ABSTRACT
The aim of the present study was to investigate the volatile fraction and discriminate 34 commercial thyme honeys from Morocco, Egypt, Spain, and Greece according to geographical origin, using key volatile compounds in combination with chemometrics. Sixty-two volatile compounds belonging to different classes were identified and semi-quantified using headspace solid phase micro-extraction coupled to gas chromatography/mass spectrometry (HS-SPME-GC/MS). Applying chemometric analyses to 17 volatiles (p < 0.05), honeys were successfully discriminated according to geographical origin. Correct classification rate was 88.2% using the cross-validation method. Volatile compounds proved to be a powerful tool for discriminating commercial thyme honeys from different countries.
Introduction
Aroma is one of the most important quality parameters of foodstuffs. The pleasant aroma of foodstuffs comprises a major criterion for their acceptance by consumers. Honey aroma is owed to complex mixtures of volatile and semi-volatile compounds, which may differ depending on the nectar, processing, and storage conditions, as well as honey origin. Unifloral honeys have a distinctive aroma of the dominant plant, due to the presence of specific volatile compounds from nectars.[Citation1] On the other hand, honeybees can also produce or convert plant constituents to other volatile compounds. Moreover, these compounds can be affected by postharvest processing conditions as well as from the presence of micro-organisms.[Citation2,Citation3]
It has been documented that 400–600 different compounds have been identified in the volatile fraction of honey.[Citation4,Citation5] Volatile compounds are categorized into different classes such as esters, carboxylic acids, aldehydes, ketones, terpenoids, phenylpropanoids, hydrocarbons, ethers, alcohols, etc. Such compounds, often referred to as chemical markers, have been used in numerous studies to determine the botanical and geographical origin of honey.[Citation6–Citation14] As a matter of fact, in geographical determination efforts the use of specific chemical markers has a number of advantages, over melissopalynology (the traditional approach in distinguishing the botanical origin of honey).[Citation15–Citation18]
Thyme honey is produced from 350 different species of Thyme (Thymus spp.), while the major honey producing species include: Wild Thyme (T. capitatus), Garden Thyme (T. vulgaris), and Mother-of-Thyme (T. serpyllum). Thyme is a member of the nectar-producing mint family, Lamiaceae, the source of various flavorful honeys such as mint, sage, oregano, and lavender.[Citation14] Thyme-producing Mediterranean countries include Turkey, Spain, Greece, Italy, France, Morocco, Egypt, etc.
Typical examples of previous research studies dealing with the volatile profile of thyme honey, originate from Spain,[Citation1,Citation8,Citation19] Greece,[Citation9–Citation11] Morocco,[Citation12] and Turkey.[Citation14] It should be noted that data on the volatile fraction of thyme honey from Egypt are still scarce. What is interesting is that the characterization and/or differentiation of honey from these four countries based on specific volatile compounds has not been yet investigated. Thus, the aim of the present study was to characterize the volatile fraction and differentiate Mediterranean commercial thyme honeys according to their geographical origin based on selected volatile compounds, using chemometrics. This study comprises a research effort to highlight specific chemical markers that could aid to the determination of authenticity of thyme honey produced in different Mediterranean countries.
Materials and methods
Honey samples
A total of 34 commercial thyme honeys were purchased from natural product shops in Spain (Thymus sp., 10 samples) (from Catalonia), Egypt (Thymus sp., 7 samples) (from the Cairo greater area), and Morocco (Thymus sp., 5 samples) (from the Gharb greater area) during the harvesting periods of 2012–2013 and 2013–2014. Respective samples from Greece (12 samples from Kos island) (Thymus capitatus (L)) were donated by Attiki Honey S.A. Samples were packaged in glass containers, shipped to the laboratory, and maintained at 4 ± 1°C until analysis.
