ABSTRACT
The goal of the present study was to investigate the antioxidant activity and total phenolic content (TPC) of Maya nut (Brosimum alicastrum) in comparison with commercially available nuts (i.e. walnut, almond, and peanut). Results indicated that Maya nut had the highest TPCs among these nuts. Maya nut also possessed strong 2,2-diphenyl-1-picrylhydrazyl radical (DPPH) and 2.2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) scavenging activities and ferric reducing antioxidant power (FRAP) (p < 0.05) as compared to walnut, almond, and peanut. Five phenolic acids (gallic acid, p-hydroxybenzoic acid, vanillic acid, caffeic acid, and p-coumaric acid) and one flavonoid ((-)-epicatechin) were identified and the phenolic content ranged from 6.5 to 326.2 µg/g.
Introduction
Phytochemicals are known as bioactive plant chemicals in nuts, fruits, vegetables, grains, and other plant foods due to their various roles as an antioxidant, antimicrobial, anti-inflammatory, and anticancer factors.[Citation1] Phenolic compounds constitute one of the most widespread groups of substances in phytochemicals. More than 8000 phenolic structures were characterized with significant roles as defence agents against pathogens, parasites, and predators.[Citation2,Citation3] In addition, phenolic compounds serve as antioxidants due to the reactivity of the phenol group and play a role on the colour, sensory qualities, and nutritional properties of food. The redox properties of phenolic substances are based on their antioxidant activities like scavenging of free radicals, degrading peroxides, or quenching oxygen. In recent years, owing to the growing interest in natural health-benefiting compounds, these have been studied from raw materials in order to evaluate their potential health benefits.[Citation4] The analysis of those different phytochemicals might be hard since many of them are bound through ester or ether bonds to either structural elements of the plant such as cellulose, proteins, and lignin or smaller organic molecules such as glucose.[Citation5] Acidic hydrolysis and saponification are commonly used procedures in order to release the phenolic acids even though the methods might decompose the target compounds.
Maya nut is the seed of Brosimum alicastrum, a fast-growing and huge tropical rainforest tree indigenous in Central and Northern South America.[Citation6] Maya nut is also known as Ramon nut, Breadnut, Ojoche, Ox, Ash, Ujuxte, Ojite, Ojushte, Ujushte, Capomo, Pisbawaihka, and Masica and falls under the Moraceae family, often called the mulberry or fig family.[Citation7,Citation8] Historical studies have shown that Maya nut was one of the main staple foods for ancient Mayas and other Neotropical pre-Columbians, and also used as an alternative food when the traditional crop production diminished.[Citation9,Citation10] The seeds can be dried, roasted, and ground into powder and used for baking, hot and cold drinks, and sauces.[Citation6] Additionally, it is rich in protein, folate, calcium, potassium, magnesium, phosphorus, fibre, and vitamins A and C.[Citation11]
Although many studies have been performed on proximates, vitamins, minerals, and fatty acid composition of Maya nut, we lack a detailed understanding of the antioxidant activity and phenolic composition of Maya nut. Therefore, we have aimed to evaluate the antioxidant capacity and total phenolic content (TPC) of Maya nut utilizing 2,2-diphenyl-1-picrylhydrazyl radical (DPPH) scavenging, ferric-reducing antioxidant power (FRAP), and ABTS radical scavenging assays and to compare the data with commercial tree nuts including walnut, peanut, and almond. We have also studied to optimize the extraction methods for phenolic compounds of Maya nut by applying acidic and alkaline hydrolyses, and to quantify the individual phenolic compounds.
