ABSTRACT
Trans-free mango kernel fat stearins and oleins were produced by three-stage acetone fractionation to achieve the sufficient utilization of fat. Fatty acid and triacylglycerol compositions, slip melting points, iodine values, micronutrient (tocopherol, sterol, and squalene) levels, as well as oxidative stability indexes of the fractions were analyzed to evaluate their qualities. The most abundant fatty acids in the stearins were saturated fatty acids (57.3–65.1%, mainly including palmitic, stearic, and arachidic acids), and the major triacylglycerols were symmetrical monounsaturated types (78.3–93.7%). The unique properties make the stearins show highest slip melting points 34.7–38.3°C and oxidative stability indexes (12.0–14.2 h), and very suitable for the manufacturing of hard chocolate fats. The oleins contained high percentages of monounsaturated (48.3–53.7%) and polyunsaturated fatty acids (7.8–8.5%). Their oxidative stability indexes (6.3–6.7 h) were lower than the stearins but higher than common commercial oils. About 85.2% of tocopherol, 99.2% of sterol, and 79.2% of squalene were transferred to the liquids after fractionation, which could improve their antioxidant abilities. Further multiple linear regression analyses between oxidative stability indexes and fat compositions revealed that polyunsaturated fatty acid and sterol were the main factors that affect the oxidative stabilities of the fractions. The results suggested that the moderated refining techniques should be developed to retain more sterol to improve the oxidative stabilities and nutritive values of the fats.
Introduction
Mango kernel fat (MKF) is extracted from the kernels of Mango (Mangifera indica, Linn), the second largest tropical fruit belonging to the family of Anacardiaceae.[Citation1] The fat has attracted considerable interests nowadays for its biological activities and qualities as a natural food, which were benefited from its heterogenous composition (40.7–69.0% of unsaturated fatty acids, 29.7–61.0% of saturated fatty acids (SFAs), and 4082–8942 of mg kg–Citation1 micronutrients).[Citation1–Citation3] In particular, the high content of symmetrical monounsaturated triacylglycerols (SUS-TAGs, 52.2–81.5%) makes MKF very suitable for the manufacturing of chocolate products.[3,4] Furthermore, there have been no studies showing that MKF contains any toxic or allergenic compounds.[Citation5] Recent research showed that MKF could exhibit remarkable antioxidant and antimicrobial activities in some food systems, for example, sunflower oil, potato chips, and pasteurized cow milk.[Citation6] Thus, it makes the fat very suitable to mix with other vegetable oils or food ingredients to prepare for food ingredients, especially the confectionary products.[Citation7,Citation8]
For the past few years, chocolate fats were produced by blending of MKF with some vegetable fats, for example, palm mid-fractions, palm stearins, and mahua fats.[Citation9–Citation11] However, the thermal properties, crystallographic forms, and microstructures of the mixed fats might be quite different from those of cocoa butter due to the presence of triunsaturated and diunsaturated triacylglycerols (UUU-TAGs and SUU-TAGs).[Citation3,Citation11–Citation13] In addition, a high diacylglycerol content (2.7–5.8%) in MKF would also give chocolate its undesirable physical properties.[Citation3,Citation14,Citation15] Thus, it is important to fractionate the fat to obtain the SUS-TAG-rich fraction, while the other fraction was enriched in SUU- and UUU-TAGs. The latter is usually an ideal source for producing cooking oils, frying fats, and hard structured lipids by further blending or interesterification.[Citation16,Citation17] In this regard, comparably with palm oil, the composition makes MKF very suitable for being separated into various fats and oils characterized as different physicochemical properties by fractionation.[Citation18,Citation19] But only the stearin, which is obtained from one-stage fractionation, has achieved industrialized production and application.[Citation20] Although the fractions produced from multi-stage crystallization are usually considered high-quality products due to their more unique components, the characteristics and usages of them have seldom been studied. In particular, there is very little information about the oxidative/antioxidative properties of the fractions to the best of our knowledge, while oxidative stability has been recognized as the key characteristic, which directly influences the shelf life of the lipid-containing foods.
