactions and Applications of Indoles, Topics in Heterocyclic Chemistry; Series Editor: Bert U.W. Maes: springer-Verlag Berlin Heidelberg, 2010.
- Papazoglou, E.S. Parthasarathy, A. Bionanotechnology; A Publication in the Morgan & Claypool (Publishers), Connecticut, 2007.
- Marrelli, M.; Cachet, X.; Conforti, F.; Sirianni, R.; Chimento, A.; Pezzi,V.; Michel, S.; Statti, G.A.; Menichini, F. Synthesis of a new bis(indolyl)methane that Inhibits Growth and Induces Apoptosis in Human Prostate Cancer Cells. Natural Product Research 2013, 27, 2039–2045.
- Yunpeng, S.U.; Kathryn,V.; Courtney, I.; Janelle, O.; Henry, G.; Stephen, S.; Frankel. E. 1,1-Bis(3’-indolyl)-1-(p-biphenyl)methane Inhibits Basal-like Breast Cancer Growth in Athymic Nude Mice. Breast Cancer Research 2007, 9, R 56.
- Li, X.; Lee, S.O.; Safe, S. Structure-Dependent Activation of NR4A2 (Nurr1) by 1, 1-bis(3’-indolyl)-1-(aromatic)methane Analogs in Pancreatic Cancer Cells. Biochemical Pharmacology 2012, 83, 1445–1455
- Kassouf,W.; Chintharlapalli, S.; Abdelrahim, M.; Nelkin, G.; Safe, S.; Kamat, A.M. Inhibition of Bladder Tumor Growth by 1, 1-bis(3’-indolyl)-1-(p-substitutedphenyl)methanes: A New Class of Peroxisome Proliferator-Activated Receptor Gamma Agonists. Cancer Research 2006, 66, 412–418.
- York, M.; Abdelrahim, M.; Chintharlapalli, S.; Lucero, S. D.; Safe S. 1, 1-bis (3’-indolyl)-1-(p-substitutedphenyl)methanes Induce Apoptosis and Inhibit Renal Cell Carcinoma Growth. Clinical Cancer Research 2007, 15, 6743–6752.
- Terrick, A.; Apurva, P.; Tanise, J.; Stephen, S.; Mandip, S. 1,1-Bis (3′-indolyl)-1-(p-substitutedphenyl)methane Compounds Inhibit Lung Cancer Cell and Tumor Growth in a Metastasis Model. European Journal of Pharmaceutical Sciences 2013, 50, 227–241.
- Cho, S.D.; Chintharlapalli, S.; Abdelrahim, M. Papineni, S.; Liu, S.; Guo, J.; Lei, P.; Abudayyeh, A.; Safe, S. 5,5’-Dibromo-bis(3’-indolyl)methane Induces Kruppel-like Factor 4 and p21 in Colon Cancer Cells. Molecular Cancer Therapeutics 2008, 7, 2109–2120.
- Cho, S.D.; Lei, P.; Abdelrahim, M.; Yoon, K.; Liu, S.; Guo, J.; Papineni, S.; Chintharlapalli, S.; Safe, S. 1,1-bis(3’-indolyl)-1-(p-methoxyphenyl)methane Activates Nur77-Independent Proapoptotic Responses in Colon Cancer Cells. Molecular Carcinogenesis 2008, 47, 252–263.
- Kamal, A.; Khan, M.N.; Srinivasa, R.K.; Srikanth, Y.V.; Kaleem, A.S.; Pranay, K.K.; Murthy, U.S. An Efficient Synthesis of bis(indolyl)methanes and Evaluation of their Antimicrobial Activities. Journal of Enzyme Inhibition and Medicinal Chemistry 2009, 24, 559–565.
- Imran, S.; Taha, M.; Ismail, N.H.; Khan, K.M.; Naz, F.; Hussain, M.; Tauseef, S. Synthesis of Novel Bisindolylmethane Schiff Bases and their Antibacterial Activity. Molecules 2014, 19, 11722–11740.
- Oliveira, E.; Baptista, R.M.F.; Costa, S.P.G.; Manuela, M.; Raposo, M.; Lodeiro, C. Synthesis and Solvatochromism Studies of Novel bis(indolyl)methanes Bearing Functionalized Arylthiophene Groups as new Colored Materials. Photochemical and Photobiological Sciences 2014, 13, 492–498.
- Wanga, L.; Weia, W.; Guoa, Y.; Xu, J.; Shao, S. Nitro-substituted 3, 3_-bis(indolyl)methane Derivatives as Anion Receptors: Electron-Withdrawing Effect and Tunability of Anion Binding Properties. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2011, 78, 726–731.
