Abstract
A water-soluble polysaccharide SJ-A presenting a relatively narrow molecular weight distribution was prepared by ethanol precipitation and purified from the pods of S. japonica. Chemical composition, molecular weight distribution, and glycosidic configurations of SJ-A were elucidated by sugar analysis, molecular determination, Fourier-transform infrared spectroscopy (FTIR), and nuclear magnetic resonance (NMR) analyses. The impacts of the concentration (0.5–2.0%) of SJ-A on the rheological parameters were investigated to clarify the molecular crosslinking features of SJ-A in relation to its rheological properties. The experimental results indicated that the microstructural coupling of the gel induced at higher concentrations of SJ-A required a higher frequency of the rheometer.
Introduction
Sophora japonica belongs to the family of Leguminosae and is widely cultivated in all parts of China to exploit the pharmacological properties of its organs such as the stem, leaves, flowers, fruits, and seeds. Indeed, these parts contain flavonoids, which have been previously investigated.[Citation1,Citation2] Besides the numerous bioactive substances present in the plant, S. japonica may be a potential source of polysaccharides. According to a previous study,[Citation3] galactomannan isolated from the endosperm of its seeds has been identified as the main energy storage component in legumes. Galactomannan is a heteropolysaccharide, which can form at relatively low concentrations (0.04–0.1/%, w/v) viscous solutions exhibiting a non-Newtonian behaviour.[Citation3,Citation4] Therefore, S. japonica is a very interesting alternative and renewable source for food colloids.
Notably, ripe S. japonica fruits (Chinese name, Huaijiao) flesh out a sticky colloidal substance different from the one secreted from the fruit named achene from other Sophora species. Interestingly, the content of the colloidal substance in the pods of the fruits is increased when the seed is stunted or totally damaged by insects. To the best of our knowledge, the exact composition of the colloidal substance is not yet known.
The aims of this study were to extract and characterize the colloidal compound contained in S. japonica pods. Isolation and purification proceeded via a hot water extraction, precipitation with ethanol, lyophilization, and chromatographic purification using DEAE-52 cellulose and Sephadex G-100. The isolated compound was characterized by high-performance gel-permeation chromatography (HPGPC), gas chromatography (GC), Fourier-transform infrared spectroscopy (FTIR), and nuclear magnetic resonance (NMR). Moreover, the rheological properties of the polysaccharide were evaluated for the potential application of the polysaccharide in foods.
Materials and methods
Materials
Ripe fruits of S. japonica were collected after a 5-month blooming of the trees from the Tianjin University of Science and Technology, China. The seeds were manually removed from the fruits as described by Bourbon et al.[Citation5] The deseeded fruits as pods were dried under sunlight and stored at room temperature for further use.
Isolation and purification of the polysaccharide
Unlike the common Leguminosae species, the pods of S. japonica exhibited a green or slightly yellow colour and were fleshed and beaded. The deseeded pods were covered with a green sticky substance, supposedly composed of polysaccharides or some viscous proteins. The seed could be divided into three parts: a seed coat presenting a smooth surface of black to brownish-black colour, a transparent endosperm slightly elastic, and a typical leguminous double green cotyledon. According to a published procedure, the pods (50 g) were heated in ethanol (80%, v/v) at a ratio of 1:4 (w/v, pods/ethanol) at 70°C for 30 min to remove ethanol-soluble substances, such as lipids and pigments, and to inactivate the enzymes.[Citation6] The ethanol was separated by filtration, followed by a washing with water to avoid the residual alcohol. The distilled water at a proportion of 1:1 (v/w) was added to the sample, which was then placed at 25°C for 12 h to allow the husks to expand. The polysaccharide was extracted three times with distilled water (10:1, w/v) at 60°C, under stirring (2 h each time). The suspension fractions were pooled and stored at 4°C for 12 h. The obtained viscous solution was filtered through a nylon mesh to remove the pods residues and then concentrated by evaporation of the water under vacuum. The polysaccharide was precipitated by ethanol (80%, v/v). After centrifugation, the precipitate was successively washed with ethanol (78%, v/v), acetone, and anhydrous ethanol, to obtain the crude polysaccharide free of low molecular weight impurity such as pigments but still containing proteins. The extract was named S. japonica SJ-Pr fraction.