Melissopalynological analysis
In order to confirm the floral origin of honey, the melissopalynological method was applied to samples according to a previous work.[Citation11]
HS-SPME-GC/MS analysis
Headspace volatile compounds were extracted from honey, using a divinyl benzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) fiber 50/30 μm (Supelco, Bellefonte, PA, USA). Prior to use, the fiber was conditioned following the manufacturer’s recommendations. The samples (2 g honey in 2 mL of distilled water, plus 0.20 g NaCl (Merck, Darmstadt, Germany)) plus 20 μL of internal standard (benzophenone, 100 μg/mL, Sigma Aldrich) were placed in 15 mL screw-cap vials equipped with PTFE/silicone septa. The vials were maintained at 45°C in a water bath under stirring at 600 rpm during the entire extraction procedure. A cross-shaped PTFE-coated magnetic stirrer (diameter 10 mm) (Semadeni, Ostermundigen–Bern, Switzerland) was placed inside the vials. The sample preparation conditions were: 15 min equilibration time, 30 min sampling time, 4 mL sample volume, and 45°C water bath temperature. Test samples were prepared daily prior to headspace solid phase micro-extraction coupled to gas chromatography/mass spectrometry (HS-SPME-GC/MS) analysis. Blank runs were carried out before sample analysis to make sure that there was no contamination that could cause memory effects. Each sample was run in duplicate.[Citation11]
Identification and semi-quantification of volatile compounds
GC/MS instrumentation and conditions
An Agilent 7890 A GC unit coupled to an Agilent 5975 MS detector was used to analyze the honey solutions. A DB-5MS (cross-linked 5% PH ME siloxane) capillary column (60 m × 320 μm i.d., × 1 μm film thickness) was used, with helium as the carrier gas (purity 99.999%), at 1.5 mL/min flow rate. The injector and MS-transfer line were maintained at 250°C and 270°C, respectively. For the SPME analysis, oven temperature was held at 40°C for 3 min, increased to 260°C at 8°C/min (6 min hold). Electron impact mass spectra were recorded at the 50–550 mass range. An electron ionization system was used with ionization energy of 70 eV.
Mass spectral data processing
The identification of compounds was achieved using the Wiley 7, NIST 2005 mass spectral library.[Citation20] For the determination of linear retention indices, a mixture of n-alkanes (C8–C20) dissolved in n-hexane was employed. The mixture was supplied by Supelco (Bellefonte, PA, USA). The calculation was carried out for components eluting between n-octane and n-eicosane. Benzophenone (m/z = 182) was chosen as an internal standard since it did not naturally occur in any of the isolated compounds.[Citation9,Citation11] Volatile compounds having ≥80% similarity with the Wiley mass spectral library were tentatively identified using GC-MS spectra. For semi-quantification, compound concentration was expressed as micrograms per gram based on the ratio of peak area of analyte to peak area of internal standard.[Citation1]
Statistical analysis
All data processing was performed using the SPSS 20.0 software. Comparison of the means was achieved by multivariate analysis of variance (MANOVA) in order to determine those volatile compounds that were significant (p < 0.05) in discriminating thyme honeys of different geographical origin. Volatile compounds were taken as the dependent variable, while geographical origin was taken as the independent variable. The Pillai’s trace and Wilks’ lambda indices were computed to determine a possible significant effect of volatile compounds on geographical origin of thyme honeys. Linear discriminant analysis (LDA) was then applied using the selected dependent variables to explore the possibility of differentiating thyme honey samples according to geographical origin.[Citation11]
Results and discussion
Pollen characteristics of mediterranean thyme honeys
Differences in the pollen characteristics of commercial thyme honeys originating from different regions (% pollen grains of Thymus spp.) were observed during the melissopalynological analysis. More specifically, the pollen grains (%) in thyme honey samples ranged 29–94% for Greece, 19–29% for Spain, 1–18% for Morocco, and 6–8% for Egypt.
Volatile compounds of mediterranean thyme honeys
Sixty-two volatile compounds, belonging to different classes, were identified and semi-quantified (). For the qualitative analysis, the NIST 7 Wiley library (2005) was employed along with spectral data and retention index values published previously in the literature. Numerous volatile compounds were identified in the present study such as esters (formic acid ethyl ester, hexanoic acid ethyl ester, octanoic acid ethyl ester, nonanoic acid ethyl ester, and decanoic acid ethyl ester), hydrocarbons (heptane, octane, undecane, 1-nonene, 5-methyl-4-nonene), linear aldehydes (octanal, nonanal, decanal), alcohols (3-octanol, 3-methyl-1-butanol, 2-ethyl-1-hexanol, etc.), ketones (1-2-furanyl-ethanone, 4,7,7-trimethyl-byciclo(3,3,0)-octan-2-one, 3-hydroxy-4-phenyl-2-butanone), terpenoids (alpha-pinene, alpha-terpinene, dl-limonene, sabinene, 1,8-cineole, gamma-terpinene, alpha-terpinolene, cis-linalool oxide, terpinen-4-ol, caryophyllene, etc.), and aromatic hydrocarbons (p-cymene, etc.). Such compounds have been reported previously in European unifloral honeys analyzed.[Citation6–Citation9,Citation11,Citation21,Citation22] Furthermore, numerous of these compounds (alpha-terpinolene, alpha-pinene, linalool, hotrienol, benzaldehyde, lilac aldehyde, and eugenol) have also been reported in unifloral native Chilean honeys.[Citation2,Citation4]
Table 1. Volatile compounds (μg/g) of commercial thyme honeys produced in Mediterranean countries.
