ABSTRACT
Ultra-performance liquid chromatography coupled with electrospray ionization tandem quadrupole time-of-flight mass spectrometry (UHPLC−Q-TOF-MS) and HPLC-DAD with an ultrasonic extraction method was developed for qualitative and quantitative compositions and contents in black soybean sprouts. Operational conditions of ultrasonic extraction were optimized by single-factor experiment. Phytochemical compositions of black soybean sprouts were identified by UHPLC−Q-TOF-MS in positive ion mode. A total of 23 compounds were tentatively characterized and identified by means of accurate mass and characteristic fragment ions. Among of them, the six isoflavones in different germinated black soybean sprouts were further quantified by HPLC-DAD. The results indicated that the calibration curves showed good linearity (r2 > 0.9994). The intra-day and inter-day precision variations were less than 1.65% and 1.73%, respectively. The recoveries were in the range of 94.95–106.45%. The results indicated that the maximum amounts of isoflavones were produced on the fourth to fifth day germinated black soybean sprouts.
Introduction
Black soybean (Glycine max L. Merrill) belongs to the Leguminosae family. Black soybean is a black hulled soybean with seed coats. It has been used as a health food and traditional medicine in Asian countries. Several pharmacological studies have demonstrated that black soybeans have antioxidant activity against 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical, low density lipoprotein (LDL) oxidation, and ferric reducing antioxidant power (FRAP).[Citation1–Citation3] Black soybean extract may promote wound healing and prevent inflammation.[Citation4–Citation6] In addition, black soybeans show anti-diabetic activity involving the suppression of hepatic endoplasmic reticulum stress, restoration of insulin resistance, and the induction of differentiation of pre-adipocytes into smaller and insulin-sensitive adipocytes.[Citation7–Citation10] The literatures reported that black soybean anthocyanins had neuroprotective effects.[Citation11,Citation12] Recently, germinated black soybean sprouts are popularly consumed as a nutritional legume in many parts of the world, due to its high level of isoflavones content and nutritive values. These isoflavones are associated with biological activities such as antioxidant[Citation13] and anti-inflammatory properties.[Citation14] In addition, isoflavones are well-known phytoestrogen glycosides. The dietary intake of black soybean foods has been associated with the prevention of cardiovascular disease and the improvement of blood circulation.[Citation15]
Previous phytochemical studies reveal that black soybean contains various components such as isoflavone aglycones (daidzein, genistein, and glycitein), glucoside conjugated forms (daidzin, genistin, and glycitin), their acetyl glucoside derivatives (6’’-O-acetyldaidzin, 6’’-O-acetylgenistin and 6’’-O-acetylglycitin), and malonyl glucosides (6’’-O-malonyldaidzin, 6’’-O-malonylgenistin and 6’’-O-malonylglycitin), anthocyanins including cyanidin-3-O-glucoside, cyanidin-3-O-galactoside, delphinidin-3-O-glucoside, delphinidin-3-O-galactoside, petunidin-3-O-glucoside, peonidin-3-O-glucoside, pelargonidin-3-O-glucoside, cyaniding, and catechin-cyanidin-3-O-glucoside, proanthocyanidins consisting of procyanidin B2, procyanidin C1, and cinnamtannin A2.[Citation16–Citation19]
Germination is a complex metabolic process, which can promote the nutritional value of legumes by increasing protein digestibility and amino acids.[Citation20,Citation21] Germination can significantly enhance the antioxidant and ACE inhibitory activities of legumes grains (chickpea, red lentil, mung bean, kidney bean, and soybean).[Citation22] In particular, germination contributed to increase the amount of isoflavone aglycones, B soyasaponins, phytosterols, inositol metabolites, antioxidants, and amino acids in soybeans.[Citation23] Lopez-Amoros reported that the germination process of beans, peas, and lentils modified their phenolic composition. Peas and beans undergo a significant increase in antioxidant activity after germination, whereas lentils show a decrease.[Citation24] Furthermore, the contents of isoflavones varied with different germination times in black soybean sprouts. However, to date, there have been few reports on the identification and determination for the changes of isoflavone contents during the germination of black soybean sprouts.