The crude fraction was dialyzed and the proteins were removed as described by Sevag .[Citation7] The sample was then lyophilized before further analysis and named SJ. The SJ fraction was purified with an anion-exchange DEAE-52 column (Pharmacia, 2 cm, ID × 40 cm). The product was eluted stepwise with distilled water followed by a sodium chloride (NaCl) gradient (0–0.4 M) at a flow rate of 1.0 mL.min−1. The main fractions containing the polysaccharide eluted from the DEAE-52 column were pooled to form the SJ-1 fraction, and then dialyzed for 48 h against distilled water (cut-off Mw 7000 Da) and lyophilized. The obtained substrate (80 mg) was dissolved in deionized water (2.0 mL). After centrifugation, the supernatant was further purified on a Sephadex G-100 column (Pharmacia, 1 cm ID × 80 cm) using ultra-pure water as the eluting solvent.
The total polysaccharide content of each elution was detected by the phenol-sulphuric spectrophotometric method,[Citation8] using galactose as the standard sample. According to the obtained polysaccharide contents, the main fractions were pooled, concentrated, dialyzed for 48 h against distilled water (cut-off Mw 7000 Da), and the retentate was finally lyophilized to yield the sample named SJ-A.
Physicochemical characterization
The moisture content was determined by gravimetric analysis as follows. The sample (1 g) was heated at 105°C until reaching a constant weight. The total carbohydrate content was determined by the phenol-sulphuric acid method, using galactose as the standard.[Citation8] The protein content was estimated by a Bradford assay[Citation9] using BSA (Sigma-Aldrich, St Louis, MO, USA) as a standard. The uronic acid content was measured following the method described in the literature[Citation10] and the absorbance values were recorded at 520 nm. A standard curve of the absorbance of glucuronic versus its concentration was established to quantify the uronic acid content.
The monosaccharide composition was analysed on an Agilent 7890A GC system equipped with an OV-1701 column (30 m × 0.32 mm × 0.5 μm, Agilent Technologies Inc., USA) as described in the literature[Citation11] but with some modifications. Briefly, the polysaccharide (10 mg) was completely hydrolysed into monosaccharides with 0.5 mL of 2 M trifluoroacetic acid at 110°C for 5 h. The excess of acid was removed by three co-distillations with methanol under a nitrogen atmosphere. The released monosaccharides were then converted into alditol acetate by successive reduction with sodium boron hydride (NaBH4) and acetylation with a mixture containing acetic anhydride and anhydrous pyridine (1:1, v/v), and subsequently analysed by GC. The mobile phase was a nitrogen gas at a flow rate of 1 mL.min−1 and the column temperature was set to 190°C. The temperatures of the injector and detector were 250°C and 280°C, respectively. The standard monosaccharides glucose (Glc), galactose (Gal), mannose (Man), arabinose (Ara), xylose (Xyl), rhamnose (Rha), and ribose (Rib) were also subjected to the GC system as references. The internal standard was inositol (Sigma-Aldrich, St Louis, MO, USA).
Determination of the purity and molecular weight
The weight average molecular weight was determined by HPGPC carried out on a Waters 515 instrument (Waters Corp., Milford, MA, USA) fitted with a Waters 2410 refractive index detector and a TSK G4000-SWXL column (30 cm × 7.8 mm, 13 µm, Tosoh Bioscience Co., Kyoto, Japan). The mobile phase was ultrapure water at a flow rate of 0.8 mL min−1 and the sample concentration was 1 mg.mL−1. The HPGPC system was calibrated with T-series Dextran standards (Sigma-Aldrich, St Louis, MO, USA) having definite molecular masses ranging from 10 to 500 kDa. During the experiment, the column was kept at 30°C. The sample solution was prepared under the same conditions used in a previous study.[Citation12] All acquired data were recorded and analysed using the gel permeation chromatography software (GPC, version M32).
Infrared spectral analysis
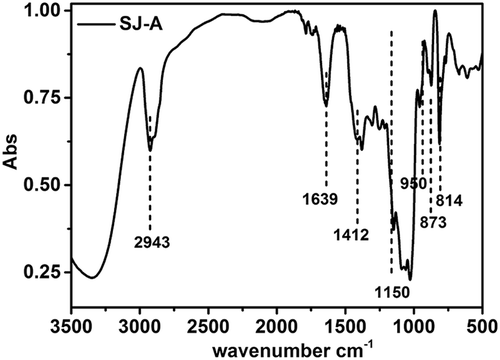
The FTIR spectrum of the SJ-A sample was recorded on a Shimadzu IR spectrophotometer (Model 8300, Kyoto, Japan) in the range of 4000–400 cm−1. Before detection, the samples were mixed to potassium bromide (KBr, spectroscopic grade) and pressed into a 1-mm pellet. Each FTIR spectrum was processed using the OMNIC software (version 8.2, Thermo Fisher Scientific Inc., Madison, WI, USA).