Materials and methods
Materials
Maya nut (roasted and grounded seed) was obtained as a ready-to-use powder from Maya Nut Institute (Maya Nut Institute, Crested Butte, CO, USA) through the website.[Citation6] Unsalted dry roasted peanuts (Wal-Mart Stores, Inc, Bentonville, AR, USA), walnuts, and almonds (Hines Nut Company, Dallas, Texas, USA) were purchased from a local store in Clemson, SC, USA. Potassium carbonate was from the Alfa Aesar (Massachusetts, MA, USA). The Folin-Ciocalteu reagent, 2,4,6-tripyridyl-s-triazine (TPTZ), 2,2’-diphenyl-1-picrylhydrazyl (DPPH), ferric chloride, gallic acid (≥98% purity), vanillic acid (97% purity), caffeic acid (≥99% purity), p-hydroxybenzoic acid (≥99% purity), and p-coumaric acid (≥98% purity) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Sodium carbonate, sodium acetate, HPLC-grade and laboratory-grade solvents were purchased from Fisher Scientific (Suwanee, GA, USA). Ferrous sulphate was from J.T. Baker Chemical Company (Phillipsburg, NJ, USA). Hydrochloric acid and glacial acetic acid were obtained from EM Science (Gibbstown, NY, USA). Butylated hydroxytoluene (BHT) of 99% purity and sodium methoxide were purchased from Acros Organics (New Jersey, NJ, USA). (-)-Epicatechin (≥98% purity) was from MP Biomedicals (Santa Ana, California, USA).
Preparation of defatted samples
The oil extraction method was modified from the method reported by Shahidi et al.[Citation12] All samples were ground separately in a spice and nut grinder (Model SG 10C, Blank and Cuisinart Company, Stamford, CT) for 5 min. Then, they were defatted by sonication with hexane (1:5, w/v, 3 × 10 min) using the Branson sonicator (Model 5510, Bransonic Tabletop Ultrasonic Cleaners, Branson Ultrasonics Corporation, Danbury, CT) at an ambient temperature. The samples were centrifuged at 3,024 g (Model Eppendorf Centrifuge 5810 R, Eppendorf North America Inc., Westbury, NY) for 10 min before the upper layer was removed. Subsequently, defatted samples were air-dried for 1 h and then stored for further analysis.
Extraction of phenolic compounds
The preparation of the antioxidant extracts was modified from the method previously reported.[Citation13] Defatted samples were extracted by a Soxhlet extractor using 80:20 (v/v) ethanol/water mixtures (6 g of sample /100 mL of solvent) at 80°C for 30 min. The residue was re-extracted twice under the same condition and the remaining slurries were collected in the flask. A rotary evaporator at 40°C (Model R-200; Buchi, Switzerland) was used to remove the solvent in the slurries. The prepared crude extracts were redissolved in methanol and filtrated by a Whatman filter paper (No. 5). The final concentration was expressed as mg of sample equivalent per mL of methanol. The solutions were then stored at −20°C in the dark for the measurements of TPC and antioxidant activity.
Determination of total phenolic content
Content of phenolics in the extracts was determined by a modified method described by Singleton et al.[Citation14] Briefly, 1 mL of Folin-Ciocalteu phenol reagent (1:10 diluted with distilled water) was added to each centrifuge tube containing 200 μL of the extract and 800 μL saturated sodium carbonate (75.05 g/L). The tubes were thoroughly mixed by vortexing before the tubes were allowed to stand at ambient temperature for 2 h until the characteristic blue colour developed. Absorbances of the clear supernatants were measured at 750 nm using a UV spectrophotometer (Genesys 20 Model 4001/4; ThermoSpectronic, Rochester, NY, USA). The blank, devoid of any extract, was used for background subtraction. The contents of total phenolics in each extract were determined from a series of standard solutions at concentrations of 25, 50, 100, 200, and 400 μg/mL of gallic acid as a standard. The TPC value was expressed as mg gallic acid equivalents (GAE) per dry weight of the sample extract.