The purposes of this study were designed to identify the representative isoflavone aglycones and glycosides in black soybean sprout by UHPLC−Q-TOF-MS using accurate mass weight and characteristic fragment ions, and to determine the optimal germination time for the maximum production of isoflavone content during the germination process by HPLC-DAD.
Experimental
Chemicals and materials
Black soybeans were purchased from RT-MART (Rizhao, China). Daidzin, genistin, glycitin, daidzein, genistein, and glycitein (purity≥98.0%) were purchased from Spring & Autumn Biological Engineering Co., Ltd. (Nanjing, China). HPLC analytical-grade acetonitrile was purchased from Honeywell, Burdick & Jackson. LC/MS-grade acetonitrile was purchased from Mallinckrodt Baker, Inc. (Phillipsburg, NJ, USA). HPLC-grade acetic acid was purchased from TEDIA, Inc (Fairfield, USA). Deionized water was purified by Millipore purification system (Millipore, MA, USA).
Black soybean sprout cultivation
Black soybean seeds were soaked in 70% ethanol solution for 15 min for disinfection. After washing out the ethanol from the seeds with distilled water, dry black soybean seeds were soaked for 8 h in distilled water. Then black soybean seeds were cultivated for 9 days. They were located in trays at room temperature (at 25°C) and 90% relative humidity. For the first and second days, the trays were covered with cotton gauzes in the dark. For the other days, the cotton gauzes were removed with natural light. Soybean sprouts were harvested on different days. For each germination time, soybean sprouts were randomly collected as shown in .
Sample preparation
The fresh black soybean sprouts were cut into small pieces. Black soybean sprout samples of about 1 g were weighed into a 50 mL centrifuge tube with 15 mL 70% methanol in water (solid-liquid ratio 1:15). The detailed data are shown in the online supplementary information (Table S1). It was sonicated at 80 kHz for 15 min in a water bath (60°C). The extract was centrifuged at 10,000 rpm for 10 min. The supernatant was filtered with a 0.22 μm filter and then analysed by UHPLC−Q-TOF-MS and HPLC-DAD.
Analysis of isoflavones by UHPLC−Q-TOF-MS analysis
Agilent 1290 series Rapid Resolution LC system was coupled to Agilent 6530 Accurate-Mass quadrupole time of flight (Q-TOF) mass spectrometer (Agilent Technologies, CA, USA) equipped with an electrospray ionization (ESI) interface. The chromatographic separation was performed on a TOSOH TSK gel ODS-100 V (4.6 × 150 mm, 3.0 µm) column (Tosoh Bioscience, Japan). The column temperature was maintained at 35°C. The injection volume was 5 μL. The mobile phase consisted of 0.2% acetic acid (v/v) (A) and acetonitrile (B). The gradient program was applied as follows: 0–20 min at 10–40% B; 20–25 min at 40–50% B; 25–45 min at 50–95% B; 45–48 min at 95% B; 48–51 min at 95–10%; and 51–60 min at 10%. Elution was set to a flow rate of 0.8 mL/min. The outlet of UHPLC was split (1:4) and introduced into the ESI source.
The MS conditions were set as follows: drying gas temperature 350°C; drying gas flow rate 10 L/min; pressure of nebulizer gas pressure 45 psig; sheath gas temperature 350°C; sheath gas flow rate 11 L/min; capillary voltage 4000 V; nozzle voltage 500 V in positive ion modes; and the mass range from m/z 100 to 1200 Da. The MS/MS spectra were acquired with auto MS/MS mode at the acquisition rate of 2 spectra/s and 500 ms/spectrum. The isolation width MS/MS was set as narrow (~1.3 amu). The reference masses were selected as follows: m/z 118.0861, m/z 194.1170, m/z 121.0508, and m/z 922.0098. The data were acquired with Mass Hunter Acquisition B.06.00 and analysed by means of Mass Hunter Qualitative Software, Version B.06.00 (Agilent Technologies, CA, USA).
Quantification and validation for the isoflavones by HPLC−DAD
The contents of six isoflavones in black soybean sprouts were determined by HPLC-DAD. The experiments were performed using a Shimadzu liquid chromatography system. The instrument was configured with two LC-20AT pumps, a SIL-20A auto sampler, a CTO-20AC column oven, an SPD-M20A diode array detector, a CBM-20A communications bus module, and a DGU-20A3 degasser. LC solution software version 1.24 SP1 was used to control the HPLC system and process the data.