NMR spectroscopy analysis
The 1H and 13C nuclear magnetic resonance (1H NMR and 13C NMR) spectra of the SJ-A sample (40 mg.mL−1) dissolved in D2O were recorded at 25°C on a 400 MHz Bruker spectrometer (AVANCE III, Bruker, Germany) equipped with a 5-mm inverse probe. Chemical shifts were expressed in ppm (δ) using tetramethylsilane (TMS) as reference for the 1H and 13C signals.
The 13C NMR spectrum of the purified polysaccharide presenting a high molecular weight was better resolved after partial hydrolysis than that of the native polysaccharide. The polysaccharide was partially hydrolysed by dissolving the polysaccharide in D2O and sealing the NMR tube and then heating at 90°C for 12 h under intermittent agitation of the tube.
Rheological measurements
Samples at different concentrations (0.5–2.0% w/v) of the SJ-A polysaccharide in distilled water were prepared and left overnight at room temperature to obtain a fully dissolved system. The experiments were performed with a HAAKE MARS-III rheometer (Thermo Fisher Scientific Inc., Germany) equipped with stainless steel parallel plates (Φ=20 mm) and an intelligent control temperature system set at 25°C. A gap of 2 mm was kept during all the experiments and a serrated surface was applied to avoid slippage. The data were recorded and analysed using the HAAKE PolySoft software.
Steady-state viscosity measurements were performed by subjecting the solutions to linear sweep procedures at shear rates ranging from 0.1 to 100 s−1. The apparent viscosity at a given shear rate was calculated as the ratio of shear stress to shear rate.[Citation13] The dynamic rheology frequency sweep was run from 0.1 to 100 rad s−1 within the strain range of 1%. This range was selected after a preliminary strain sweep test performed over a frequency range of 0.01–10 Hz and at a constant temperature of 25°C to determine the linear viscoelastic region. The storage modulus (G′) and loss storage modulus (G″) values were recorded.[Citation14] The power-law correlation constants related to G′ and G″ and the angular frequency (rad s−1) for the SJ-A solution were determined using Eq. (1) and Eq. (2), respectively:
where k′ (Pa.sn) and k″ (Pa.sn) were intercepts, n′ and n″ were slopes of the frequency dependence of G′ and G″, respectively, and ω was the angular frequency (rad s−1).
Statistical analysis
The statistical analysis was performed by a one-way analysis of variance using the SPSS Statistics software (version 17.0, SPSS Inc., Chicago, IL, USA). Duncan’s multiple range tests were used to compare the mean values at a significance level of p<0.05.
Results and discussion
Isolation and purification
The extraction yield of the SJ polysaccharide was 19.7% (), which is lower than the galactomannan yield (>30%) obtained from the endosperm seeds.[Citation15] The value of the yield depends on the separation method and the source as described by Haddarah et al.[Citation16] and may explain why S. Japonica is not yet a well-known source of galactomannan.
Table 1. The composition and yield analysis of the polysaccharides from S. japonica pods.
According to the elution profile obtained from the DEAE-52 column (), the main fraction SJ-1 was eluted with distilled water, indicating that SJ-1 was primarily composed of a neutral polysaccharide. A further purification of SJ-1 on a Sephadex G-100 column yielded the polysaccharide SJ-A (fraction 2 in ), which displayed a symmetric peak as shown in Fig. 1c. According to the calibration curve, the weight-average (Mw) and number-average (Mn) molecular weights of SJ-A were estimated to be 1.5×105 Da and 1.4×105 Da, respectively. The polydispersity (Pd) was 1.07. The Mw value is less than the one determined for locust bean gum (galactomannan gum, 1.0×106 Da) but similar to the one of guar gum (galactomannan gum, 2.1×105 Da). These slight differences in weight may be related to the source, isolation, and purification methods.
Figure 1. The purification profile of polysaccharide isolated from S. japonica pods. (a) The elution curve of SJ on DEAE-52; (b) the elution curve of SJ-1 on Sephadex G-100; (c) the HPGPC result of SJ-A.