Determination of antioxidant capacity
DPPH radical scavenging assay
The method described by Kitts et al.[Citation15] was used with some modifications to evaluate the DPPH radical scavenging activity of the extracts. A volume of 0.5 mL of antioxidant extract solution (10, 100, 200, 300, 400, 500, 1000 µg/mL in methanol) was mixed with 0.5 mL of 0.125 mM DPPH solution dissolved in methanol. The absorbance of the reaction mixture was measured at 515 nm after 1 h. Finally, the absorbance of the resulting solution was read spectrophotometrically at 515 nm. The results were expressed as the content of extract (mg per assay) versus absorbance at 515 nm. DPPH scavenging of the extracts was calculated as described before.[Citation15]
FRAP assay
The ferric reducing power of extracts was determined by a followed method.[Citation16] The working FRAP reagent was prepared daily by a mixture of 300 mM acetate buffer at pH 3.6, 10 mM TPTZ (2,4,6-tri(2-pyridyl)-striazine) in 40 mM hydrochloric acid and 20 mM ferric chloride at a 10:1:1 ratio, respectively. A standard curve was prepared using various concentrations (0.5, 1, 2, 4, 5 mM) of FeSO4•7H2O. All solutions were freshly used. Around 100 µl of the sample and 300 μl of deionized water were added to 3 ml of freshly prepared FRAP reagent. The reaction mixture was incubated at 37°C for 30 min in a water bath. Then, the absorbances of the samples were measured at 593 nm. The FRAP values were expressed as mmol Fe+2/100 g of sample. All measurements were carried out in triplicate.
ABTS radical scavenging assay
ABTS radical cation (ABTS•+) was produced by reacting 7 mM ABTS with 2.45 mM (final concentration) potassium persulphate in the dark at room temperature for 12–16 h. This solution was diluted in deionized water to an absorbance of 0.7 at 734 nm. An appropriate solvent blank reading was taken (AB). After the addition of 100 µl of aqueous extract solutions to 3 mL of ABTS•+ solution, the absorbance reading was taken at 30°C for 20 min after initial mixing (AE).[Citation17] All solutions were freshly used and all measurements were carried out in triplicate. Results were expressed as the percentage of ABTS inhibition.
Extraction and hydrolysis of phenolic acids
Extraction of phenolic acids was determined with some modifications of the method described by Mattila et al.[Citation18] A homogenized dried (0.2–0.5 g) sample was mixed with 7 mL of methanol (containing 2 g/L of 2,3-tert-butyl-4-hydroxyanisole and 10% acetic acid (85:15)) using a vortex for 1 min. Next, 4 g sample of the mixture was then added to 5 mL of the extraction solution (4.25 mL of methanol and 0.75 mL of acetic acid) and mixed by vortexing. The sample extract was ultrasonicated for 30 min, made up to 10 mL with distilled water. Now, 1 mL of the final extract was filtered through a membrane filter (0.45 μm, 25 mm; Fisher Scientific) for first acid hydrolysis. In order to hydrolyse the sample in a sealed test tube, the extract was mixed with 12 mL of distilled water and 5 mL of 10 M NaOH and stirred overnight at room temperature (about 16 h). The solution pH was then adjusted to 2. The liberated phenolic acids were extracted three times with 15 mL of a mixture (1:1) of cold diethyl ether (DE) and ethyl acetate (EA) by manually shaking and centrifuging. DE/EA layers were combined and evaporated to dryness, followed by dissolving in 1.5 mL of methanol. After the sample was filtered through a membrane filter (0.45 μm, 25 mm; Fisher Scientific), it was analysed by HPLC. After the alkaline hydrolysis, a second acidic hydrolysis was performed by adding 2.5 mL of concentrated HCl into the test tube followed by incubating the tube in a water bath (85°C) for 30 min. After the acidic hydrolysis, the sample was allowed to cool down and the pH adjusted to 2. The DE/EA extraction layers were prepared as before and injected into the HPLC system.