The chromatographic separation was performed on TOSOH TSK gel ODS-100Z (4.6 × 150 mm, 3 µm) column (Tosoh Bioscience, Japan). The column temperature was maintained at 35°C, the injection volume was 5 μL, and UV absorbance was detected at a wavelength of 254 nm. The mobile phase consisted of 0.2% aqueous acetic acid (v/v) (A) and acetonitrile (B). The gradient program was applied as follows: 0–15 min at 4–24% B; 15–28 min at 24–36% B; 28–35 min at 36–95% B; 35–37 min at 95–4% B; and 37–45 min at 4% B. Elution was set to a flow rate of 1.0 mL/min.
Standard solutions and method validation
Six reference isoflavones were accurately weighed and dissolved in dimethylsulfoxide (DMSO, Sigma-Aldrich, USA). The mixed stock solutions (daidzin, genistin, glycitin, daidzein, genistein, and glycitein) were prepared at the concentration of 1000 μg/mL. The mixed solution was diluted with 80% aqueous ethanol to obtain a series of standard working solutions at eight different concentrations (0.5, 1, 5, 10, 25, 50, 100, and 250 μg/mL), which were used to establish the calibration curves (1–250 μg/mL). All the mixed stock solutions and standard working solutions were stored in the refrigerator at 4°C.
The standard working solutions at concentrations of 5, 50, and 100 μg/mL were used for the intra-day and inter-day precision experiments. The black soybean sprouts (germination of 9 days) used in the repeatability and stability experiments were prepared, according to the procedure in the section for sample preparation. The accurate qualification has been examined according to International Council for Harmonisation guidelines Q2 (R1). Accuracy may be assessed on samples spiked with known amounts of the analyte. A recovery test was carried out with low, medium, and high concentrations of the six reference substances spiked into the black soybean sprouts (germination of 9 days). The spiked samples were pretreated, according to the procedure in the section for sample preparation.
Results and discussion
Optimization of ultrasonic-assisted extraction method
The ultrasonic extraction method has been widely used to extract the active compounds from the plants. The efficiency of ultrasonic extraction is influenced by many conditions. To optimize the ultrasonic extraction method such as ultrasonic temperature, ultrasonic time, solid-–liquid ratio, and ultrasonic frequency were investigated using a single-factor test. Ultrasonic temperature was assessed at 30°C, 40°C, 50°C, 60°C, 70°C, and 80°C, while other extraction conditions were given as follows: ultrasonic time of 20 min, solvent to raw material ratio of 1:20 (g/mL), and ultrasonic frequency of 80 KHz. As shown in the online SI (Fig. S1), the peaks area of isoflavone aglycones (daidzein, genistein, and glycitein) and glucoside conjugated forms (daidzin, genistin, and glycitin) increased with the increasing ultrasonic temperature and reached the maximum value when ultrasonic temperature was at 60°C. When the ultrasonic temperature was over 70°C, the six peaks area decreased slightly. Therefore, 60°C was chosen as the optimum temperature. Ultrasonic time was carried out with different times including 1, 5, 10, 15, and 30 min, respectively. The results indicated that the extraction of six isoflavones did not require longer time; a short time such as 15 min is enough as shown in Fig. S2. Furthermore, different solid–liquid ratios including 1:10, 1:15, 1:20, 1:30, and 1:40 (g/mL) were investigated in parallel experiments. It was found that the optimum ratio of 1:15 (g/mL) was the most efficient extraction, as shown in Fig. S3. In addition, the available ranges of ultrasonic frequency (50, 60, 70, 80, 90, and 100 KHz) were tested. Figure S4 shows that the extraction yield increased when the ultrasonic frequency increased from 50 to 80 KHz, and then the yield slightly decreased from 80 to 100 KHz. Thus, the optimum ultrasonic frequency was chosen as 80 KHz.
