ABSTRACT
Sonchus brachyotus DC. (S. brachyotus) is considered a vegetable, and rarely considered to be medicinal. However, S. brachyotus is used as a kind of traditional folk herb in Europe. Pharmacological studies are necessary in order to provide a scientific basis to substantiate their use. This study investigated the antimicrobial effect and mechanism of action of the ethanol extract from S. brachyotus. Ethanol extract from S. brachyotus exhibited antimicrobial activity against Escherichia coli, Enterobacter cloacae, Klebsiella pneumonia, Salmonella enteric, Staphylococcus aureus, and Micrococcus luteus; this is especially so in the case of E. coli. This study investigated the novel antibacterial mechanism of ethanol extract from S. brachyotus that shows an apoptosis-like response in E. coli. At all the tested concentration, it was found that ethanol extract from S. brachyotus–treated E. coli cells displayed various apoptotic markers, such as membrane damage, reactive oxygen species accumulation, and membrane depolarization. Expression of caspases protein, which mediates a bacterial apoptosis–like response, was also increased by ethanol extract from S. brachyotus. In order to evaluate the influence of caspases on the appearance of other apoptotic markers, phosphatidylserine exposure and DNA fragmentation were detected and compared with untreated and with ethanol extract from S. brachyotus. These characteristics were detected in E. coli cells, but not in untreated, with ethanol extract from S. brachyotus. In conclusion, the data obtained demonstrated that ethanol extract from S. brachyotus induces an apoptosis-like response in E. coli that associates with caspase-like protein.
Introduction
Infections by pathogenic microorganisms are constantly increasing, and drug-resistant microorganisms are rapidly emerging.[Citation1] These are major public health problems, and antibacterial agents with novel modes of action are required for effective therapies against these microorganisms.[Citation2] Many antimicrobials extracted from plant have exhibited enormous therapeutic potentials. They could dramatically alleviate infectious diseases, but lack adverse side-effects which are often associated with traditional antimicrobial agents, such as hypersensitivity, allergic reaction, and immunosuppression. Hence, there is an increased interest to obtain natural and safe antibacterial compounds from various natural sources.
In eukaryotic cells, apoptosis is a process of programmed cell death, involving specialized cellular machinery. It is processed by accompanying specific hallmarks (ROS accumulation, caspase activation, DNA fragmentation, and so on) and has the advantage of occurring without the release of harmful substances.[Citation3] Recently, a novel bacterial cell death mechanism was reported, which proves that bacterial cells could also undergo an apoptosis-like response, and features of apoptosis have been detected in Escherichia coli after treatment with norfloxacin that induces DNA damage by inhibiting DNA gyrase.[Citation4–Citation6] Therefore, it is important to develop natural antibacterial agents with an apoptosis-like response for commercial use, and for more applicable purposes.
Sonchus brachyotus DC. (S. brachyotus) belongs to the Sonchus genus which can be found in China, Japan, Mongolia, and Russia’s far east. S. brachyotus is considered a vegetable, and rarely considered to be medicinal. However, S. brachyotus is commonly used as a kind of traditional folk herb in Europe.[Citation7] A previous study found that S. brachyotus has antimicrobial activities against a number of pathogenic microorganisms.[Citation8] This study evaluated the antibacterial potential of the ethanol extract from S. brachyotus (SBE) against pathogenic microorganisms, as well as its mechanism of action.
Materials and methods
Plant material
All the samples of S. brachyotus were collected from Beijing, P. R. China, in May 2015. All specimens were authenticated by Xiu-Mei Li (Feed Research Institute, Chinese Academy of Agricultural Sciences, Beijing, P. R. China). A voucher specimen was deposited at the Feed Research Institute (specimen number: P20150501).
Preparation of SBE
SBE was prepared using the following method. The aerial parts of the dried plants were harvested and then sieved through a 60-mesh sieve. Each sample of powder (1.00 g) was accurately weighed and extracted using 30 mL 5.5:4.5 (v/v) ethanol–water solution by ultrasonic extraction for 30 min at 50°C. After centrifugation at 5000 rpm for 10 min, the supernatant was evaporated by rotary evaporation at 40 ± 2°C, lyophilization was carried out, and the sample was stored at –20°C.