HPLC analysis
HPLC analysis was performed using a Shimadzu UFLC- 20AT HPLC system (Kyoto, Japan) equipped with a model SPD-20A UV/VIS detector, a model DGU-20A5 degasser, and a model SIL-20 AHT auto sampler, and all of which were connected and controlled by the LC Solution software. Identification wavelength of phenolic acids with the UV detector was 280 nm. Separation of phenolic acids was conducted on a Premier C18 column (150 × 4.6 mm; 5 μm particle size) with a C-18 guard column. The mobile phase consisted of 50 mM H3PO4, pH 2.5 (Solution A) and acetonitrile (Solution B). Gradient elution was employed as follows: isocratic elution 5% B, 0–5 min; linear gradient from 5% B to 50% B, 5–55 min; isocratic elution 50% B, 55–65 min; linear gradient from 50%B to 5% B, 65–67 min; post-time 6 min before the next injection. Flow rate of the mobile phase was maintained at 0.7 mL/min, and the injection volumes of the standards and sample extracts were 10 μL. Quantification was performed based on the external standard. The compounds in the chromatograms were identified by comparing their retention times (RT) with those of the standards. All quantifications were determined based on the peak area and the samples were analysed in triplicate.
Statistical analysis
Data was presented as the means ± standard deviation of three–four independent samples. All statistical analyses were performed on the SAS V9.2 software for Windows (SAS Institute Inc., Cary, NC, USA). Differences between the means were calculated by analysis of variance (one-way analysis of variance, ANOVA) at p < 0.05.
Results and discussion
Phenolic compounds could react with the molybdenum-containing Folin-Ciocalteu reagent and might be induced by an electron transfer during the TPC assay. With the electron transfer, the deep yellow colour was converted to a blue colour, which can be measured spectroscopically. As shown in , the TPCs of the samples were illustrated as GAE per gram of Maya nut/tree nuts extracts. The mean values of the total phenolics ranged from 87.5 ± 2 to 2467 ± 85 mg GAE/100 g and were in the following order: Maya nut ˃ walnut ˃ peanut ˃ almond. Based on the Folin-Ciocalteu method, Yang et al.[Citation1] and Kornsteiner et al.[Citation19] reported that the TPCs of these tree nuts ranged from 1020 to 2052 mg GAE/100 g for walnut, from 130 to 459 mg GAE/100 g for almond, and from 326 to 552 mg GAE/100 g for peanut. The reason why TPC values vary in the nuts may lie in the different geographical locations of the nuts, the extraction solvent, or the time used in their studies. Consistent with these results, our study showed the same order as in the TPCs of these tree nuts.[Citation1,Citation19] Surprisingly, we found that Maya nut had much higher TPC than these tree nuts in an aqueous-ethanol-based extraction system, even more than those of various kinds of fruits and vegetables.[Citation20,Citation21]
Table 1. Antioxidant capacities of Maya nut, walnut, almond, and peanut extracts.
Table 2. Recoveries of phenolic acids after different hydrolysis conditions at 280 nm (µg per g of Maya nut).
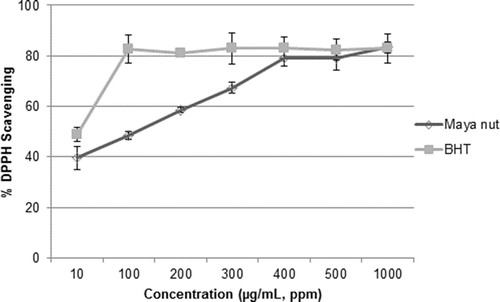
To determine the antioxidant capacity of the extracts, we utilized DPPH, FRAP, and ABTS assays. The DPPH radical scavenging assay is commonly used in evaluating the ability of antioxidants to scavenge free radicals. The change in absorbance at 515 nm presents a measure of the scavenging effect of a particular extract for DPPH radicals.[Citation22] The absorbance at 515 nm decreases as the reaction between antioxidant molecules and DPPH radicals progresses. Therefore, more potent antioxidant activity indicates a rapid decrease in the absorbance of the extract.[Citation23] As shown in , the percentages of DPPH scavenging activities were 79 ± 2.7 (Maya nut), 83 ± 0.5 (walnut), 47 ± 1.3 (peanut), and 45.5 ± 0.6 (almond). Our results are comparable to results previously found on the scavenging activity of DPPH radicals from walnut kernels,[Citation24] almond seed,[Citation13] and peanut kernel.[Citation25] Several researchers showed that walnut has the highest levels of free and total polyphenols, and the highest DPPH scavenging activity among commercially available nuts.[Citation26–Citation28] Interestingly, we found that Maya nut extract has potent antioxidant activity as high as walnut extract and it might be a good source of natural antioxidants. In addition, the comparison of DPPH scavenging values of the Maya nut extract and standard antioxidant BHT is indicated in . After 60 min of the reaction, the percentage DPPH values of the remaining Maya nut extract at concentrations of 10–1000 µg/mL ranged from 42 to 83%, respectively. Aqueous-ethanol extract of Maya nut revealed a strong concentration-dependent antioxidant activity. Even at low extract concentration, the DPPH activity of Maya nut was found to be very high (79% at 400 µg/mL), which was comparable to the one achieved for BHT (83% at 400 µg/mL). Among tree nuts, Maya nut had as high DPPH free radical scavenging activity as walnut, indicating consistency with its rich TPC.