Recent studies have reported that the oxidative stabilities of edible lipids depend primarily on the fatty acids incorporated in the triacylglycerol molecules (the predominant form in lipids).[Citation14] Double bonds presented in the fatty acids are the predominant factors of the oxidation of fats and oils, as they can act as free radical acceptors.[Citation14,Citation21] Oxidative molecules, for example, hydroperoxides, ketones, alkanes, aldehydes, and alcohols, etc., are produced from the oxidation, which have a negative influence on body health.[Citation14] Some minor components which belong to the unsaponifiable matters in fats and oils (<20,000 mg kg–Citation1) have received considerable attention due to their excellent antioxidant activities. Most of these minor components are called micronutrients, which are industrially important compounds due to their nutritional and pharmaceutical values. Tocopherol, sterol, and squalene are perhaps the most common micronutrients which are vital to stabilize the double bonds of fatty acids against oxidative deterioration.[Citation14] However, their dominant forms depend on various types of fats and oils. As an example, soybean oil and palm oil contain mainly γ-tocopherol with decreasing amounts of other tocopherols, whereas sunflower oil contains mainly α-tocopherol and very small amounts of other isomers.[Citation22] The antioxidant activities of α- and γ-tocopherols decrease in the order of α > γ in both vivo and bulk oils, resulting in different antioxidative behaviors in related lipid systems.[Citation14] Furthermore, α-tocopherol could act as an antioxidant or pro-oxidant depending on its concentration.[Citation21] In addition to the differences in the activities of individual antioxidants, a synergistic or antergic effect will be shown when the micronutrients are mixed together. The antioxidative activity of individual antioxidant will be increased or decreased in different kinds of lipids. For instance, squalene exhibited an antioxidative activity in the mixture with α-tocopherol and β-sitosterol, but no significant effect was tested for squalene alone.[Citation23] Ceci et al. [Citation24] suggested that polyphenol content was more effective to prevent the olive oil from oxidation than carotenes, β-tocopherol, and other compounds. The major unsaponifiables presented in MKF are also tocopherol, sterol, and squalene.[Citation5] But their content changes during the fat fractionation, and antioxidant capacities in the fractions are not clear yet, which limits their application in the foods.
In this work, the fatty acids (including the trans-isomers) and micronutrient levels of the fractions separated from MKF by three-stage fractionation were evaluated, and their relations between oxidative stability index (OSI) and compositions were analyzed to explore the oxidative and antioxidative properties for the mango fats.
Materials and methods
Materials
Mango seeds were provided by local mango processing factory in Guangxi Province, and were cut open to obtain kernels. Crude MKF was extracted from the kernels as Sonwai et al.[Citation11] described, and then refined (free fatty acid, 0.6 %; hydroperoxides, 0.52 mmol kg–Citation1) as our previous method.[Citation3] The fat was kept refrigerated at 4°C prior to fractionation and analysis.
Standard 37 fatty acid methyl esters, tocopherols (α-, β-, γ-, and δ-isomers, purity >95%), and 5α-cholestane were purchased from Sigma-Aldrich Chemical Co. Ltd. (Shanghai, China). Triacylglycerol standards, StOSt, StOO, and OOO (St, stearic; O, oleic), were obtained from Larodan Fine Chemicals AB (Malmö, Sweden). Other reagents and solvents were bought from Sinopharm Chemical Regent (Shanghai, China).
Mango kernel fat fraction preparation
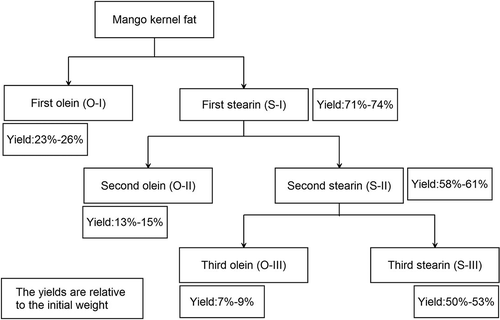
The fractionation including three-stage processes () was conducted in a laboratory fractionation crystallization unit with a capacity of 1000 mL. One hundred grams of MKF was melted uniformly at 65°C to remove any previous crystal memories and then mixed with acetone in a 1:5 (w:v) ratio. The first process was done at 13°C for 100 min to collect the first stearin (S-I) and olein (O-I). The fractions were separated by vacuum filtration, and acetone remaining in them was removed by a rotavapor under vacuum. Then, S-I was maintained at 15°C for 180 min to produce the second stearin (S-II) and olein (O-II), and S-II was further separated at 18°C for 180 min to obtain the third stearin (S-III) and olein (O-III). Both of the processes were carried out in the same manner as the first fractionation. The fractionation was carried out in duplicate. Fatty acid (FA) compositions, micronutrient levels, and OSIs of the fractions were analyzed, and the yields of them with respect to the initial weight were calculated.
Determination of fatty acid composition
Fat/oil of 50 mg was dissolved in 2.0 mL hexane and methyl esterified by adding 0.5 mL 2 mol L–Citation1 KOH–CH3OH to prepare fatty acid methyl esters. The separation was performed on a trace TR-FAME capillary column (0.25 µm, 60 m × 0.25 mm, Thermo Fisher, USA) installed in a gas chromatograph (7820A, Agilent, USA). The analysis conditions were: nitrogen (carrier gas), 1 mL min–Citation1; split ratio, 1:100; flame ionization detector (FID) and injector temperature, 250°C; oven temperature, 60°C (3 min)−175°C (5°C min–Citation1, 15 min)−220°C (2°C min–Citation1, 10 min); injection volume, 1.0 µL. Fatty acids were identified by comparing the retention times with those of standards, and their levels were reported in terms of the relative proportions.