Phenolic volatiles with a pleasant “honey” or “sweet” aroma such as benzaldehyde and phenylacetaldehyde were identified in Spanish, Moroccan, and Greek thyme honeys in good agreement with the results reported in previous studies.[Citation8,Citation12,Citation18] Phenylethylalcohol, a compound with a soft “rose-like” odor, has been also identified in several honeys.[Citation2,Citation4,Citation9,Citation10,Citation11,Citation14,Citation22]
Lilac aldehyde (isomer II) and hotrienol (3,7-dimethyl-1,5,7-octatrien-3-ol) were identified only in Spanish thyme honey samples, in excellent agreement with previous results.[Citation19] Lilac aldehydes (isomer I and III) were identified only in two Spanish and Moroccan thyme honeys, respectively. This is in general agreement with the results of previous work involving Spanish thyme honeys.[Citation8, Citation19]
p-Cymene (1-methyl-4-(1-methylethyl)-benzene) was identified at much higher concentrations (μg/g) in Egyptian thyme honeys and at much lower concentrations in Greek thyme honeys; it was not identified in Moroccan and Spanish thyme honeys (). Octanal, nonanal, and decanal were not identified in Spanish thyme honey samples, in agreement with previous work on Spanish thyme honeys,[Citation8, Citation19] while their presence in Moroccan and Egyptian thyme honeys was limited. Furthermore, such compounds were present in Greek thyme honey samples, in good agreement with previous work on Greek thyme honeys.[Citation11] More recently,[Citation12] the above compounds were identified at low concentrations in commercial Moroccan honeys of different botanical origins (acacia, bitter, black, mountain, and forest honeys).
Alpha,4-dimethyl-cyclohex-3-ene-1-acetaldehyde (p-menth-1-en-9-al) was identified only in Moroccan thyme honey samples. This compound was identified in Spanish thyme honey samples as well as in Portuguese multifloral honeys (wild flora, eucalyptus, hissed, and rosemary).[Citation19, Citation22] Linalool was identified in Egyptian and Spanish thyme honeys, while it was not detected in Moroccan and Greek thyme honeys. cis-Linalool oxide was identified in Moroccan thyme honeys, and at much lower concentrations (μg/g) in Spanish thyme honeys, whereas it was not detected in Egyptian and Greek thyme honeys. This compound was identified at higher concentrations in eucalyptus and herbal Moroccan honeys, and at lower concentrations in forest, black, acacia, and mountain honeys.[Citation12]
Terpinen-4-ol was identified in much higher concentrations (μg/g) in Egyptian thyme honeys compared with Spanish and Greek thyme honeys, while it was not detected in Moroccan thyme honeys. Previous works on Spanish and Greek thyme honeys have confirmed the presence of this terpenoid.[Citation11,Citation19] Beta-damascenone, a cyclic carotenoid derivative, was identified only in Greek thyme honey samples in good agreement with a previous work.[Citation11] This compound has been reported previously contributing to the volatile fraction of Spanish artisanal honeys.[Citation23] Of the carboxylic acids, formic, acetic, and pentanoic acid were identified only in Moroccan and Greek thyme honey samples. This finding is in very good agreement with previous work on Spanish and Greek thyme honeys.[Citation8,Citation11]
Octanoic, nonanoic, and decanoic acid ethyl esters were identified in thyme honey samples from Morocco, Spain, and Greece, while they were not identified in Egyptian thyme honey samples. It should be noted that these compounds recorded higher concentrations (μg/g) in Moroccan compared with Spanish and Greek thyme honey samples; formic acid ethyl ester was identified only in Greek thyme honey samples, while hexanoic acid ethyl ester was identified in Spanish and Moroccan thyme honey samples at higher concentrations compared with Greek thyme honey samples. This compound was not identified in Egyptian thyme honey samples (). Present results are in very good agreement with those reported previously for Greek thyme honeys,[Citation11] whereas these compounds were not identified in Spanish thyme honey samples or commercial Moroccan honey samples.[Citation8,Citation12,Citation19]