Strains
E. coli (ATCC 25922), Enterobacter cloacae (ATCC 13047), Klebsiella pneumoniae (ATCC 10031), Staphylococcus aureus (ATCC 25923), and Salmonella enterica (ATCC 14028) were acquired from Jianhua Wang (Feed Research Institute, Chinese Academy of Agricultural Sciences). Micrococcus luteus (ATCC 10240) was acquired from Xuegang Luo (Tianjin Key Lab of Industrial Microbiology; College of Biotechnology, Tianjin University of Science and Technology, Tianjin, P. R. China). All strains were maintained in Luria-Bertani (LB) agar (Hopebio, P. R. China) and stored at –20°C.
Minimum inhibitory concentration
MTT assay was used for measuring the proliferation of bacterial cells.[Citation9] The bacterial cells (106 cells/mL) were incubated into LB at 200 μL/well in 96-well microtiter plates. Twofold serial dilutions of SBE were added to wells containing bacterial cells. After 24 h of incubation at 37°C, each concentration was assayed in triplicates (n = 3). Twenty-four hours later, 10 μL of the MTT (5 mg/mL) reagent was added to each well and the plates were incubated for 1 h at 37°C. Then, DMSO (100 μL) was added to terminate the reaction, and the plates were shaken slightly to redissolve the crystals formed. The absorbance of each well was measured using Synergy 4 microplate reader (BioTek Instruments, Winooski, VT, USA) (Li et al., 2015). All the results were expressed as the inhibition ratio of cell proliferation, calculated as [(A – B)/A] ×100%, where A and B are the average numbers of visible bacterial cells of the control and samples, respectively. The MIC is the lowest concentration of the test sample required to inhibit any visible growth.
Minimum bactericidal concentration
MBC, defined as the minimum concentration required to exterminate 99.9% of bacteria inoculums, was determined by re-inoculating 20 μL of each culture medium from the microtiter plate wells onto LB-agar plates. After 18 h of incubation at 37°C, MBCs were determined by visually inspecting the agar plates for bacterial growth. All MIC and MBC measurements were performed, at least, in duplicates.
Analysis of intracellular reactive oxygen species (ROS) accumulation
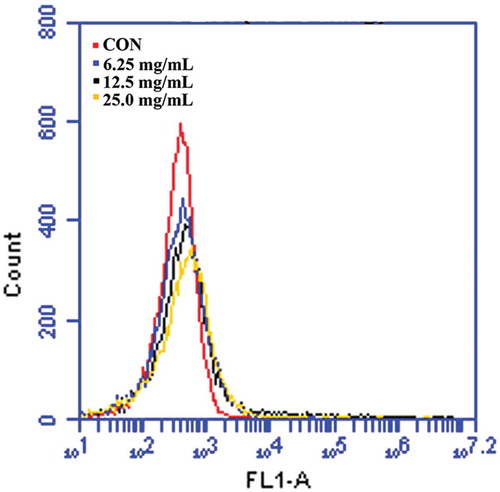
E. coli was treated with SBE (6.25, 12.5, and 25.0 mg/mL). After 1 h, E. coli was harvested via centrifugation at 12,000 rpm and re-suspended in phosphate buffer saline (PBS). In order to analyze intracellular ROS accumulation, the cells were incubated with a fluorescent dye, 2,7-dichlorodihydrofluorescein diacetate (H2DCFDA) (10 μM) (Invitrogen, Molecular Probes, Eugene, OR, USA), which reacts with intracellular ROS, at 37°C for 1 h. The samples were suspended in PBS, and their relative fluorescence intensity was analyzed by the FACSCalibur flow cytometer (FACScan, BD Biosciences).[Citation10,Citation11]
Analysis of membrane depolarization
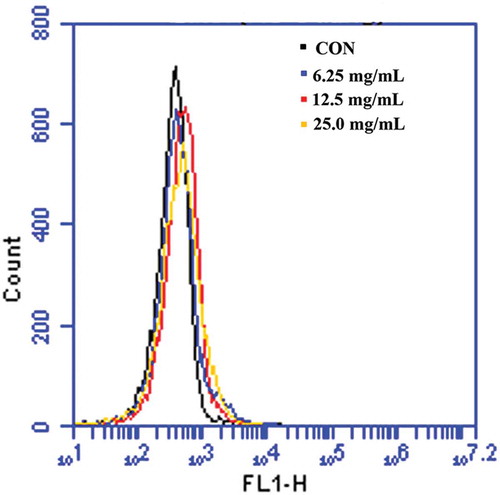
E. coli cells were incubated with SBE (6.25, 12.5, and 25.0 mg/mL) at 37°C. One hour later, E. coli cells were collected, washed twice with cold PBS, and detected with Mitochondrial Membrane Potential and Annexin V apoptosis kit, according to the manufacturer’s recommendations (Invitrogen, Molecular Probes). E. coli cells were analyzed by FACScanto (BD Biosciences), they were then stained with Rhodamine 123 (1 μM, Sigma, Germany) for 30 min at 37°C, then a re-analysis was carried out by FACScanto (BD Biosciences).[Citation12,Citation13]