Determination of triacylglycerol composition
Triacylglycerol composition of each fat/oil sample was determined based on the AOCS Official Method Ce 5c-93 using a high-performance liquid chromatograph (Agilent 1200) system.[Citation25] Triacylglycerols were separated on two LiChroCART 18e columns (5 µm, 4.6 × 250 mm each; Merck KGaA, Germany) and detected by a refractive index detector. The operating conditions were: mobile phase, acetone/acetonitrile (75:25, v:v); flow rate, 1.0 mL min–Citation1; sample concentration, 30 mg mL–Citation1; injection volume, 20 µL. Triacylglycerol groups, SUS, SUU, and UUU, were identified by comparing the retention times with that of relative standards and soybean oil of known triacylglycerol composition. Contents were reported in terms of the relative proportions.
Determinations of slip melting point and iodine value
Slip melting point (SMP) and iodine value (IV) were detected as the AOCS Official Method Cc 1–25 and Cd 1–25, respectively.[Citation25]
Determination of tocopherol
Tocopherols were separated on a silica column (5 µm, 4.6 × 250 mm, Hanbon, China) mounted in a high-performance liquid chromatograph (LC-20AT, Shimadzu, Japan) and detected by an ultraviolet detector (SPD-20A, Shimadzu, Japan) according to our pervious method.[Citation26] Conditions of analysis were: mobile phase, hexane/isopropanol (98.5:1.5, v:v); rate, 1.0 mL min–Citation1; detection wavelength, 295 nm; column temperature, 30°C; sample concentration, 100 mg mL–Citation1; injection volume, 20 µL. Tocopherol isomers were identified and quantified by comparing the standards, and the contents were reported in milligrams per kilogram.
Determination of sterol and squalene
Sterol and squalene were detected by a gas chromatograph–mass spectrum system (Thermo Fisher) equipped with an FID as described by Li et al. [Citation27] Two hundred milligrams of the fat/oil together with 0.2 mL of 0.825 mg mL–Citation1 5α-cholestane was saponified using 2 mL of 2 mol L–Citation1 KOH–CH3CH2OH solution. The mixture was then heated at 85°C for 1 h and cooled to room temperature, and 5 mL of hexane followed by 2 mL of distilled water were added to extract the unsaponifiables three times. The extract was dried by nitrogen and silylated with 400 µL BSTFA + TMCS at 70°C for 30 min, and finally dissolved by 1 mL hexane. The analysis was performed by a gas chromatograph–mass spectrum system (Thermo Fisher) equipped with an FID and a DB-5 capillary column (0.25 µm, 30 m × 0.25 mm, Agilent). Gas chromatograph conditions were: helium (carrier gas), 1 mL min–Citation1; split ratio, 1:100; FID and injector temperature, 290°C; oven temperature = 200°C (1 min)−300°C (1°C min–Citation1, 18 min); injection volume, 1.0 µL. Mass spectrum conditions were: source temperature, 280°C; transmission line temperature, 250°C; ionization mode, EI; mass range (m/z), 50–500. The internal standard, 5α-cholestane, was used to quantify the contents of sterol and squalene (mg kg–Citation1).
Determination of oxidative stability index
OSI was detected by a Metrohm Rancimat model 743 (Herisau, Switzerland) according to the AOCS Cd 12b-92 method.[Citation25] Fat/oil (3 g) was heated at 120°C, and 20 L h–Citation1 of the cleaned and dried air was bubbled into the hot sample. Effluent air containing volatile organic acids from the sample was collected in a measuring vessel containing 50 mL of distilled water. The conductivity of the water was measured automatically as oxidation proceeded, and the result was recorded in hours (h).
Statistical analysis
All determinations were carried out in triplicates, and were reported as mean ± standard deviation. The results were analyzed by analysis of variance (ANOVA) and an LSD test at 95% confidence interval using the SPSS program, version 19.0. The correlations between OSI and FA composition, tocopherol, sterol, and squalene were calculated by multiple linear regression analysis using a stepwise method. The use probability of F values p-to-enter and p-to-remove new variables into statistical model were p < 0.05 and p > 0.10, respectively.
Results and discussion
Chemical compositions and physical properties of the fractions
The FA compositions of MKF and its fractions are given in . The major FAs found in MKF were stearic (C18:0, 41.9%) and oleic (C18:1, 42.6%) acids, also the fat contained appreciable quantities of palmitic (C16:0, 6.0%), linolenic (C18:2, 4.5%), and arachidic (C20:0, 2.2%) acids. Jahurul et al. [Citation28] also concluded that stearic and oleic acids are the dominant fatty acids in MKF extracted from Manila, Kaew, Kenya, and Malaysia mango varieties. SFAs (palmitic acid, 62.9°C; stearic acid, 70.1°C) usually have higher melting points than unsaturated ones (oleic acid, 16.3°C; linolenic acid, –5.0°C), which would result in the SFAs gradually precipitate from the oleins by the effect of low-temperature crystallization.[Citation14] Specifically, S-I, S-II, and S-III contained significantly higher levels of SFAs (57.3–65.1%) and were solid at room temperature (SMPs, 34.7, 36.3, and 38.3°C, respectively), whereas the oliens with more monounsaturated fatty acids (MUFAs, 8.3–53.7%) and polyunsaturated fatty acids (PUFAs, 7.8–8.5%) were melted completely as the temperature increased to 20°C (SMPs for the O-I, O-II, and O-III were 10.4, 14.9, and 19.3°C, respectively). In addition, almost no trans-fatty acids were observed (<0.1%) among the samples. The trans-isomers are known to increase low-density lipoprotein cholesterol and decrease high-density lipoprotein cholesterol, which might lead to coronary heart disease.[Citation29] The values detected in the MKF fractions were within the maximum limit of 2% determined in the edible oils market surveys conducted in China and India.[Citation30,Citation31] Some structural fats and oils containing 0.2–0.5% of trans-isomers were considered as the trans-free lipids.[Citation32,Citation33] Strictly speaking, products containing 2 g trans-fatty acids per 100 g serving may be declared as “zero” or “trans-fat free.”[Citation34] Thus, it was presumed that the prepared MKF fractions were also known as trans-free fats.