Compounds such as thymol (2-isopropyl-5-methylphenol), thymoquinone (2-isopropyl-5-methylbenzo-1,4-quinone), 1-octen-3-ol, and eugenol (4-allyl-2-methoxyphenol) were identified only in Egyptian thyme honey samples, while 4,7,7-trimethylbyciclo (3,3,0)-octan-2-one and 1-(2-furanyl)-ethanone were identified only in Greek thyme honey samples in very good agreement with the results of previous work.[Citation11]
Finally, compounds such as (1R)-1,7,7-trimethyl-bicyclo-(2,2,1)-heptan-2-one((+)-camphor) and borneol, a compound with “camphor–like” odor, were identified only in Egyptian thyme honey samples. The same holds for carvacrol and thymol methyl ethers. It should be mentioned that these compounds have not been reported previously contributing to the volatile fraction of thyme honeys from different geographical origin.[Citation8,Citation9,Citation11,Citation19] (+)-Camphor and borneol have been previously reported contributing to the volatile fraction of artisanal honeys from Madrid.[Citation23]
Total volatile compounds of mediterranean thyme honeys
Total volatile content (μg/g) followed the order Egypt > Greece > Morocco > Spain (). What is remarkable is that significant variations were observed in the percent contribution of each compound class to the total volatile content, according to geographical origin of thyme honeys. More specifically, results were for Egyptian thyme honeys: other compounds (71.89%) > terpenoids (20.05%) > alcohols (4.90%) > ethers (1.93%) > hydrocarbons (0.65%) > aldehydes (0.58%); for Greek thyme honeys: aldehydes (63.83%) > alcohols (10.52%) > carboxylic acids (8.51%) > hydrocarbons (2.96%) > esters (2.36%) > ketones (2.01%) > other compounds (1.89%) > terpenoids (1.42%); for Moroccon thyme honeys: aldehydes (47.61%) > esters (29.57%) > hydrocarbons (8.10%) > carboxylic acids (6.56%) > terpenoids (6.20%) > other compounds (4.75%); and for Spanish thyme honeys: esters (31.94%) > terpenoids (30.62%) > aldehydes (29.31%) > hydrocarbons (3.59%) > other compounds (3.24%) > ethers (1.32%). Present results indicate a remarkable effect of geographical origin on the total volatile content of thyme honeys.
Table 2. Total volatile compounds (μg/g) of commercial thyme honeys according to geographical origin.
The much higher total volatile content (μg/g) of Egyptian thyme honeys (), compared with Moroccan, Spanish, and Greek thyme honeys, is attributed to thymol and thymoquinone content (μg/g). Thymol and thymoquinone are phytochemical compounds found in many Lamiaceae family (i.e., Monarda fistulosa) and Ranunculaceae plants (i.e., Nigella sativa), respectively. These plants are characterized as “honey plants,” and such compounds are also found in thyme essential oil along with gamma terpinene, p-cymene, and carvacrol.[Citation24]
Classification of mediterranean thyme honeys according to geographical origin based on volatile compounds
The 34 thyme honey samples were subjected to MANOVA to determine which volatile compounds were significant for their geographical discrimination. Dependent variables included the 62 volatiles, while geographical origin was taken as the independent variable. Pillai’s trace (=2.931, F = 13.529, p = 0.001 < 0.05) and Wilks’ lambda (=0.001, F = 170.382, p = 0.001 < 0.05) index values showed that there was a significant multivariable effect of volatile compounds on geographical origin of honey.
Seventeen of the 62 volatiles () (–) were found to be significant (p < 0.05) for the geographical discrimination of Mediterranean thyme honeys. Thus, these 17 volatiles were subjected to LDA. Results showed that two statistically significant discriminant functions were formed (Wilks’ lambda = 0.001, X2 = 214.911, df = 45, p = 0.001 < 0.05 for the first function, and Wilks’ lambda =0.010, X2 = 108,246, df = 28, p = 0.001 < 0.05 for the second). A significant value of Wilks’ lambda index showed that the discriminant function was basic for the differentiation of the investigated groups. Testing of the uniformity of variability (Box’s M =257.314, F = 2.749, p = 0.055 > 0.05) was insignificant at the 95% confidence level, showing the existence of uniformity of sample variability for each geographical origin. The first discriminant function accounted for 80.4% of total variance and the second accounted for 16.1%.