- Boland, M.; Golding, M.; Singh, H. Food Structures, Digestion and Health; Riddet Institute, Massey University: Palmerston North, New Zealand: Elsevier, 2014.
- Livney, Y.D. Milk Proteins as Vehicles for Bioactives. Current Opinion in Colloid and Interface Science 2010, 15, 73–83.
- FOX, P.F.; McSWeeny, P.L.H. Dairy Chemistry and Biochemistry; Blackie Academic & Professional: London, U.K, 1998.
- Elzoghby, A.O.; El-Fotoh, W.S.A.; Elgindy. N.A. Casein-Based Formulations as Promising Controlled Release Drug Delivery Systems. Journal of Controlled Release 2011, 153, 206–216.
- Barth, C.A.; Schlimme, E. Milk Protein: Nutritional, Clinical, Functional and Technological Aspects; Steinkopff: Darmstadt, Springer: New York, 1988.
- Haratifar, S.; Meckling, K.A.; Corredig, M. Bioefficacy of Tea Catechins Encapsulated in Casein Micelles Tested on a Normal Mouse Cell Line (4D/WT) and its Cancerous Counterpart (D/v-src) Before and After in Vitro Digestion. Food & Function 2014, 5, 1160–1166.
- Hasni, I.; Bourassa, P.; Hamdani, S.; Samson,G.; Carpentier, R.; Tajmir-Riahi, H.A. Interaction of Milk α- and β-caseins with Tea Polyphenols. Food Chemistry 2011, 126, 630–639.
- Bourassa, P.; Bariyanga, J.; Tajmir-Riahi, H.A. Binding Sites of Resveratrol, Genistein, and Curcumin with Milk α- and β‑caseins. Journal of Physical Chemistry B 2013, 117, 1287–1295.
- Jiang, Z.; Wang, L.; Wu, W.; Wang, Y. Biological Activities and Physicochemical Properties of Maillard Reaction Products in Sugar–Bovine Casein Peptide Model Systems. Food Chemistry 2013, 141, 3837–3845.
- Sahu, A.; Kasoju, N.; Bora, U. Fluorescence Study of the Curcumin-Casein Micelle Complexation and Its Application as a Drug Nanocarrier to Cancer Cells. Biomacromolecules 2008, 9, 2905–2912.
- Rahimi-Yazdi, S.; Bonomi, F.; Iametti, S.; Miriani, M.; Brutti, A.; Corredig, M. Binding of the Curcumin to Casein Micelles Increases After Static High Pressure Treatment of Skim Milk. Journal of Dairy Research 2014, 80, 152–158.
- Semo, E.; Kesselman, E.; Danino, D.; Livney, Y.D. Casein Micelle as a Natural Nano-Capsular Vehicle for Nutraceuticals. Food Hydrocolloids 2007, 21, 936–942.
- Bourassa, P.; N’soukpoé-Kossi, C.N.; Tajmir-Riahi H. A. Binding of Vitamin A with Milk α- and β-caseins. Food Chemistry 2013, 138, 444–453.
- Bourassa, P.; Bekale, L.; Tajmir-Riahi, H.A. Association of Lipids with Milk α- and β-caseins. International Journal of Biological Macromolecules 2014, 70, 156–166.
- Thorn, D.C.; Meehan, S.; Sunde, M.; Rekas, A.; Gras, S.L.; MacPhee, C.E. Dobson, C.M.; Wilson, M.R.; Carver, J.A. Amyloid Fibril Formation by Bovine Milk қ-casein and its Inhibition by the Molecular Chaperones αs- and β-casein. Biochemistry 2005, 44, 17027–17036.
- Khorshidi, A.; Mardazad, N.; Shaabanzadeh, Z. Zirconium (IV)-catalyzed one-pot Synthesis and Oxidation of bis- and tris(indolyl)methanes into Conjugated Chromophores as New pH Indicators or Calorimetric Chemosensors for Transition Metals. Tetrahedron Letters 2014, 55, 3873–3877.
- Chakraborty, A.; Basak, S. Interaction with Al and Zn Induces Structure Formation and Aggregation in Natively Unfolded Caseins. Journal of Photochemistry and Photobiology B 2008, 93, 36–43.
- Schwede, T.; Kopp, J.; Guex, N.; Peitsch, M.C. Swiss-Model: An Automated Protein Homology-Modeling Server. Nucleic Acids Research 2003, 31, 3381–3385.
- Mehranfar, F.; Bordbar, A. Kh.; Fani, N.; Keyhanfar, M. Binding Analysis for Interaction of Diacetylcurcumin with β-casein Nanoparticles by Using fluorescence Spectroscopy and Molecular Docking Calculations. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2013, 115, 629–635.