Figure 1. The comparison of % DPPH free radical scavenging capacity of Maya nut and BHT. Each value represents the mean ± SD of three independent assays.
Moreover, the FRAP assay was used to determine the ferric reducing ability of the samples. The antioxidant capacities of the samples were estimated from their ability to reduce the TPTZ-Fe3+ to the TPTZ-Fe2+ complex.[Citation16] The calculation of the FRAP value was performed utilizing iron (II) sulphate (). The mean values of walnut, Maya nut, peanut, and almond for the FRAP assay were 22.64 ± 1.1, 8.08 ± .0.8, 1.61 ± 0.3, and 1.52 ± 0.9 mmol Fe2+/100 g sample, respectively. Walnut had the greatest ability to induce the reduction of TPTZ-Fe3+ to TPTZ-Fe2+ complex, followed in order of Maya nut, peanut, and almond. FRAP of Maya nut was much higher than those of other nuts including chestnuts (4.7 mmol/100 g), sunflower seeds (6.4 mmol/100 g), peanuts (2 mmol/100 g), and pistachios (1.7 mmol/100 g).[Citation29] Additionally, the FRAP value of Maya nut was preferable to various kinds of beverages such as orange juice (0.64 mmol/100 g), pomegranate juice (2.1 mmol/100 g), green tea (1.5 mmol/100 g), and to antioxidant-rich berries such as blueberries (3.5 mmol/100 g), strawberries (2.1 mmol/100 g), and several types of fruits and vegetables.[Citation29] The free radical scavenging capacities of extracts were also analysed using the ABTS radical cation decolourization assay,[Citation17] which is based on the reduction of ABTS radicals by antioxidants of the extracts tested. In the ABTS assay, Maya nut extract (92.55%) and walnut extract (92.1%) had the highest antioxidant capacity, followed by peanut extract (91.1%) and almond extract (58.96%) ().
In this study, when we correlate the TPC and antioxidant activities of ethanolic extracts of tree nuts, typically there is a correlation of an increase in phenolic content to an antioxidant power. When we excluded walnut extract, there was a direct relationship between TPC and FRAP (R2 = 0.9853) and there was a significant linear correlation between TPC and DPPH scavenging activity (R2 = 0.9976) in the extracts. In addition, a strong correlation was observed between FRAP and DPPH scavenging activity (R2 = 0.9947). However, there was no considerable linear correlation between ABTS radical scavenging capacity and TPC (R2 = 0.4099).