Figure 1. A typical gas chromatogram of thyme honey (no.2) from Egypt. Key volatile compounds of geographical origin are numbered (1-7) and indicated in bold. Th1: Thymoquinone, Th2: Thymol. IS: internal standard.
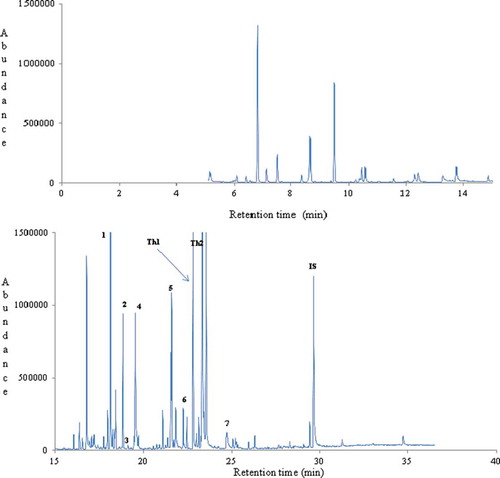
Figure 2. A typical gas chromatogram of thyme honey (no.1) from Morocco. Key volatile compounds of geographical origin are numbered (8-12) and indicated in bold. IS: internal standard.
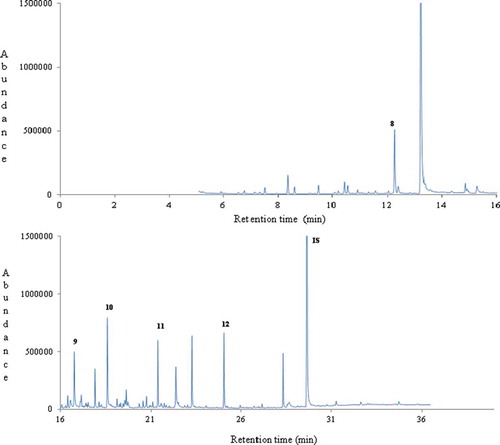
Figure 3. A typical gas chromatogram of thyme honey (no.3) from Spain. Key volatile compounds of geographical origin are numbered (13-14) and indicated in bold. IS: internal standard.
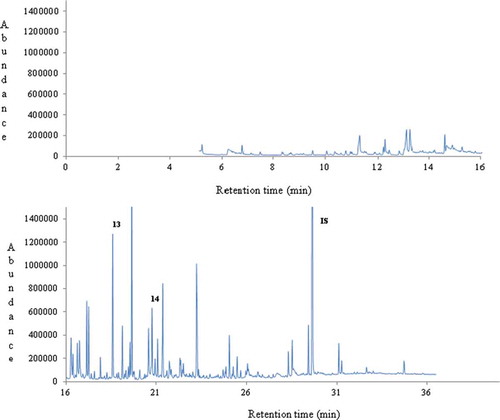
Figure 4. A typical gas chromatogram of thyme honey (no.2) from Greece. Key volatile compounds of geographical origin are numbered (15-17) and indicated in bold. IS: internal standard.
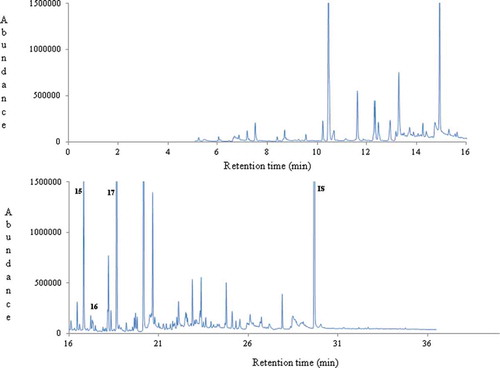
Figure 5. Geographical discrimination of commercial thyme honeys produced in Mediterranean countries based on 17 key volatile compounds.