Analysis of bacterial caspase-like protein
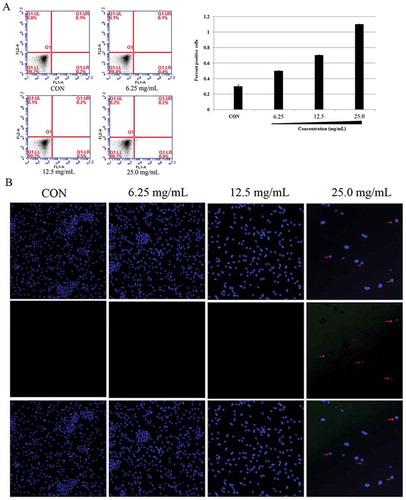
CaspACE FITC-VAD-FMK In Situ Marker (Promega, Fitchburg, WI, USA) was used to detect a homolog of eukaryotic caspase. E. coli was harvested by centrifugation at 12,000 rpm and washed with PBS. The suspensions were stained with the pan-caspase inhibitor FITC-VAD-FMK (2.5 μM) and then incubated at 37°C for 30 min. The samples were centrifuged at 12,000 rpm and suspended in PBS. The relative percentages of fluorescent cells were analyzed using the FACSCalibur flow cytometer (FACScan, BD Biosciences).[Citation10,Citation11]
Analysis of phosphatidylserine exposure
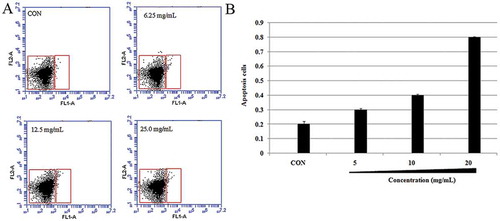
Annexin V-FITC apoptosis kit (Invitrogen, Molecular Probes) was used to detect phosphatidylserine (PS) exposure. E. coli cells were treated with SBE (6.25, 12.5, and 25.0 mg/mL) for 1 h. E. coli cells were harvested and re-suspended in PBS. Then, 100 μL of Annexin V binding buffer, 5 μL of Annexin V-FITC, and 5 μL of PI were added to the cell suspension; the mixtures were incubated at room temperature for 15 min. The cells were pelleted, re-suspended in 400 μL of Annexin V binding buffer, according to the manufacturer’s recommendations. The analysis of the cells was done using the FACSCalibur flow cytometer (FACScan, BD Biosciences). Cells were sorted into living, necrotic, early apoptotic, and late apoptotic cells. The relative ratio of early to late apoptotic cells was estimated for further comparison. This assay was repeated three times.[Citation10–Citation13]
Analysis of DNA structure
E. coli (106 cells/mL) cells were incubated with SBE (6.25, 12.5, and 25.0 mg/mL) for 1 h. E. coli cells were performed using the DNA-specific stain, Hoechst 33342 (Invitrogen, Molecular Probes), according to kit instructions. Counterstaining was performed using PI. After staining, cells were analyzed using the FACSCalibur flow cytometer (FACScan, BD Biosciences).[Citation4]
DNA fragmentation assay
The 3′-OH of the DNA fragments in apoptotic cells were labeled and stained by terminal dexynucleotidyl transferase (TdT)-mediated dUTP nick end labeling method using an apoptosis in situ detection kit (Promega, Germany), according to the manufacturer’s instructions. Fluorescent microscopy (Olympus, FV1000, Japan) was used to capture the image of the fluorescein-labeled TUNEL-positive cells. Moreover, TUNEL assay was performed as a complementary method to flow cytometric assay in order to demonstrate the apoptosis level more accurately. The cell nucleus was labeled in blue by Hoechst 33342 (Invitrogen, Molecular Probes) and the nick-ends were labeled in green. A merge between the nucleus (blue) and nick-end (green) labeling showed up as purple.[Citation4]
Statistical analysis
All the data were expressed as mean ± SE (standard error) and MIC values were obtained using SPSS 17.0 statistical software package (SPSS, Inc., Chicago, IL, USA).