Table 1. Chemical compositions (fatty acids, triacylglycerols) and physical properties (iodine values and slip melting points) of mango kernel fat and its fractions.
also shows the triacylglycerol compositions of the samples. The major type found in MKF was SUS-TAG (63.1%), which has already been confirmed by many researchers.[Citation3,Citation28] Related studies have reported that blending of SUS-TAG (especially StOSt) fats into chocolate products can increase the hardness and inhibit the fat bloom.[Citation20,Citation35] Therefore, higher SUS-TAG content is of interest to the confectioners, and such ideal products are usually produced by fractionation. Similarly with the changes of FA compositions, SUS-TAG levels increased in the stearins through the separation process (S-I, 78.3%; S-II, 87.2%; and S-III, 93.7%), while triacylglycerols with more unsaturated FAs (SUU- and UUU-TAGs) were transferred into the oleins. The oleins showed triacylglycerol compositions similar to the palm olein or super palm oil, indicating they were appropriate to prepare frying fats, cooking oils, and filler fats.[Citation16] They also could be used to produce chocolate fats by interesterification with palmitic or/and stearic acids.[Citation17]
IV reflects the unsaturation of lipids. MKF showed a value of 50.0 g/100 g (), which fell within the range of 40.9–60.7 g/100 g and was similar to that of palm oil (50.4–53.7 g/100 g).[Citation16,Citation20,Citation35] Palm oil is widely used in the forms of various fractions, thus the property also indicates that MKF has the potential fractional value. IVs decreased in the stearins while increased in the oleins. The changes were also in agreement with that of FAs. The unique compositions and trans-free properties showed that the fractions could be widely used as cocoa butter equivalent, margarine, shortening, frying fat, cooking oil, and other lipid-containing food ingredients.[Citation5] In particular, the stearins are the high added value alternatives to the trans-containing fats produced by hydrogenation.
Micronutrient levels
Contents of tocopherol, sterol, and squalene of the studied samples are shown in . MKF contained 259 mg kg–Citation1 of tocopherol and was mostly dominated by α-tocopherol (256 mg kg–Citation1). Previous research revealed that α-tocopherol occurs primarily (>65%) in most of MKFs.[Citation3] The fractionation of MKF helped to enrich tocopherol in O-I (588 mg kg–Citation1), and left 149 mg kg–Citation1 in S-I. Further process showed that 428 mg kg–Citation1 of tocopherol was distributed in O-II, and only 53.8 mg kg–Citation1 was distributed in S-II. Similar trend was also observed for the third separation, the tocopherol level for O-III was 153 mg kg–Citation1, whereas for S-III 53 was mg kg–Citation1. Most of the total tocopherol (85.2%) was transferred to the liquid fractions after the three-stage process. Alim et al. [Citation36] also found that the olein contained higher amounts of tocopherol (504 mg kg–Citation1) than the stearin (199 mg kg–Citation1). The high solubility of tocopherol in liquids as compared with solids might be responsible for the changes.[Citation37]
Table 2. Tocopherol, sterol, and squalene levels of mango kernel fat and its fractions.
Sterol makes up 72.4% (1895 mg kg–Citation1) of the micronutrients in MKF. β-Sitosterol is the most abundant sterol with the content of 1195 mg kg–Citation1, followed by stigmasterol (430 mg kg–Citation1), campesterol (139 mg kg–Citation1), lupeol (69 mg kg–Citation1), and 24-methylenecycloartanol (61 mg kg–Citation1). The levels also showed significant decreases from S-I (1379 mg kg–Citation1) to S-III (308 mg kg–Citation1) based on fractionation, but no significant differences were observed among liquid fractions (3953–4039 mg kg–Citation1). The contents in the oleins accounted for 99.2% of the total sterol, indicating that sterol might have a better solubility in liquid fractions than tocopherol. Similarly, most of the sterol (1290.4 mg kg–Citation1) was accumulated in the stearin produced from two-stage fractionation of palm oil, while only 115.4 mg kg–Citation1 was detected in the olein.[Citation38] Sterol occurs in fats and oils in esterified form in relative levels that are dependent on the lipid.[Citation14] MKF consists of 33.3–62.5% of esterified sterol in terms of the total sterol content, the solubility of the esterified one in the oil is extremely high compared with that of free type.[Citation39,Citation40] In addition, a study with respect to rapeseed oil fractionation showed that 80% of the free sterols were enriched in the liquid fraction.[Citation41] In this regard, both of the two types exhibited high solubilities in the liquid lipids.