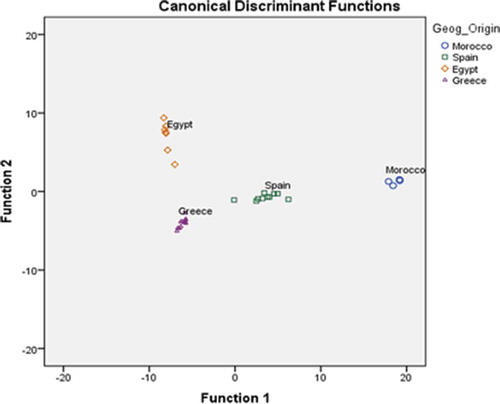
Table 3. Volatile compounds of commercial thyme honeys produced in Mediterranean countries, serving as key markers of geographical origin.
In , it is shown that all the geographical regions are well separated. The first discriminant function clearly differentiates Morocco and Spain from all the other regions, while the second discriminant function clearly differentiates Greece and Egypt. The volatiles (independent variables) that contribute to the first and second discriminant functions are shown in . The overall correct classification rate was 100% using the original and 88.2% using the cross-validation method, which was considered a satisfactory value especially for the second method. The geographical classification rate was 100% for Morocco, Spain, Greece, and Egypt using the original, and 60%, 90%, 100%, and 85.7% for Morocco, Spain, Greece, and Egypt using the cross-validation method, respectively.
Present results are in excellent agreement with previous work on Greek thyme honeys.[Citation9] These authors classified thyme honey from different regions in Greece reporting a classification rate of 85.7%, while similar studies dealing with Moroccan or Egyptian thyme honeys are scarce. It should also be noted that in previous work on Spanish honeys, dealing with the differentiation of botanical origin, the reported classification rates were 83% and 90%, respectively.[Citation19,Citation23]
Conclusion
Results of the present study showed the existence of a rich volatile fraction in commercial thyme honey produced in specific Mediterranean countries. Significant variations among volatiles determined were observed according to geographical origin. Volatile compounds in combination with chemometrics may successfully differentiate the geographical origin of thyme honey from different countries (correct classification rate 88.2% using the cross-validation method). To the best of our knowledge, this is the first study on the geographical differentiation of commercial thyme honeys from Morocco, Egypt, Spain, and Greece using volatile compound data and chemometrics, thus constituting the novelty of the present work. Such studies aid to authentication efforts and may comprise a useful tool in this sense for producers, exporters, or even consumers of honey, at an international level.
Acknowledgments
The authors are grateful to Mr. Christos Nikolaou for his excellent technical assistance and to Attiki Honey S.A. for the donation of honey samples from Greece.
References
- Castro-Vázquez, L.; Díaz-Maroto, M.C.; González-Viñas, M.A.; Perez-Coello, M.S. Aroma Composition and New Chemical Markers of Spanish Citrus Honeys. Food Chemistry 2007, 103, 601–606.
- Barra, M.P.G.; Ponce-Diaz, M.C.; Venegas-Gallegos, C. Volatile Compounds in Honey Produced in the Central Valley of Ňuble Province, Chile. Chilean Journal of Agricultural Research 2010, 70, 75–84.
- Iglesias, M.T.; Martian-Alvarez, P.J.; Polo, M.C.; Lorenzo, C.; Gonzalez, M., Pueyo, E.N. Changes in the Free Amino Acid Contents of Honeys During Storage at Ambient Temperature. Journal of Agricultural and Food Chemistry 2006, 54, 9099–9104.
- Montenegro, G.; Gómez, M.; Casaubon, C.; Belancic, A.; Mujica, A.M.; Peña, R.C. Analysis of Volatile Compounds in Three Unifloral Native Chilean honeys. International Journal of Experimental Botany 2009, 78, 61–65.
- da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical Composition, Stability and Authenticity. Food Chemistry 2016, 196, 309–323.
- Radovic, B.S.; Careri, M.; Mangia, A.; Musci, M.; Gerboles, M.; Anklam, E. Contribution of Dynamic Headspace GC–MS Analysis of Aroma Compounds to Authenticity Testing of Honey. Food Chemistry 2001, 72, 511–520.
- Tananaki, Ch.; Thrasyvoulou, A.; Giraudel, J.L.; Montury, M. Determination of Volatile Characteristics of Greek and Turkish Pine Honey Samples and Their Classification by Using Kohonen Self Organising Maps. Food Chemistry 2007, 101, 1687–1693.
- Soria, A.C.; Martínez-Castro, I.; Sanz, J. Some Aspects of Dynamic Headspace Analysis of Volatile Components in Honey. Food Research International 2008, 41, 838–848.