- Mehranfar, F.; Bordbar, A. Kh.; Keyhanfar, M.; Behbahani M. Spectrofluoremetric and Molecular Docking Study on the Interaction of Bisdemethoxycurcumin with Bovine β-casein Nanoparticles. Journal of Luminescence 2013, 143, 687–692.
- Bourassa, P. Tajmir-Riahi, H. A. Locating the Binding Sites of Folic Acid With milk α- and β-caseins. Journal of Physical Chemistry B 2012, 116, 513–519.
- Zhao, X.; Sheng, F.; Zheng, J. Liu, R. Composition and Stability of Anthocyanins from Purple Solanum Tuberosum and their Protective Influence on Cr (VI) Targeted to Bovine Serum Albumin. Journal of Agricultural and Food Chemistry 2011, 59, 7902–7909.
- Lakowicz, J. R. Principles of fluorescence spectroscopy; 3nd repr. Center for fluorescence spectroscopy, University of Maryland School of medicine, Baltimore, MD, USA: Springer, 2006.
- Zhao, X.; Hao, F.; Lu, D.; Liu, W.; Zhou, Q.; Jiang, G. Influence of the Surface Functional Group Density on the Carbon- Nanotube-Induced α‑chymotrypsin Structure and Activity Alterations. ACS Applied Materials & Interfaces 2015, 7, 18880–18890.
- Mote, U.S.; Han, S.H.; Patil, S.R.; Kolekar G.B. Effect of Temperature and pH on Interaction Between Bovine Serum Albumin and Cetylpyridinium Bromide: Fluorescence Spectroscopic Approach. Journal of Luminescence 2010, 130, 2059–2066.
- Zhao, X.; Liu, R.; Chi, Zh.; Teng, Y.; Qin, P. New Insights into the Behavior of Bovine Serum Albumin Adsorbed onto Carbon Nanotubes: Comprehensive Spectroscopic Studies. The Journal of Physical Chemistry B 2010, 114, 5625–5631.
- Albani J.R. Principles and applications of fluorescence spectroscopy, 1nd repr. Blackwell Science, Blackwell Publishing, Oxford, 2007.
- Zhang, X.P.; HeHou, Y.; Ye-ZhongZhang, L.; Liu, Y. Exploring the Mechanism of Interaction Between Sulindac and Human Serum Albumin: Spectroscopic and Molecular Modeling Methods. Journal of Luminescence 2013, 138, 8–14.
- Zhang, G.; Ma, Y.; Mechanistic and Conformational Studies on the Interaction of Food Dye Amaranth with Human Serum Albumin by Multispectroscopic Methods. Food Chemistry 2013, 136, 442–449.
- Liu, E.H.; Qi, L.W.; Li, P. Structural Relationship and Binding Mechanisms of Five Flavonoids with Bovine Serum Albumin. Molecules 2010, 15, 9092–9103.
- Philip, D.R.; Subramanian S. Thermodynamics of Protein Association Reactions: Forces Contributing to Stability. Biochemistry 1981, 20 (11), 3096–3102.
- Yu, D.D.; Zhang, H.; Ding, Q.B.; H.Y.; Wu, J.P.; Zhang. L.D. Spectral Properties of Interaction Between Caffeic Acid and Milk Protein and the Change in Antioxidant Capacity Spectroscopy and Spectral Analysis 2012, 32, 1061–1067.
- He, Z.; Xu, M.; Zeng, M.; Qin, F.; Chen, J. Interactions of milk α- and β-casein with Malvidin-3-O-glucoside and Their Effects on the Stability of Grape Skin Anthocyanin Extracts. Food Chemistry 2016, 199, 314–322.
- Zhao, X.; Lu, D.; Hao, F.; Liu, R. Exploring the Diameter and Surface Dependent Conformational Changes in Carbon Nanotube-Protein Corona and the Related Cytotoxicity. Journal of Hazardous Materials 2015, 292, 98–107.
- Jana, S.; Dalapati; S.; Ghosh, S.; Guchhait N. Binding Interaction Between Plasma Protein Bovine Serum Albumin and Flexible Charge Transfer Fluorophore: A spectroscopic Study in Combination with Molecular Docking and Molecular Dynamics Simulation. Journal of Photochemistry and Photobiology, A 2012, 231, 19–27.

![Figure 5. Synchronous fluorescence spectra: (a) and (b) of α-casein solution by BIM in PBS of pH 7, at 25°C and [α with [BIM]= 0, 0.66, 2, 4, 6, 8, 15, 30, and 40 µM.](/cms/asset/90b95d4d-ad55-4815-ad12-2847316cce20/ljfp_a_1247857_f0005_b.gif)