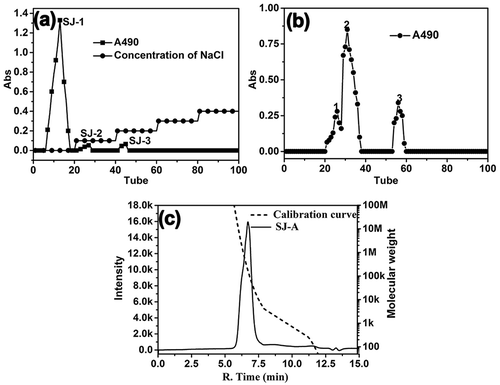
The lyophilized SJ-A polysaccharide exhibited no significant absorption at 280 nm or 260 nm as observed in the UV spectrum. These results suggested the absence of protein and nucleic acid inside the isolated fraction. The purified SJ-A appeared as a white powder, suggesting fewer pigment contents in contrast to the published work reporting a light yellow colour.[Citation17]
Main physicochemical composition analysis
shows the chemical composition of the two polysaccharides. The total carbohydrate content of the polysaccharide from the pods increased from 70.7% to 86.3% after removing proteins. Only 3.05% of uronic acid was detected for SJ-A, suggesting that it mainly consisted of a neutral polysaccharide. This result was different from the one obtained for the pectin extracted from the cell walls of fruits of C. japonica.[Citation18,Citation19] However, Gorbacheva et al. (1995) estimated that the substance consisted of mainly pectin (85%) and fewer amounts of a neutral polysaccharide (15%) from a roughly isolated sample, which was not further purified.[Citation20]
The GC analysis showed that mannose (60.7%) and galactose (35.1%) were the major monosaccharides present in the polysaccharide of S. japonica pods. Other monosaccharides, such as Rha, Ara, Xyl, and Glc, presented at a lesser extent in the sample. The value of the Man/Gal ratio has a fundamental effect on the physicochemical properties and health benefits of the polysaccharide, such as flexibility of chain conformation, synergistic interaction, polymeric solubility, rheological and emulsifying characteristics, and relief of chronic diseases.[Citation21] In our study, we have obtained a value of the Man/Gal ratio for S. japonica pods of 1.73. The value was lower than the ones for tara (2.5–3.0), C. pulcherrima (2.8), G. triacanthos (3.2), locust bean (3.5–4.0), and cress seed (8.2). However, the value was higher than the ones reported for fenugreek (1.0–1.1), A. pavonina (1.35), and mesquite seed gums (1.1–1.50).[Citation22] The obtained M/G ratio of 1.73 was relatively lower than that of a galactomannan isolated from Gleditsia triacanthos previously determined (M/G 5.75:1) by Cerqueira et al.[Citation23] According to the previous reports, the Galactopyranosyl (Galp) in galactomannan distributed on the Mannopyranosyl (Manp) main chain, and the Man/Gal ratio value depend on the degree of maturation of the pod, source, and extraction and purification procedures. The relatively lower M/G value indicated an enhanced substitution, which could reduce the flexibility and non-ordered entanglement of the molecular chains. In addition, the galactose content was 35%, and this could make the isolated fraction an interesting ingredient for commercial applications. Indeed, it has been recognized that galactose in galactomannan has an important role on the microstructure, gelation property, and aggregation behaviour of the gum.[Citation24,Citation25]
Infrared spectral analysis
As presented in , SJ-A exhibited a typical FTIR spectrum of polysaccharides.[Citation22] The absorptions in the wavenumber range of 950–1150 cm−1 were attributed to the bending vibrations of the C–O, C–O–C glycosidic, and C–O–H bonds of the pyranose ring. The bands at 814 cm−1 and 873 cm−1 have been observed in β-linked D-Manp units and α-linked D-Galp units, respectively. The intensity of the peaks varied between two absorption bands, confirming that the contents of Man and Gal were different. The small peaks observed around 1700 cm−1 on the FTIR spectrum suggested the slight content of uronic acid in SJ-A. This observation was consistent with the aforesaid result that SJ-A mainly consisted of a neutral polysaccharide.