Results and discussion
Antibacterial effect of SBE against pathogenic microorganisms
The results of the antibacterial activity of SBE against different pathogenic microorganisms are presented in . The MIC values of SBE for E. coli, E. cloacae, K. pneumonia, S. aureus, S. enterica, and M. luteus were 25, >50, >50, 50, 50, and >50 mg/mL, respectively. The MBC values of SBE for E. coli and S. enterica were 25 and 50 mg/mL, respectively (). The results showed that SBE possessed antibacterial effect and mainly inhibited the proliferation of Gram-negative bacteria. Numerous plant extracts exhibited inhibition against Gram-negative bacteria. Gram-negative bacteria have a hydrophilic membrane because of the presence of lipopolysaccharides. Thus, a small hydrophilic molecule can pass through the outer membrane. Inversely, this outer membrane also permits passage of lipophilic compounds and macromolecules. Seeing the permeation properties of the outer membrane of the microorganisms is a precondition for understanding the antibacterial activity of a solute. Therefore, since the ethanol extracts used in this work are partially soluble in water, they penetrated the outer membrane of Gram-negative bacteria and perturbed the inside of the cell, hampering cellular function and metabolism; thereby causing loss of cellular constituents, and ultimately resulting in cell death.[Citation14–Citation16] Hence, E. coli was used as the model bacteria for the studies of apoptosis-like response of SBE.
Table 1. Determination of MIC and MBC values of SBE against selected pathogenic microorganisms.
Intracellular ROS accumulation
It has been shown that ROS directly contributes to cellular damage and death in E. coli.[Citation4] Additionally, ROS is contained of crucial molecules that attack proteins, nucleic acids, and lipid membranes, but it is usually scavenged by intracellular enzymatic antioxidants before ROS affect cells.[Citation17] However, superfluous generation of ROS can attack bacterial cell membrane lipids and then lead to collapse of bacterial cell membrane function.[Citation18] Therefore, the study first investigated whether SBE induces ROS accumulation in E. coli cells. Through an H2DCFDA assay, it was confirmed that ROS accumulated in E. coli in response to various concentrations of SBE treatment, in a dose-dependent manner, as shown in . The results suggested that SBE could induce a bacterial apoptosis-like response by promoting ROS accumulation.
Effect of SBE on the membrane potential of E. coli
Membrane depolarization is also an apoptotic hallmark. When cells undergo apoptosis, the membrane potential (Δψm) is perturbed and membrane depolarization is induced.[Citation19] To test whether SBE induces apoptosis of E. coli through destroying the cell membrane, cell membrane potential was analyzed using Mitochondrial Membrane Potential/Annexin V apoptosis kit and Rhodamine 123. As demonstrated in , when E. coli was treated with 6.25, 12.5, and 25.0 mg/mL of SBE for indicated periods, the collapse of Δψm in a dose-dependent manner occurred. The observation indicated that SBE might exert antibacterial activity through a bacterial apoptosis-like response.
Figure 2. Analysis of membrane depolarization in E. coli following treatment with SBE. (A, B) Flow cytometric analysis of membrane depolarization using Mitochondrial Membrane Potential/Annexin V apoptosis kit. (C) Membrane potential was determined by flow cytometric analysis using Rhodamine 123 staining.