Squalene is usually present in shark liver oil and olive oil (410–730 and 1500–7000 mg kg–Citation1, respectively), and is also found in less amounts (<300 mg kg–Citation1) in common vegetable lipids.[Citation14] As shown in , MKF is an ideal squalene-source food ingredient, as the content was as high as 388 mg kg–Citation1. The distribution of squalene during the fractionation was similar to sterol. About 79.2% of the squalene was enriched in the oleins, the highest levels were observed in O-I, O-II, and O-III, reaching 605–682 mg kg–Citation1. The enrichment pattern of squalene in the fractions was in agreement with that of tocopherol and sterol and related study.[Citation38]
Characteristics of oxidative stabilities
OSI is a significant parameter for evaluating the shelf life of lipids. The values of MKF, its fractions, and some commercial oils detected at 120°C are shown in . OSIs of the stearins (12.0–14.2 h) were significantly higher than those of the oleins (6.3–6.7 h). Although the values in the oleins are nearly half than those in the stearins, they were significantly higher than that in common edible oils (peanut, canola, corn, soybean, and sunflower oils, 3.1–5.0 h).
Table 3. Oxidative stability indexes of mango kernel fat, its fractions, and some common edible oils.
Further multiple linear regression analysis between OSI and FA compositions revealed that PUFA was the most significant independent variable (R = 0.878; p < 0.05) (), suggesting that higher PUFA contents would more easily lead to oxidation. The oxidative stabilities of the oleins greatly decrease with the increasing of PUFA from 5.1% to 7.8%–8.5%. There is no doubt that almost all of PUFA-rich lipids have a low resistance to oxidative deterioration.[Citation6] But the issue for MUFA is different. In spite of high MUFA contents (33.0%–53.7%) were found in the fractions, they showed no significant relationships with the OSIs. Virgin olive oil even has a high oxidation stability due to its high oleic acid level.[Citation42] For some other oils and fats (e.g., canola oil, shea fat, cocoa butter equivalent, and medium chain triacylglycerols), MUFA and PUFA together demonstrated strong correlations with oxidative stabilities.[Citation43,Citation44] The differences might be affected by the micronutrient species and concentrations present in the lipids except the FA compositions.
Table 4. Multiple linear regression analysis between oxidative stability indexes and fatty acid compositions.
When micronutrients were fitted into the OSI model, the best highly significant variable(s) was sterol for the stearins (R = 0.957; p < 0.05) and were sterol and tocopherol for the oliens (R = 0.984; p < 0.05) (). Therefore, sterol could be the main factor that affected the oxidative stabilities in both solid and liquid fractions. Not only the number and location of the double bond in the sterol skeleton but also the presence of an ethylidene group in the sterol side chain could provide the lipid with anti-polymerization.[Citation45,Citation46] Winkler et al. [Citation47] also found that phytosterols will have an impact on the oxidative stability of oils. Of which, β-sitosterol is the primary sterol present in the MKF, and its fractions are as shown in . Higher synergism in the antioxidative between β-sitosterol and certain lipid systems was also confirmed by Hidalgo et al. [Citation48] However, sterol is easy to be washed with the soap stock generated from the neutralization process, and a significant reduction will also be caused by deodorization.[Citation14,Citation49] Crude MKF extracted from hexane contained high levels of free fatty acids (2.52–6.61%).[Citation3] It is necessary to remove them by refining, which would cause a loss of sterol to some extent. Therefore, moderate refining techniques should be developed to retain more sterol. As proposed, degumming is carried out as the first step to reduce the phosphorus content to less than 5 mg kg–Citation1, the value meets the requirement for physical refining;[Citation50] the fat was then deacidified by steam refining or molecular distillation to remove the free fatty acids; and other residual impurities, for example, metals, pigments,and polyaromatic hydrocarbons, could be adsorbed by small amounts of clays (usually <1% of the fat weight); the refined fat will be obtained with light yellow color and mango flavor.
Table 5. Multiple linear regression analyses between oxidative stability indexes and micronutrient levels (tocopherol, sterol, and squalene).
Conclusion
MKF fractions produced from three-stage acetone fractionation were proved valuable as food ingredients because of their trans-free properties, unique compositions, and high oxidative stabilities. The stearins with rich SFAs showed high contents of SUS-TAGs (78.3–93.7%) and high SMP values (34.7–38.3°C), indicating the fats are the ideal hard chocolate fat sources. The SFA-rich properties also make the stearins more stable (OSIs, 12.0–14.2 h). The oleins were characteristic of MUFAs and PUFAs, but their oxidative stabilities (6.3–6.7 h) were still higher than most commercial oils. Micronutrients (tocopherol, sterol, and squalene), which could prevent the oils from oxidation, were accumulated in the oleins during the fractionation due to their high solubilities in the liquids. Especially, sterol is the most significant factor to prevent the MKF from oxidation according to the correlation analyses between OSIs and micronutrients compositions, suggesting that refining techniques of alkali refining and deodorization which might cause the massive loss of sterol should be moderated.