NMR spectroscopy analysis
According to previously reported structural investigations, SJ-A was initially considered as a galactomannan.[Citation26] The preliminary structure of seed galactomannan consisted of D-Galp distributed randomly on the D-Manp backbone. The fine structure of the purified polysaccharide was characterized by NMR spectroscopy. The 1H and 13C NMR spectra are shown in and , respectively. Three well-resolved signals were observable in the typical regions of the anomeric groups in the 13C (δ90–110 ppm) and the 1H (δ=4.5–5.5 ppm) NMR spectra. We could assign the C-1/H-1 of the substituted and non-substituted β-D-Manp (peaks C and D, δ13C=96.43 ppm, δ1H=4.64 ppm, and δ13C=95.88 ppm, δ1H=4.57 ppm), and of the α-D-galp (peaks A, δ13C=92.26 ppm, δ1H=5.18 ppm) of the SJ-A sample. The specific chemical shifts of these signals were different from the ones reported by published studies.[Citation27] Indeed, the spectra presented in this study were obtained from the partially hydrolysed polysaccharide to avoid ambiguous superposition of the signals. In addition, the various substitution patterns, namely the D-Galp residues distribution on the D-Manp backbone, could explain the difference in the recorded chemical shifts. Therefore, the fine structural characterization of SJ-A, related to the distribution of the Galp residues, deserves further investigation.
Figure 3. NMR spectrum of the SJ-A in D2O at 25°C. (a) 1H NMR spectrum; (b) 13C NMR spectrum. A, B, C, and D represented α-D-galp, solvent, substituted, and non-substituted β-D-Manp. E represented the signals 1H signal of C2-C6 for the monosaccharide.
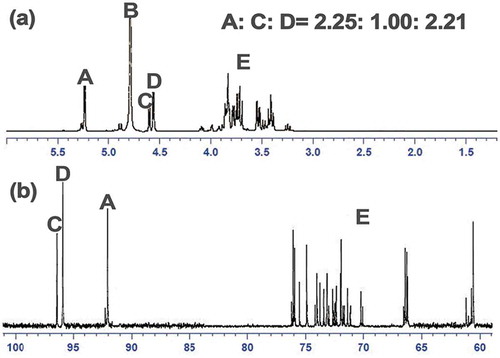
The value of the Man/Gal ratio calculated from the integrations of the relative peak areas from the 1H NMR signals of the anomeric protons of the mannose and galactose units (δ1H=1.43 ppm, , signals (C+D)/A) confirmed the high substitution rate, but the value was lower than that obtained by GC analysis (Man/Gal=1.73). This discrepancy may indicate that the hydrolysis performed prior to the NMR experiment was not complete. The signals appearing in the region of 3.3–4.1 ppm in the 1H NMR spectrum were attributed to the protons at the C2–C6 sugar carbons, which were also observable in the 13C NMR spectrum between 60 and 80 ppm.
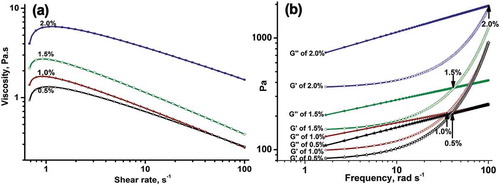
Rheological behaviour
The rheological behaviour of SJ-A aqueous solutions was studied and distinct viscoelastic profiles were observed among the different concentrations of SJ-A (). The results of the frequency sweep tests suggested that the concentration clearly affected the rheological properties of SJ-A gum. As shown in , the apparent viscosity of all the SJ-A aqueous solutions exhibited a shear-thinning behaviour of typical non-Newtonian fluids (fitted by the Cross model) at 0.5 and at 2.0% (w/v) concentration.[Citation28,Citation29] As presented in , when the concentration of SJ-A increased, the apparent viscosity magnification in the range of 0.01 to 1 s−1 changed from 40.41% times for a hydrocolloid concentration of 0.5% to 24.03% times when the concentration was 1.0%, and then continued to increase 50.96% times when the concentration reached 2.0%, indicating an increase on the chain entanglements upon being shear-induced upon the increase of the SJ-A concentration and of the yield point at a concentration of 1.0%. Then, the apparent viscosity of SJ-A decreased as the shear rate increased at the high ranges of 1–10 s−1 and 10–100 s−1. The 2% w/v SJ-A solution showed the least shear dependency and the least decreasing ratio of apparent viscosity confirmed more important chain entanglements (40.91% and 55.81% for 1–10 s−1 and 10–100 s−1, respectively).[Citation30] This behaviour was similar to the ones reported for other galactomannan or seed gums and could be attributed to the changes in the network upon shear-induced chain entanglements.[Citation3,Citation31] When the concentration increased, much more junction zones were present for intermolecular interactions due to the reduced distance between molecules. Therefore, more crosslinking networks or enhanced bond strength were formed, leading to an efficient resistance towards mechanical deformation.[Citation14]
Table 2. The rheological and the model parameters at different concentrations of the polysaccharides from S. japonica pods.