Analysis of chemical components from black soybean sprouts by UHPLC−Q-TOF-MS
The UHPLC−Q-TOF-MS is capable of providing the retention time, high resolution mass, second-stage mass fragment ions, and UV-Vis absorption data for the identification. The elemental composition was calculated by the high-resolution mass within ±5 ppm mass error. The characteristic fragment ions of the reference compounds are obtained and summarized. For the unknown compounds, the molecular formula was calculated by Mass Hunter Qualitative Software. The summarized fragmentation patterns of known compounds were utilized to propose the unknown compounds. In addition, the identification information was confirmed by comparison to the literatures.
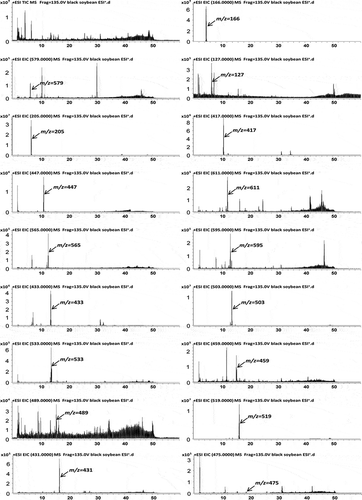
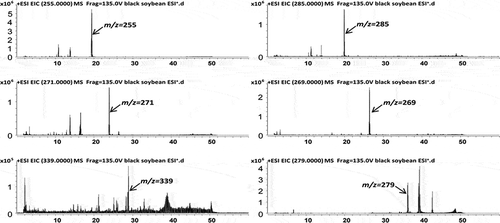
The compounds in 9-day black soybean sprouts were characterized by UHPLC−Q-TOF-MS in the positive mode. A total ion chromatogram and extract ion chromatograms are shown in . The high-resolution mass spectra and second-stage mass spectra are shown in Fig. S5. In the black soybean sprouts the major isoflavone glucosides consisted of daidzin (peak 5), glycitin (peak 6), and genistin (peak 10), which produced characteristic fragment ion at m/z 255, 285, and 271 corresponding to the characteristic loss of a glucosyl group. The three aglycone forms corresponded to daidzein (peak 18), glycitein (peak 19), and genistein (peak 20). In the MS2 spectrum, the aglycone fragment ions were produced from the fragment ions [M+H−CO2]+, [M+H−H2O]+, [M+H−CH3]+, and the retro-Diels-Alder fragment ions. Peak 5, 6, 10, 18, 19, and 20 were identified by comparison with reference compounds. In addition, the black soybean sprouts contained the acetyl glucosides and malonyl glucosides, which provided the fragment ions [M+H−malonylglucose]+ and [M+H−acetylglucose]+, respectively. Peaks 11, 12, 13, 14, 15, and 17 were, respectively, identified as malonyl daidzin, malonyl glycitin, acetyl daidzin, acetyl glycitin, malonyl genistin, and acetyl genistin by the characteristic fragment patterns of the reference compounds. These compounds are summarized along with their retention time, theoretical mass, molecular formula, observed mass, and MS/MS fragments as shown in . The method was validated for the following parameters: linearity, precision, repeatability, stability, and recovery according to International Council for Harmonisation guidelines.[Citation25]
Table 1. Identification of the chemical constituents from black soybean sprouts by UHPLC−Q-TOF-MS.
Calibration curves, limit of detection, and limit of quantification
Standard solutions containing eight different concentrations were analysed for calibration. The calibration curves were constructed by plotting the corresponding peak areas (Y) versus the concentrations (X, mg/mL). The standard solutions were diluted and detected until the signal-to-noise (S/N) ratio corresponded to 3 and 10, defined as the limit of detection (LOD) and limit of quantification (LOQ). The calibration curves, correlation coefficients (r2), linear ranges, LOD, and LOQ are presented in ; all analytes showed good linearity with r2 greater than 0.9994. The LOD and LOQ were 0.01 and 0.05 μg/mL, respectively.
Table 2. Calibration curves, LOD, and LOQ data of six investigated compounds.