Abbreviations
| ANOVA, analysis of variance | = | |
| FA, fatty acid | = | |
| FID, flame ionization detector | = | |
| IV, iodine value | = | |
| MKF, mango kernel fat | = | |
| MUFA, monounsaturated fatty acid | = | |
| OSI, oxidative stability index | = | |
| PUFA, polyunsaturated fatty acid | = | |
| SFA, saturated fatty acid | = | |
| SMP, slip melting point | = | |
| SUS-TAG, monounsaturated triacylglycerol | = | |
| SUU-TAG, diunsaturated triacylglycerol | = | |
| UUU-TAG, triunsaturated triacylglycerol | = |
Funding
This research was financially supported by Graduate Research and Innovation Projects in Jiangsu Province (grant number KYLX16_0825), the Natural Science Foundation of Jiangsu Province (grant number BK 20150137), and Program of Science and Technology Department of Jiangsu Province (grant number BY2016022-33).
Additional information
Funding
References
- Muchiri, D.R.; Mahungu, S.M.; Gituanja, S.N. Studies on Mango (Mangifera indica, L.) Kernel fat of Some Kenyan Varieties in Meru. Journal of the American Oil Chemists’ Society 2012, 89, 1567–1575.
- Jahurul, M.H.A.; Zaidul, I.S.M.; Norulaini, N.N.A.; Sahena, F.; Jaffri, J.M.; Mohd Omar, A.K. Supercritical Carbon Dioxide Extraction and Studies of Mango Seed Kernel for Cocoa Butter Analogy Fats. CyTA-Journal of Food 2014, 12, 97–103.
- Jin, J.; Warda, P.; Mu, H.Y.; Zhang, Y.F.; Jie, L.; Mao, J.; Xie, D.; Huang, J.H.; Jin, Q.Z.; Wang, X.G. Characteristics of Mango Kernel Fats Extracted from 11 China-Specific Varieties and Their Typically Fractionated Fractions. Journal of the American Oil Chemists’ Society 2016, 93, 1115–1125.
- Jahurul, M.H.A.; Zaidul, I.S.M.; Norulaini, N.A.N.; Sahena, F.; Kamaruzzaman, B.Y.; Ghafoor, K.; Omar, A.K.M. Cocoa Butter Replacers from Blends of Mango Seed Fat Extracted by Supercritical Carbon Dioxide and Palm stearin. Food Research International 2014, 65, 401–406.
- Solís-Fuentes, J.A.; Durán-de-Bazúa, M.C. Mango (Mangifera indica L.) Seed and Its Fats. Nuts and Seeds in Health and Disease Prevention 2011, 88, 741–748. DOI: 10.1016/B978-0-12-375688-6.10088-X
- Abdalla, A.E.M.; Darwish, S.M.; Ayad, E.H.E.; El-Hamahmy, R.M. Egyptian Mango by-Product 2: Antioxidant and Antimicrobial Activities of Extract and Oil from Mango Seed Kernel. Food Chemistry 2007, 103, 1141–1152.
- Torres-Leon, C.; Rojas, R.; Contreras-Esquivel, J.C.; Serna-Cock, L.; Belmares-Cerda, R.E.; Aguilar, C.N. Mango Seed: Functional and Nutritional Properties. Trends in Food Science & Technology 2016, 55, 109–117.
- Kittiphoom, S. Utilization of Mango Seed. International Food Research Journal 2012, 19, 1325–1335.
- Jahurul, M.H.A.; Zaidul, I.S.M.; Norulaini, N.A.N.; Sahena, F.; Abedin, M.Z.; Ghafoor, K.; Omar, A.M. Characterization of Crystallization and Melting Profiles of Blends of Mango Seed fat and Palm Oil Mid-fraction as Cocoa Butter Replacers using Differential Scanning Calorimetry and Pulse Nuclear Magnetic Resonance. Food Research International 2014, 55, 103–109.
- Jahurul, M.H.A.; Zaidul, I.S.M.; Norulaini, N.A.N.; Sahena, F.; Abedin, M.Z.; Mohamed, A.; Omar, A.M. Hard Cocoa Butter Replacers from Mango Seed Fat and Palm Stearin. Food Chemistry 2014, 154, 323–329.
- Sonwai, S.; Kaphueakngam, P.; Flood, A. Blending of Mango Kernel fat and Palm Oil Mid-fraction to Obtain Cocoa Butter Equivalent. Journal of Food Science and Technology 2014, 51, 2357–2369.
- Miura, S.; Konishi, H. Crystallization Behavior of 1,3-Dipalmitoyl-2-Oleoyl-Glycerol and 1-Palmitoyl-2, 3-Dioleoyl-Glycerol. European Journal of Lipid Science and Technology 2001, 103, 804–809.