- Alissandrakis, E.; Tarantilis, P.A.; Pappas, C.; Harizanis, P.C.; Polissiou, M. Ultrasound-Assisted Extraction Gas Chromatography-Mass Spectrometry Analysis of Volatile Compounds in Unifloral Thyme Honey from GREECE. European Food Research and Technology 2009, 229, 365–373.
- Aliferis, K.A.; Tarantilis, P.A.; Harizanis, P.C.; Alissandrakis, E. Botanical Discrimination and Classification of Honey Samples Applying Gas Chromatography/Mass Spectrometry Fingerprinting of Headspace Volatile Compounds. Food Chemistry 2010, 121, 856–862.
- Karabagias, I.K.; Badeka, A.; Kontakos, S.; Karabournioti, S.; Kontominas, M.G. Characterization and Classification of Thymus capitatus (L.) Honey According to Geographical Origin Based on Volatile Compounds, Physicochemical Parameters and Chemometrics. Food Research International 2014, 55, 363–372.
- Petretto, G.L.; Tuberoso, C.I.G.; Vlahopoulou, G.; Atzei, A.; Mannu, A.; Zrira, S., Pintore, G. Volatiles, Color Characteristics and Other Physico-Chemical Parameters of Commercial Moroccan Honeys. Natural Product Research 2016, 30, 286–292.
- Oroian, M.; Prisacaru, A.; Hretcanu, E.C.; Stroe, S.G.; Leahu, A.; Buculei, A. Heavy Metals Profile in Honey as a Potential Indicator of Botanical and Geographical Origin. International Journal of Food Properties 2016, 19, 1825–1836.
- Mannas, D.; Altuğ, T. SPME/GC/MS and Sensory Flavour Profile Analysis for Estimation of Authenticity of Thyme Honey. International Journal of Food Science and Technology 2007, 42, 133–138.
- Tan, S.T.; Wilkins, A.L.; Holland, P.T.; McGhie, T.K. Extractives from New Zealand Unifloral Honeys. 2. Degraded Carotenoids and other Substances from Heather Honey. Journal of Agricultural and Food Chemistry 1989, 37, 1217–1221.
- Molan, P.C. The Limitations of the Methods of Identifying the Floral Source of Honeys. Bee World 1998, 79, 59–68.
- Anklam, E. A Review of the Analytical Methods to Determine the Geographical and Botanical Origin of Honey. Food Chemistry 1998, 63, 549–562.
- Dimou, M.; Katsaros, J.; Tzavella-Klonari, K.; Thrasyvoulou, A. A Study on the Botanical and Geographical Discrimination of Pine and Fir Honeydew Honeys by their Microscopical Characteristics. Journal of Apicultural Research 2006, 45, 16–21.
- Castro-Vázquez, L.; Díaz-Maroto, M.C.; Perez-Coello, M.S. Differentiation of Monofloral Citrus, Rosemary, Eucalyptus, Lavender, Thyme, and Heather Honeys Based on Volatile Composition and Sensory Descriptive Analysis. Food Chemistry 2009, 112, 1022–1030.
- NIST 2005. National Institute of Standards and Technology; J. Wiley & Sons Ltd.: West Sussex, England, 2005.
- Blank, I.; Fischer, K.; Grosch, W. Intensive Neutral Odorants of Linden Honey. Differences from Honey of Other Botanical Origin. Zeitschrift für Lebensmittel Untersuchung und Forschung 1989, 189, 426–433.
- Pontes, M.; Marques, J.C.; Câmara, J.S. Screening of Volatile Composition from Portuguese Multifloral Honeys using Headspace Solid-Phase Microextraction-gas Chromatography-Quadrapole Mass Spectrometry. Talanta 2007, 74, 91–103.
- Soria, A.C.; González, M.; de Lorenzo, C.; Martínez-Castro, I.; Sanz, J. Characterization of Artisanal Honeys from Madrid (Central Spain) on the Basis of their Melissopalynological, Physicochemical and Volatile Composition Data. Food Chemistry 2004, 85, 121–130.
- Senatore, F. Influence of Harvesting Time on Yield and Composition of the Essential oil of a Thyme (Thymus pulegioides L.) Growing wild in Campania (Southern Italy). Journal of Agricultural and Food Chemistry 1996, 44, 1327–1332.