Precision, repeatability, stability, and recovery of quantification
The precision was validated by determining the intra- and inter-day variations. The intra- and inter-day precisions were evaluated by measuring the standard solutions at three concentration levels (low, medium, and high) six times a day and once a day for six sequential days. The intra- and inter-day precisions were determined with the relative standard deviation (RSD) in the range of 0.32–1.65% and 0.64–1.73%, respectively. The injection of six consecutive times using black soybean sprouts was determined for the repeatability. The RSD values were less than 2.81%. In the stability test, black soybean sprouts were analysed at 0, 2, 4, 8, 12, and 24 h at room temperature. The stabilities indicated the analytes were stable with the RSD of the peak area less than 1.93%. In the recovery investigation, the known amounts of mixed standard solution with three concentration levels (low, medium, and high) were spiked into the known amounts of black soybean sprouts. The equation used to define recovery was (amount detected - original amount)/amount spiked × 100. The recovery ranged from 94.95% to 106.45% with an RSD from 1.1% to 4.6%. The precision, repeatability, stability, and recovery are shown in .
Table 3. Intra-, inter-day precision, stability, repeatability, and recovery of investigated compounds.
HPLC-DAD quantitative analysis of samples
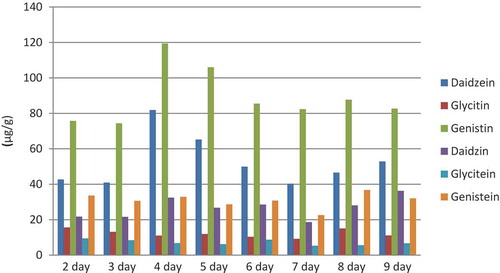
The proposed HPLC-DAD method has been used to determine the six isoflavones in the germinated black soybean sprouts. The germination time might contribute to the isoflavone contents in black soybean sprouts. The quantitative results are presented in . Compared to day 2 of germination, the daidzin, genistin, and daidzein contents significantly increased on day 4 of germination by 92%, 58%, and 49%, respectively. The contents of daidzin and genistin decreased by 35% and 31% from days 4 to 9. However, the daidzein increased by 12% from days 4 to 9. Throughout the germination, the daidzin and daidzein concentrations ranged from 41.0 to 81.9 μg/g and from 21.6 to 36.3 μg/g, respectively. The glycitin and glycitein contents were in the range of 9.2–15.6 and 5.3–9.4 μg/g, respectively. The genistin and genistein contents were in the range of 75.7–119.3and 22.6–36.8 μg/g, respectively. The highest contents of total isoflavones were up to 284 μg/g on day 4 of germination. From the 5th to 9th day of germination, the six isoflavone contents decreased slightly (about 9% decrease). Among the six isoflavones, genistin and daidzein were dominant with germination time. The contents of the six isoflavones in the black soybean sprouts were different. Lower contents of the compounds were found on 2–3 and 6–9 days of germination, and higher on 4–5 days of germination. Similar growth pattern was reported in the literatures. The black soybean had yielded significantly higher glycosides than that in the original soybean for germination with light.[Citation26,Citation27] Shweta Kumari reported that glycitein was not detected in the sprouts, contrary to our results. The difference may be due to the different varieties of soybeans used for germination[Citation26]. In addition, the amounts of daidzin and genistin are yielded by the hydrolysis of glycosides through β-glucosidase; the increase of glycosides in this study may have resulted from the inhibition of the activity of β-glucosidase during germination between days 2 and 4.[Citation27] On days 4 and 5 of germination, it showed an increase in the maximum concentration of the isoflavones and recommended for consumption for potential health benefits.
Conclusion
UHPLC−Q-TOF-MS and HPLC-DAD with an optimum extraction method were developed for qualitative and quantitative compositions and contents of isoflavones in germinated black soybean sprouts. A total of 23 compounds were identified on the basis of accurate mass, two-stage mass fragments, and characteristic fragmentation pathway. In addition, the research has evaluated the six isoflavones in black soybean sprouts. On the 4th day of germination, it showed an increase in the maximum concentration of the six isoflavones. The developed method was a simple, rapid, and sensitive method for the quality control of black soybean sprouts.
LJFP_A_1256303_Supplementary_material.zip
Download Zip (1.1 MB)Funding
This work was supported by the Jining Medical University Doctor Scientific Research Foundation Project (grant numbers JY14QD05); the Medicine and Health Science Technology Development Plan Funds (grant numbers 2014WS0508).
Supplemental data
Supplemental data for this article can be accessed on the publisher’s website.