- Ng, W.L. Nucleation Behaviour of Tripalmitin from a Triolein Solution. Journal of the American Oil Chemists’ Society 1989, 66, 1103–1106.
- Shahidi, F. Baley’s Industrial Oil and Fat Products, 6th edn; Wiley: New York, 2005; 1, 431–575; 2, 303–429; 3, 309–359.
- Ray, J.; Smith, K.W.; Bhaggan, K.; Nagy, Z.K.; Stapley, A.G. Crystallization and Polymorphic Behavior of Shea Stearin and the Effect of Removal of Polar Components. European Journal of Lipid Science and Technology 2013, 115, 1094–1106.
- Sue, T.T. Pocketbook of Palm Oil Uses, 6th edn; Malaysian Palm Oil Board: Malaysian, 2009; 19–32; 45–109.
- Kim, B.H.; Akoh, C.C. Recent Research Trends on the Enzymatic Synthesis of Structured Lipids. Journal of Food Science 2015, 80, C1713–C1724.
- Yella, R.S.; Jeyarani, T. Trans-free Bakery Shortenings from Mango Kernel and maHua Fats by Fractionation and Blending. Journal of the American Oil Chemists’ Society 2001, 78, 635–640.
- Baliga, B.P.; Shitole, A.D. Cocoa Butter Substitutes from Mango Fat. Journal of the American Oil Chemists’ Society 1981, 58, 110–114.
- Tran, P.D.; Van de Walle, D.; Hinneh, M.; Delbaere, C.; De Clercq, N.; Tran, D.N.; Dewettinck, K. Controlling the Stability of Chocolates Through the Incorporation of Soft and Hard StOSt-rich Fats. European Journal of Lipid Science and Technology 2015, 117, 1700–1713.
- Cao, J.; Li, H.; Xia, X.; Zou, X.G.; Li, J.; Zhu, X.M.; Deng, Z.Y. Effect of Fatty Acid and Tocopherol on Oxidative Stability of Vegetable Oils with Limited Air. International Journal of Food Properties 2015, 18, 808–820.
- Gunstone, F.D. Vegetable Oils in Food Technology Composition, Properties and Uses, 2nd edn; Wiley: New York, 2011; 25–65; 138–146.
- Finotti, E.; D’Ambrosio, M.; Paoletti, F.; Vivanti, V.; Quaglia, G. Synergistic Effects of α-Tocopherol, β-Sitosterol and Squalene on Antioxidant Activity Assayed by Crocin Bleaching Method. Food/Nahrung 2000, 44, 373–374.
- Ceci, L.N.; Carelli, A.A. Relation Between Oxidative Stability and Composition in Argentinian Olive Oils. Journal of the American Oil Chemists’ Society 2010, 87, 1189–1197.
- AOCS. Official Method and Recommended Practices of the American Oil Chemists’ Society, 5th edn; AOCS Press: Champaign, IL, 1998.
- Shi, C.; Chang, M.; Liu, R.J.; Jin, Q.Z; Wang, X.G. Trans-free Shortenings Through the Interesterification of Rice Bran Stearin, Fully Hydrogenated Soybean Oil and Coconut Oil. International Journal of Food Engineering 2015, 11, 467–477.
- Li, C.M.; Yao, Y.P.; Zhao, G.Z.; Cheng, W.; Liu, H.L.; Liu, C.Y., Shi, Z.; Chen, Y.; Wang, S. Comparison and Analysis of Fatty Acids, Sterols, and Tocopherols in Eight Vegetable Oils. Journal of Agricultural and Food Chemistry 2011, 59, 12493–12498.
- Jahurul, M.H.A.; Zaidul, I.S.M.; Ghafoor, K.; Al-Juhaimi, F.Y.; Nyam, K.L.; Norulaini, N.A.; Norulaini N.A.N.; Mohd Omar, A.K. Mango (Mangifera indica L.) by-Products and Their Valuable Components: A Review. Food Chemistry 2015, 183, 173–180.
- Willett, W.C.; Stampfer, M.J.; Manson, J.E.; Colditz, G.A.; Speizer, F.E.; Rosner, B.A.; Sampson, L.A.; Hennekens, G.H. Intake of trans Fatty Acids and Risk of Coronary Heart Disease Among Women. Lancet 1993, 341, 581–585.
- Kala, A.L.A. Cis-, trans- and Saturated Fatty Acids in Selected Hydrogenated and Refined Vegetable Oils in the Indian Market. Journal of the American Oil Chemists’ Society 2012, 89, 1813–1821
- Jiang, Y.R.; Xia, S.H.; Zhang, Y.Q.; Yu, J.G.; Hu, P. Trans Fatty Acid Content of Food in the Chinese Market. Journal of the Chinese Cereals and Oils Association 2013, 28, 112–115, 123. ( in Chinese)
- Farmani, J.; Safari, M.; Hamedi, M. Trans-free Fats Through Interesterification of Canola Oil/Palm Olein or Fully Hydrogenated Soybean Oil Blends. European Journal of Lipid Science and Technology 2009, 111, 1212–1220.