The enhanced storage modulus G′ and loss modulus G″ for different polysaccharide concentrations ranging from 0.5% to 2.0% w/v were observed when increasing the angular frequency (rad s−1, 25°C, 1% strain), as depicted in . At low frequency, G′ was lower than G″ (liquid-like behaviour); as the frequency increased, the value of G′ clearly increased and the difference between G′ and G″ values diminished or the G′ value overtook the G″ value (solid-like behaviour).[Citation32] Therefore, the SJ-A fraction produced a viscoelastic structure (a weak gel) induced by the higher frequency at 25°C. The sample containing 2% w/v of the hydrocolloid exhibited the highest values of G′ and G″, reflecting that it yielded higher structural strength due to more chains entanglements or macromolecules connections. The 1.0% w/v SJ-A solution exhibited a typical behaviour of entangled polymer solutions, with the G′ value dominating over the G″ one in the high-frequency range. A crossover point at 35 Hz appeared at a relative lower frequency than that of the crossover frequency of 40 Hz of the 0.5% w/v SJ-A solution. The results corroborated with the ones obtained from steady shear measurements (shear dependency of the apparent viscosity shown in ), and as confirmed by the reported results for other galactomannan gums.[Citation33] Indeed, this behaviour has been attributed to the dependency of the molecular crosslinking on concentration. The frequency value at the transition point increased when further polysaccharide was added at proportions of 1.0–2.0% w/v. These results could be explained by the fact that the polysaccharide started to exhibit the behaviour of an entangled polymer at 1.0% w/v, but the microstructural coupling of the gel at higher concentrations of SJ-A required a higher frequency. The high apparent viscosity of the 2.0% w/v SJ-A samples suggested less flexibility and hence more expanded coil geometries than the 1.0% w/v SJ-A samples. The origin of the restricted flexibility chains could be due to the steric crowding and other local interactions as the polysaccharide concentration increased.[Citation34] In all samples, the G′ and G″ values exhibited less dependency and decreased as the concentration of SJ-A increased, as indicated by a decreasing value of the power law index (n′ and n″) at the frequency range of 0.1–100 rad s−1. This behaviour supported the elastic gel property of the 2% w/v samples.[Citation34] On the other hand, when the magnitudes of k′ and k″ increased together with the SJ-A concentration, the augmented effects were more pronounced on the k″ value. The results could be described as the ‘‘weak gel’’ characteristics of a polysaccharide gel formed of overlapped and entangled flexible random coil chains.[Citation30] From these results, the existence of two or more relaxations mechanisms of galactomannan gums such as repetition or the breakup of physical bonds could be suggested. The first model based on strong and weak associations would depend on the amount of accessible backbone units, whereas the second model could be promoted by star-like structures, caused by strong physical bonds. Both possibilities could explain the microstructural coupling of the gel observed at the higher concentrations of SJ-A requiring a higher frequency of the rheometer.[Citation33]
Conclusion
The relaxation mechanisms of galactomannan gums, which were closely associated with internal molecular structural features and concentration of the hydrocolloid, could play a very important role in moderating the microstructural couplings of the entanglement network through different interactions between the SJ-A fractions and consequently affect their rheological properties. The present paper confirmed that the gum obtained from the pods of S. japonica was a galactomannan with a weight-average molecular weight of 1.5×105 Da and a medium galactose content (Man/Gal = 1.73). Monosaccharide analysis, and FTIR and NMR characterizations, revealed that the polysaccharide in S. japonica pods consisted of β-D-mannose and α-D-galactose. The gum showed shear thinning behaviour and formed a viscoelastic gel at various concentrations. The experimental results also indicated that the microstructural coupling of the gel induced at high concentrations of SJ-A required a higher frequency of the rheometer. The study provided a better understanding of the chemical and rheological characteristics of the polysaccharide, which could promote the potential use of this galactomannan resource in foods, cosmetics, or pharmaceutical formulations.
Funding
This work was financially supported by the Special Funds for Agro-scientific Research in the Public Interest (No. 201303082), National Natural Science Foundation of China (No. 31000755), and the Ph.D. Training Foundation of the Tianjin University of Science and Technology (No. 2016002).