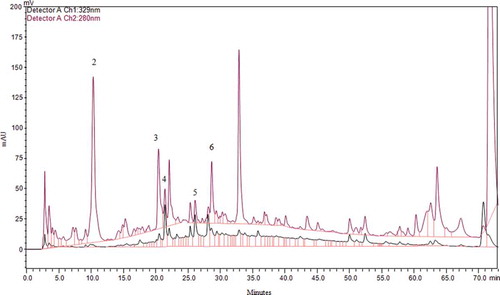
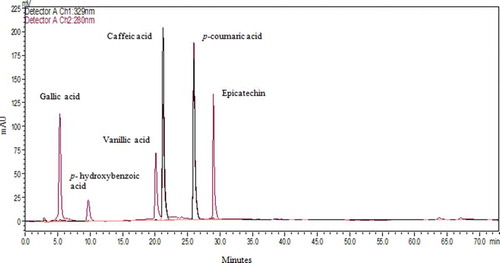
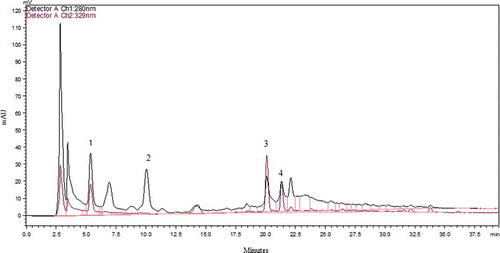
In addition to TPC and antioxidant activity, individual phenolic compounds of Maya nut were analysed by performing both an acidic and an alkaline hydrolysis. The goal of applying two different hydrolytic procedures was to compare and optimize the extraction methods for the isolation and quantification of free phenolic acids from Maya nut. The chromatographic profiles in this study correspond to the acidic hydrolysis/alkaline hydrolysis samples of Maya nut and standard mixture collected into the DE/EA solution and detected at 280 nm (–). Under the same chromatography conditions, sample peaks were detected by comparison of their RT (in minutes) with known standard compounds (). Along with epicatechin, hydroxybenzoates, gallic, p-hydroxybenzoic, and vanillic acids, and hydroxycinnamates, caffeic and p-coumaric acids were identified, accordingly. After the acidic hydrolysis, peaks of 1, 2, 3, and 4 yielded gallic acid (RT = 5.3), p-hydroxybenzoic acid (RT = 10.1), vanillic acid (RT = 20.2), and caffeic acid (RT = 21.3), respectively (). The alkaline hydrolysis revealed the peaks of 2, 3, 4, 5, and 6, respectively, in turn of p-hydroxybenzoic acid, vanillic acid, caffeic acid, p-coumaric acid (RT = 26.2), and epicatechin (RT = 28.8). Each individual phenolic was reported in µg per g of Maya nut. Among the polyphenolic compounds, p-hydroxybenzoic acid was the most abundant, followed by vanillic acid, epicatechin, gallic acid, caffeic acid, and p-coumaric acid. After the first acidic hydrolysis, concentrations of gallic acid, p-hydroxybenzoic acid, vanillic acid, and caffeic acid were sequentially 27.1, 45, 21.6, and 6.5 µg/g. Moreover, in conduction of alkaline hydrolysis, HPLC-UV detection gave larger amounts of free phenolic acids including p-hydroxybenzoic acid (326.2 µg/g), vanillic acid (103.9 µg/g), caffeic acid (17.1 µg/g), p-coumaric acid (13.5 µg/g), and epicatechin (53 µg/g) (). Besides, epicatechin was detected in an amount of 53 µg/g after the alkaline hydrolysis, which is higher than that of several black tea species ranging from 1.52 to 14.9 µg/g among different varieties.[Citation30] In addition, second acidic hydrolyses of the remaining bound phenolic acids were performed to obtain more p-hydroxybenzoic acid (16 µg/g) in Maya nut extract. These results indicate that most of the phenolic acids were in the bound form and p-hydroxybenzoic acid may be the main contributor to the antioxidant activity of Maya nut. Similarly, bound phytochemicals were the main contributors to the antioxidant activities of wheat, corn, rice, and oats, and may survive stomach and intestinal digestion, which is crucial for the prevention of colon, breast, and prostate cancer.[Citation31] The presence of rich phenolic compounds in Maya nut is an important characteristic for its various biological and nutritional applications. Altogether, further investigation is required for the nutraceutical application of these secondary metabolites present in Maya nut.
Figure 2. HPLC Chromatogram of free phenolic acids after methanol-acetic acid extraction at 280 nm and 329 nm. Gallic acid (peak 1), p-hydroxybenzoic acid (peak 2), vanillic acid (peak 3), caffeic acid (peak 4).