References
- Astadi, I.R.; Astuti, M.; Santoso, U.; Nugraheni, P.S. In vitro Antioxidant Activity of Anthocyanins of Black Soybean Seed Coat in Human Low Density Lipoprotein (LDL). Food Chemistry 2009, 112, 659–663.
- Jeng, T.L.; Shih, Y.J.; Wu, M.T.; Sung, J.M. Comparisons of Flavonoids and Antioxidative Activities in Seed Coat, Embryonic Axis and Cotyledon of Black Soybeans. Food Chemistry 2010, 123, 1112–1116.
- Liu, J.; Wen, X.Y.; Zhang, X.Q.; Pu, H.M.; Kan, J.; Jin, C.H. Extraction, Characterization and in vitro Antioxidant Activity of Polysaccharides from Black Soybean. International Journal of Biological Macromolecules 2015, 72, 1182–1190.
- Nizamutdinova, I.T.; Kim, Y.M.; Chung, J.I.; Shin, S.C.; Jeong, Y.K.; Seo, H.G.; Lee, J.H.; Chang, K.C.; Kim, H.J. Anthocyanins from Black Soybean Seed Coats Stimulate Wound Healing in Fbroblasts and Keratinocytes and Prevent Inflammation in Endothelial Cell. Food and Chemical Toxicology 2009, 47, 2806–2812.
- Sohn, D.W.; Bae, W.J.; Kim, H.S.; Kim, S.W.; Kim, S.W. The Anti-inflammatory and Anti fibrosis Effects of Anthocyanin Extracted from Black Soybean on a Peyronie Disease Rat Model. Urology 2014, 84, 1113–1116.
- Kim, S.Y.; Wi, H.R.; Choi, S.; Ha, T.J.; Lee, B.W.; Lee, M. Inhibitory Effect of Anthocyanin-rich Black Soybean Testa (Glycine max (L.) Merr.) on the Inflammation-induced Adipogenesis in a DIO Mouse Model. Journal of Functional Foods 2015, 14, 623–633.
- Jang, E.H.; Ko, J.H.; Ahn, C.W.; Lee, H.H.; Shin, J.K.; Chang, S.J.; Park, C.S.; Kang, J.H. In vivo and in vitro Application of Black Soybean Peptides in the Amelioration of Endoplasmic Reticulum Stress and Improvement of Insulin Resistance. Life Sciences 2010, 86, 267–274.
- Kim, H.K.; Kim, J.N.; Han, S.N.; Nam, J.H.; Na, H.N.; Ha, T.J. Black Soybean Anthocyanins Inhibit Adipocyte Differentiation in 3T3-L1 Cells. Nutrition Research 2012, 32, 770–777.
- Jung, J.H.; Kim, H.S. The Inhibitory Effect of Black Soybean on Hepatic Cholesterol Accumulation in High Cholesterol and High Fat Diet-induced Non-alcoholic Fatty Liver Disease. Food and Chemical Toxicology 2013, 60, 404–412.
- Matsukawa, T.; Inaguma, T.; Han, J.; Villareal, M.O.; Isoda, H. Cyanidin-3-glucoside Derived from Black Soybeans Ameliorate Type 2 Diabetes Through the Induction of Differentiation of Preadipocytes into Smaller and Insulin-sensitive Adipocytes. Life Sciences 2010, 86, 267–274.
- Kim, S.M.; Chung, M.J.; Ha, T.J.; Choi, H.A.; Jang, S.J.; Kim, S.O.; Chun, M.H.; Do, S.I.; Choo, Y.K.; Park, Y.I. Neuroprotective Effects of Black Soybean Anthocyanins via Inactivation of ASK1–JNK/p38 Pathways and Mobilization of Cellular Sialic Acids. Life Sciences 2012, 90, 874–882.
- Paik, S.S.; Jeong, E.; Jung, S.W.; Ha, T.J.; Kang, S.; Sooyeon, S.; Jeon, J.H.; Chun, M.H.; Kim, I.B. Anthocyanins from the Seed Coat of Black Soybean Reduce Retinal Degeneration Induced by N-methyl-N-nitrosourea. Experimental Eye Research 2012, 97, 55–62.