Additional information
Funding
References
- Serial, M.R.; Blanco Canalis, M.S.; Carpinella, M.; Valentinuzzi, M.C.; León, A.E.; Ribotta, P.D.; Acosta, R.H. Influence of the Incorporation of Fibers in Biscuit Dough on Proton Mobility Characterized by Time Domain NMR. Food Chemistry 2016, 192, 950–957.
- Wang, R.; Lei, F.; Ding, Y.; Xing, D.; Wang, L.; Du, L. Manufacturing Process and Quality Control of Total Flavonoid from Chrysanthemum Morifolium and Sophora Japonica and its Quality Control. Zhongguo Zhong Yao Za Zhi. Zhongguo Zhongyao Zazhi. China Journal of Chinese Materia Medica 2010, 35, 2980–2984.
- Busch, V.M.; Kolender, A.A.; Santagapita, P.R.; Buera, M.P. Vinal Gum, A Galactomannan from Prosopis Ruscifolia Seeds: Physicochemical Characterization. Food Hydrocolloids 2015, 51, 495–502.
- Richardson, P.H.; Willmer, J.; Foster, T.J. Dilute Solution Properties of Guar and Locust Bean Gum in Sucrose Solutions. Food Hydrocolloids 1998, 12, 339–348.
- Bourbon, A.I.; Pinheiro, A.C.; Ribeiro, C.; Miranda, C.; Maia, J.M.; Teixeira, J.A.; Vicente, A.A. Characterization of Galactomannans Extracted from Seeds of Gleditsia Triacanthos and Sophora Japonica through Shear and Extensional Rheology: Comparison with Guar Gum and Locust Bean Gum. Food Hydrocolloids 2010, 24, 184–192.
- Egorov, A.V.; Mestechkina, N.M.; Shcherbukhin, V.D., Determination of the Primary and Fine Structures of a Galactomannan from the Seed of Gleditsia triacanthos f. inermis L. Applied Biochemistry and Microbiology 2003, 39, 398–402.
- Sevag, M.G., Lackman, D.B., Smolens, J. The Isolation of Components of Streptococcal Nucleoproteins in Serologically Active Form. Journal of Biological Chemistry 1938, 425–436.
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F., Colorimetric Method for Determination of Sugars and Related Substances. Analytical Chemistry 1956, 28, 350–356.
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-dye Binding. Analytical Biochemistry 1976, 72, 248–254.
- Blumenkrantz, N.; Asboe-Hansen, G., New Method for Quantitative Determination of Uronic Acids. Analytical Biochemistry 1973, 54, 484–489.
- Villanueva-Suárez, M.J.; Redondo-Cuenca, A.; Rodríguez-Sevilla, M.D.; de las Heras Martínez, M. Characterization of Nonstarch Polysaccharides Content from Different Edible Organs of Some Vegetables, Determined by GC and HPLC: Comparative Study. Journal of Agricultural and Food Chemistry 2003, 51, 5950–5955.
- Shobha, M.S.; Kumar, A.B.V.; Tharanathan, R.N.; Koka, R.; Gaonkar, A.K. Modification of Guar Galactomannan with the Aid of Aspergillus Niger Pectinase. Carbohydrate Polymers 2005, 62, 267–273.
- Sun, A.; Gunasekaran, S., Yield Stress in Foods: Measurements and Applications. International Journal of Food Properties 2009, 12, 70–101.
- Cengiz, E.; Karaman, S.; Dogan, M., Rheological Characterization of Binary Combination of Gleditsia Triacanthos Gum and Tapioca Starch. International Journal of Food Properties 2016, 19, 1391–1400.
- Nwokocha, L.M.; Williams, P.A. Isolation and Rheological Characterization of Mucuna Flagellipes Seed Gum. Food Hydrocolloids 2009, 23, 1394–1397.
- Haddarah, A.; Bassal, A.; Ismail, A.; Gaiani, C.; Ioannou, I.; Charbonnel, C.; Hamieh, T.; Ghoul, M., The Structural Characteristics and Rheological Properties of Lebanese Locust Bean Gum. Journal of Food Engineering 2014, 120, 204–214.
- Ayaz, F.A.; Torun, H.; Ayaz, S.; Correia, P.J.; Alaiz, M.; Sanz, C.; Gruz, J.; Strnad, M. Determination of Chemical Composition of Anatolian Carob Pod (Ceratonia siliqua L.): Sugars, Amino and Organic Acids, Minerals and Phenolic Compounds. Journal of Food Quality 2007, 30, 1040–1055.