Conclusion
This study shows the first report showing the TPC and antioxidant activity of Maya nut in comparison with commercial tree nuts. HPLC analysis of Maya nut extract also revealed the presence of rich polyphenols, which likely contributes to its antioxidant capacity even though some detected phenolic compounds remained unidentified. We have found that Maya nut had the highest TPC (2467 mg GAE/100 g) and potent free radical scavenging capacities among the tree nuts tested. p-hydroxybenzoic acid was the major phenolic compound. Consequently, Maya nut may be a suitable ingredient in functional foods and could serve as an important natural antioxidant resource. Further investigations are required to evaluate the anti-proliferative and anti-inflammatory activities in order to improve its usage in food and dietary supplemental products for health promotion.
Declaration of interest
The author reports no conflicts of interest.
Acknowledgements
The author thanks Dr Feng Chen for his technical assistance.
Funding
The author acknowledges The Ministry of National Education, Turkey, for financial assistance.
Additional information
Funding
References
- Yang, J.; Liu, R.H.; Halim, L. Antioxidant and Antiproliferative Activities of Common Edible Nut Seeds. LWT - Food Science and Technology 2009, 42, 1–8.
- Dong, M.; He, X.; Liu, R.H. Phytochemicals of Black Bean Seed Coats: Isolation, Structure Elucidation, and Their Antiproliferative and Antioxidative Activities. Journal of Agricultural and Food Chemistry 2007, 55, 6044–6051.
- Dimitrios, B. Sources of Natural Phenolic Antioxidants. Trends in Food Science & Technology 2006, 17, 505–512.
- Singh, J.P.; Kaur, A.; Singh, N.; Nim, L.; Shevkani, K.; Kaur, H.; Arora, D.S. In vitro antioxidant and antimicrobial properties of jambolan (Syzygium cumini) fruit polyphenols. LWT-Food Science and Technology 2016, 65, 1025–1030.
- Stalikas, C.D. Extraction, Separation, and Detection Methods for Phenolic Acids and Flavonoids. Journal of Seperation Science 2007, 30, 3268–3295.
- Mayanutinstitute.org. What is Maya nut? Available from: http://mayanutinstitute.org/maya-nut/food/(accessed June 16, 2016).
- Puleston, D.E. Brosimum alicastrum as a Subsistence Alternative for the Classic Maya of the Central Southern Lowlands [Thesis]. Philadelphia, PA: University of Pennsylvania, 1968.
- Puleston, D.E. Ancient Maya Settlement Patterns and Environment at Tikal, Guatemala: Implications for Subsistence Models [Dissertation]. Philadelphia, PA: University of Pennsylvania, 1972.
- Peters, C.M. Reproduction, Growth and the Population Dynamics of Brosimum alicastrum in a Moist Tropical Forest of Central Veracruz, Mexico [Dissertation]. Connecticut: Yale University, 1989.
- Peters, C.M. Observations on Maya Subsistence and the Ecology of a Tropical Tree. American Antiquity 1983, 48, 610–614.
- US Department of Agriculture, Agricultural Research Service, Nutrient Data Laboratory. USDA National Database for Standard Reference, Release 28. Version Current: September 2015. Available from http://www.ars.usda.gov/nea/bhnrc/ndl (cited June 14, 2016)
- Shahidi, F.; Alasalvar, C.; Liyana-Pathirana, C.M. Antioxidant Phytochemicals in Hazelnut Kernel (Corylus Avellana L.) and Hazelnut ByproductsJournal of Agricultural and Food Chemistry 2007, 55, 1212–1220.
- Siriwardhana, S.S.K.W.; Shahidi, F. Antiradical Activity of Extracts of Almond and its By-Products. Journal of the American Oil Chemists’ Society 2002, 79, 903–908.
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. American Journal of Enology and Viticulture 1965, 16, 144.
- Kitts, D.D.; Yuan, Y.V.; Wijewickreme, A.N.; Hu, C. Antioxidant Properties of a North American Gingseng Extract. Molecular and Cellular Biochemistry 2000, 203, 1–10.