- Balisteiro, D.M.; Rombaldi, C.V.; Genovese, M.I. Protein, Isoflavones, Trypsin Inhibitory and in vitro Antioxidant Capacities: Comparison Among Conventionally and Organically Grown Soybeans. Food Research International 2013, 51, 8–14.
- Hämäläinen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-inflammatory Effects of Flavonoids: Genistein, Kaempferol, Quercetin, and Daidzein Inhibit STAT-1 and NF-κB Activations, Whereas Flavone, Isorhamnetin, Naringenin, and Pelargonidin Inhibit only NF-κB Activation Along with their Inhibitory Effect on iNOS Expression and NO Production in Activated Macrophages. Mediators of Inflammation 2007, 45673, 1–10.
- Ososki, A.L.; Kennelly, E.J. Phytoestrogens: A Review of the Present State of Research. Phytotherapy Research 2003, 17, 845–869.
- Lee, J.H.; Kang, N.S.; Shin, S.O.; Shin, S.H.; Lim, S.G.; Suh, D.Y.; Baek, I.Y.; Park, K.Y.; Ha, T.J. Characterisation of Anthocyanins in the Black Soybean (Glycine max L.) by HPLC-DAD-ESI/MS Analysis. Food Chemistry 2009, 112, 226–231.
- Correa, C.R.; Li, L.; Aldini, G.; Carini, M.; Chen, C.Y.O.; Chun, H.K.; Cho, S.M.; Park, K.M.; Russell, R.M.; Blumberg, J.B.; Yeum, K.J. Composition and Stability of Phytochemicals in Five Varieties of Black Soybeans (Glycine max). Food Chemistry 2010, 123, 1176–1184.
- Lee, J.H.; Cho, K.M. Changes Occurring in Compositional Components of Black Soybeans Maintained at Room Temperature for Different Storage Periods. Food Chemistry 2012, 131, 161–169.
- Ito, C.; Oki, T.; Yoshida, T.; Nanba, F.; Yamada, K.; Toda, T. Characterisation of Proanthocyanidins from Black Soybeans: Isolation and Characterisation of Proanthocyanidin Oligomers from Black Soybean Seed Coats. Food Chemistry 2013, 141, 2507–2512.
- Huang, X.Y.; Cai, W.X.; Xu, B.J. Kinetic Changes of Nutrients and Antioxidant Capacities of Germinated Soybean (Glycine max L.) and Mung Bean (Vigna radiata L.) with Germination Time. Food Chemistry 2014, 143, 268–276.
- Wang, F.Z.; Wang, H.F.; Wang, D.H.; Fang, F.; Lai, J.X.; Wu, T.; Tsao, R. Isoflavone, γ-aminobutyric Acid Contents and Antioxidant Activities are Significantly Increased during Germination of three Chinese Soybean Cultivars. Journal of Functional Foods 2015, 14, 596–604.
- Mamilla, R.K.; Mishra, V.K. Effect of Germination on Antioxidant and ACE Inhibitory Activities of Legumes. LWT - Food Science and Technology 2017, 75, 51–58.
- Gu, E.J.; Kim, D.W.; Jang, G.J.; Song, S.H.; Lee, J.I.; Lee, S.B.; Kim, B.M.; Cho, Y.; Lee, H.J.; Kim, H.J. Mass-based Metabolomic Analysis of Soybean Sprouts during Germination. Food Chemistry 2017, 217, 311–319.
- Lopez-Amoros, M.L.; Hernandez, T.; Estrella, I. Effect of Germination on Legume Phenolic Compounds and their Antioxidant Activity. Journal of Food Composition and Analysis 2006, 19, 277–283.
- I.C.H. Harmonized Tripartite Guideline. Validation of Analytical Procedure: Methodology (Q2B), International Conference on Harmonization, Geneva, Switzerland, Nov 6, 1997.
- Kumari, S.; Chang, S.K.C. Effect of Cooking on Isoflavones, Phenolic Acids, and Antioxidant Activity in Sprouts of Prosoy Soybean (Glycine max). Journal of Food Science 2016, 81, C1679–C1691.
- Quinhone Júnior, A.; Ida, E.I. Profile of the Contents of Different forms of Soybean Isoflavones and the Effect of Germination Time on these Compounds and the Physical Parameters in Soybean Sprouts. Food Chemistry 2015, 166, 173–178.