- Eige, Y.; Ishii, T., Pectic Polysaccharides from Xylem-differentiating Zone of Cryptomeria Japonica. Phytochemistry 1996, 42, 611–616.
- Thomas, M.; Guillemin, F.; Guillon, F.; Thibault, J.F. Pectins in the Fruits of Japanese Quince (Chaenomeles japonica). Carbohydrate Polymers 2003, 53, 361–372.
- Gorbacheva, L.A.; Grishkovets, V.I.; Drozd, G.A.; Chirva, V.Y.A. Isolation and Characterization of the Polysaccharide of Sophora. Chemistry of Natural Compounds 1995, 31, 626–627.
- López-Franco, Y.L.; Cervantes-Montaño, C.I.; Martínez-Robinson, K.G.; Lizardi-Mendoza, J.; Robles-Ozuna, L.E. Physicochemical Characterization and Functional Properties of Galactomannans from Mesquite Seeds (Prosopis spp.). Food Hydrocolloids 2013, 30, 656–660.
- Razavi, S.M.A.; Cui, S.W.; Guo, Q.; Ding, H., Some Physicochemical Properties of Sage (Salvia macrosiphon) Seed Gum. Food Hydrocolloids 2014, 35, 453–462.
- Cerqueira, M.A.; Lima, Á.M.; Souza, B.W.S.; Teixeira, J.A.; Moreira, R.A.; Vicente, A.A. Functional Polysaccharides as Edible Coatings for Cheese. Journal of Agricultural and Food Chemistry 2009, 57, 1456–1462.
- Monteiro, S.R.; Rebelo, S.; da Cruz e Silva, O.A.B.; Lopes-da-Silva, J.A. The Influence of Galactomannans with Different Amount of Galactose Side Chains on the Gelation of soy Proteins at Neutral pH. Food Hydrocolloids 2013, 33, 349–360.
- Jiang, J.X.; Jian, H.L.; Cristhian, C.; Zhang, W.M.; Sun, R.C. Structural and Thermal Characterization of Galactomannans from Genus Gleditsia Seeds as Potential Food Gum Substitutes. Journal of the Science of Food and Agriculture 2011, 91, 732–737.
- Guo, R.; Cao, N.N.; Wu, Y.; Wu, J.H. Optimized Extraction and Molecular Characterization of Polysaccharides from Sophora Alopecuroides L. Seeds. International Journal of Biological Macromolecules 2016, 82, 231–242.
- Guo, R.; Ai, L.; Cao, N.; Ma, J.; Wu, Y.; Wu, J.; Sun, X., Physicochemical Properties and Structural Characterization of a Galactomannan from Sophora Alopecuroides L. Seeds. Carbohydrate Polymers 2016, 140, 451–460.
- Cross, M.M. Rheology of non-Newtonian Fluids: A New Flow Equation for Pseudoplastic Systems. Journal of Colloid Science 1965, 20, 417–437.
- Anvari, M.; Tabarsa, M.; Cao, R.A.; You, S.G.; Joyner, H.S.; Behnam, S.; Rezaei, M., Compositional Characterization and Rheological Properties of An Anionic Gum from Alyssum Homolocarpum Seeds. Food Hydrocolloids 2016, 52, 766–773.
- Razavi, S.M.A.; Alghooneh, A.; Behrouzian, F.; Cui, S.W. Investigation of the Interaction Between Sage seed Gum and Guar Gum: Steady and Dynamic Shear Rheology. Food Hydrocolloids 2016, 60, 67–76.
- Nwokocha, L.M.; Williams, P.A. Rheological Characterization of the Galactomannan from Leucaena Leucocephala Seed. Carbohydrate Polymers 2012, 90, 833–838.
- Xu, C.C.; Wang, L.P.; Shao, L.Y.; Yu, C.; Yu, H.N.; Li, Y.F. Effect of Freezing/Thawing Temperature on the Viscoelastic and Nutritional Qualities of Carrots. International Journal of Food Properties 2016, 19, 1413–1424.
- Wientjes, R.H.W.; Duits, M.H.G.; Jongschaap, R.J.J.; Mellema, J., Linear Rheology of Guar Gum Solutions. Macromolecules 2000, 33, 9594–9605.
- Razavi, S.M.A.; Moghaddam, T.M.; Emadzadeh, B.; Salehi, F., Dilute Solution Properties of Wild Sage (Salvia macrosiphon) Seed Gum. Food Hydrocolloids 2012, 29, 205–210.