- Benzie, I. F. F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of Antioxidant Power: The FRAP Assay. Analytical Biochemistry 1996, 239, 70–79.
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radical Biology & Medicine 1999, 26, 1231–1237.
- Mattila, P.; Hellström, J.; Törrönen, R. Phenolic Acids in Berries, Fruits, and Beverages. Journal of Agricultural and Food Chemistry 2006, 54, 7193–7199.
- Kornsteiner, M.; Wagner, K.H.; Elmadfa, I. Tocopherols and Total Phenolics in 10 Different Nut Types. Food Chemistry 2006, 98, 381–387.
- Wolfe, K.L.; Kang, X.; He, X.; Dong, M.; Zhang, Q., Liu, R.H. Cellular Antioxidant Activity of Common Fruits. Journal of Agricultural and Food Chemistry 2008, 56, 8418–8426.
- Chu, Y.F.; Sun, J.; Wu, X.; Liu, R.H. Antioxidant and Antiproliferative Activities of Common Vegetables. Journal of Agricultural and Food Chemistry 2002, 50, 6910–6916.
- Farhoosh, R.; Kenari, R.E.; Poorazrang, R. Frying Stability of Canola Oil Blended with Palm Olein, Olive, and Corn Oils. Journal of the American Oil Chemists’ Society 2009, 86, 71–76.
- Alasalvar, C.; Karamać, M.; Amarowichz, R.; Shahidi, F. Antioxidant and Antiradical Activities in Extracts of Hazelnut Kernel (Corylus Avellana L.) and Hazelnut Green Leafy Cover. Journal of Agricultural and Food Chemistry 2006, 54, 4826–4832.
- Pereira, J.A.; Oliveira, I.; Sousa, A.; Ferreira, I.C.F.R.; Bento, A.; Estevinho, L. Bioactive Properties and Chemical Composition of Six Walnut (Juglansregia L.) Cultivars. Food and Chemical Toxicology 2008, 46, 2103–2111.
- Mar, M.W.; Abdul Hamid, A.; Sam Baharin, B.; Anwar, F.; Sabu, M.C.; Pak Dek, M.S. Phenolic Compounds and Antioxidant Activity of Peanut’s Skin, Hull, Raw Kernel and Roasted Kernel Flour. Pakistan Journal of Botany 2011, 43, 1635–1642.
- Vinson, J.A.; Cai, Y. Nuts, Especially Walnuts, have both Antioxidant Quantity and Efficacy and Exhibit Significant Potential Health Benefits. Food & Function 2012, 3, 134–140.
- Arcan, I.; Yemenicioğlu, A. Antioxidant Activity and Phenolic Content of Fresh and Dry Nuts with or without Seed Coat. Journal of Food Composition and Analysis 2009, 22, 184–188.
- Abe, L.T.; Lajolo, M., Genovese, M.I. Comparison of Phenol Content and Antioxidant Capacity of Nuts. Food Science and Technology (Campinas) 2010, 30, 254–259.
- Carlsen, M. H.; Halvorsen, B.L.; Holte, K.; Bøhn, S.K.; Dragland, S.; Sampson, L.; Willey, C.; Senoo, H.; Umezono, Y.; Sanada, C.; Barikmo, I.; Berhe, N.; Willett, W.C.; Phillips, K.M.; Jacobs Jr, D.R.; Blomhoff, R. The Total Antioxidant Content of More than 3100 Foods, Beverages, Spices, Herbs and Supplements Used Worldwide. Nutrition Journal 2010, 9, 1–11.
- Rechner, A.R.; Wagner, E.; Van Buren, L.; VandePut, F.; Wiseman, S.; Rice Evans, C.A. Black Tea Represents a Major Source of Dietary Phenolics Among Regular Tea Drinkers. Free Radical Research 2002, 36, 1127–1135.
- Lim, T.K. Edible Medicinal and Non-Medicinal Plants. In the Triticum aestivum. New York, London: Springer 2013, 385–416.