- Handa, C.; Goomer, S.; Siddhu, A. Performance and Fatty Acid Profiling of Interesterified trans, Free Bakery Shortening in Short Dough Biscuits. International Journal of Food Science & Technology 2010, 45, 1002–1008.
- Benincá, C.; Zanoelo, E.F.; de Júnior Lima Luz, L.F.; Spricigo, C.B. Trans Fatty Acids in Margarines Marketed in Brazil: Content, Labeling Regulations and Consumer Information. European Journal of Lipid Science and Technology 2009, 111, 451–458.
- Maheshwari, B.; Reddy, S.Y. Application of Kokum (Garcinia indica) Fat as Cocoa Butter Improver in Chocolate. Journal of the Science of Food & Agriculture 2005, 85, 135–140.
- Alim, M.A.; Wessman, P.; Dutta, P.C. Minor Components and Oxidative Stability as Determined by DSC of Fractionated and Lipase-catalyzed Structured Rapeseed Oil. Grasas y Aceites 2013, 64, 264–271.
- Sakina, K.; Gopala Krishna, A. G. Changes in Tocopherols, Tocotrienols and β-Carotene During Processing of Palm Oil Extracted from Indian Grown Palm Fruit. Journal of Lipid Science and Technology 2007, 39, 63–70.
- Kumar, P.K.P.; Krishna, A.G.G. Physico-chemical Characteristics and Nutraceutical Distribution of Crude Palm Oil and Its Fractions. Grasas y Aceites 2014, 65, e018. DOI: http://dx.doi.org/10.3989/gya.097413
- Ali, M.A.; Gafur, M.A.; Rahman, M.S.; Ahmed, G.M. Variations in Fat Content and Lipid Class Composition in Ten Different Mango Varieties. Journal of the American Oil Chemists’ Society 1985, 62, 520–523.
- Nagao, T.; Hirota, Y.; Watanabe, Y.; Kobayashi T.; Kishimoto. N.; Fujita. T.; Kitano, M.; Shimada, Y. Recovery of Sterols as Fatty Acid Steryl Esters from Waste Material after Purification of Tocopherols. Lipids 2004, 39, 789–794.
- Johansson, A.; Appelqvist, L.Ä. The Content and composition of Sterols and Sterol Esters in Low Erucicacid Rapeseed (Brassica napus). Lipids 1978, 13, 658–665.
- Carrasco-Pancorbo, A.; Cerretani, L.; Bendini, A.; Segura-Carretero, A.; Del Carlo, M.; Gallina-Toschi, T.; Lercker, G.; Compagnone, D.; Fernandez-Gutierrez, A. Evaluation of the Antioxidant Capacity of Individual Phenolic Compounds in Virgin Olive Oil. Journal of Agricultural and Food Chemistry 2005, 53, 8918–8925.
- Ayyildiz, H.F.; Topkafa, M.; Kara, H.; Sherazi, S.T.H. Evaluation of Fatty Acid Composition, Tocols Profile, and Oxidative Stability of Some Fully Refined Edible Oils. International Journal of Food Properties 2015, 18, 2064–2076.
- Kerrihard, A.L.; Nagy, K.; Craft, B.D.; Beggio, M.; Pegg, R.B. Oxidative Stability of Commodity Fats and Oils: Modeling Based on Fatty Acid Composition. Journal of the American Oil Chemists’ Society 2015, 92, 1153–1163.
- Jin, J.; Sheraliev, G.; Xie, D.; Zhang, W.; Jin, Q.; Wang, X. Characteristics of Specialty Natural Micronutrients in Certain Oilseeds and Oils: Plastochromanol-8, Resveratrol, 5-Hydroxytryptamine Phenylpropanoid Amides, Lanosterol, Ergosterol and Cyclolinopeptides. Journal of the American Oil Chemists’ Society 2016, 93, 155–170.
- Singh, A. Sitosterol as an Antioxidant in Frying Oils. Food chemistry 2013, 137, 62–67.
- Winkler, J.K.; Warner, K. The Effect of Phytosterol Concentration on Oxidative Stability and Thermal Polymerization of Heated Oils. European journal of lipid science and technology 2008, 110, 455–464.
- Hidalgo, F.J.; León, M.M.; Zamora, R. Effect of β-Sitosterol in the Antioxidative Activity of Oxidized Lipid-amine Reaction Products. Food Research International 2009, 42, 1215–1222.
- Gutfinger, T.; Letan, A. Quantitative Changes in Some Unsaponifiable Components of Soya Bean Oil Due to Refining. Journal of the Science Food and Agriculture, 1974, 25, 1143–1147.
- Kaimal, T.N.B.; Vali, S.R.; Rao, B.V.S.K.; Chakrabarti, P.P.; Vijayalakshmi, P.; Kale, V.; Rani, K.N.P.; Rajamma, O.; Bhaskar, P.S.; Rao, T.C. Origin of Problems Encountered in Rice Bran Oil Processing. European Journal of Lipid Science and Technology 2002, 104, 203–211.