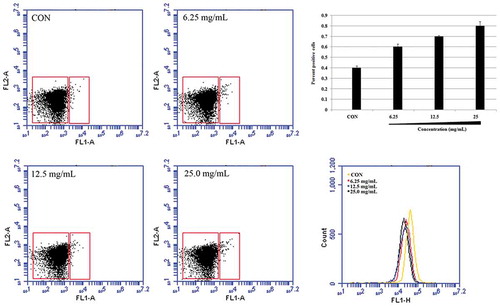
Analyzing the levels of bacterial caspase-like protein
In apoptosis, caspase activates other caspases and various apoptotic factors, amplifying the apoptotic signal and leading to cell death. Hence, caspase plays an important role in the apoptotic process.[Citation10] In order to investigate whether SBE induces E. coli death through caspase-like protein, FITC-VAD-FMK, a fluorescently labeled version of the pan-caspase substrate VAD (valine–alanine–glutamate) was used. After treatment with SBE, the fluorescence of E. coli cells increased compared with untreated cells (), indicating that SBE stimulated activation of caspase-like protein(s).
Analysis of phosphatidylserine exposure
To investigate the extent to which caspases affects the bacterial apoptosis-like response, caspase-linked apoptosis hallmarks, like PS exposure,[Citation10] were detected in E. coli. In this investigation, only a small percentage of apoptotic cells were found in the negative group (0.2 ± 0.1%) after being incubated by the vehicle for 1 h. However, the percentage of apoptotic cells significantly increased from 0.4 ± 0.1% to 0.9 ± 0.2% after being treated with different doses of SBE ().
Analysis of DNA damage
DNA fragmentation can also be induced by a caspase-activated DNase (CAD), which cleaves DNA to constant lengths (180–200 base pairs) at internucleosomal linker regions in eukaryotic cells.[Citation20] These caspase-linked apoptotic hallmarks were detected in E. coli. To monitor the structural state of the bacterial chromosome following SBE treatment, the DNA-specific and conformation-sensitive dye, Hoechst, 33342 was used. The results demonstrated that E. coli cells displayed different levels of DNA damage, in a dose-dependent manner, as shown in . Besides, TUNEL-positive cells were almost undetectable without SBE, whereas TUNEL-positive signal increased significantly after SBE treatment (). Thus, the researchers speculated that SBE induces a bacterial apoptosis-like response and caspases protein is associated with this response.
Conclusions
In conclusion, the researchers proposed that SBE targets bacterial membranes. In addition, many characteristics of a bacterial apoptosis-like response were observed after treatment with SBE at all the tested concentrations, including membrane depolarization, PS exposure, and DNA fragmentation. These results are the first to explain a novel antibacterial mechanism, a bacterial apoptosis-like response, induced by SBE. Dwyer et al. reported that ROS generated by antibiotics is central to its toxicity.[Citation20] This study further indicated that SBE also brought about ROS generation and DNA damage, leading to the induction of an apoptosis-like response associated with caspases. The isolation and chemical characterization of SBE, being carried out by this team of researchers, represent a possible, sustainable utilization of the natural resources from S. brachyotus. Further studies, relating to biological activities, should be carried out in order to better understand the health effects of S. brachyotus.
Funding
This work was supported by the Agricultural Science and Technology Innovation Program (ASTIP-FRI07), Special Fund for Agro-scientific Research in the Public Interest (201403047), and project funded by China Postdoctoral Science Foundation (2016M591306).
Additional information
Funding
Notes on contributors
Xiu-Mei Li
Xiu-Mei Li and Jing Liu contributed equally to this article.
Jing Liu
Xiu-Mei Li and Jing Liu contributed equally to this article.
References
- Bax, R.; Mullan, N.; Verhoef, J. The Millennium Bugs-the Need for and Development of New Antibacterials. International Journal of Antimicrobial Agents 2000, 16, 51–59.
- Shah, P.M. The Need for New Therapeutic Agents: What is the Pipeline? Clinical Microbiology and Infection 2005, 3, 36–42.
- Edinger, A.L.; Thompson, C.B. Death by Design: Apoptosis, Necrosis and Autophagy. Current Opinion in Cell Biology 2004, 16 (6), 663–669.
- Dwyer, D.J.; Camacho, D.M.; Kohanski, M.A.; Callura, J.M.; Collins, J.J. Antibiotic-induced Bacterial Cell Death Exhibits Physiological and Biochemical Hallmarks of Apoptosis. Molecular Cell 2012, 46, 561–572.
- Erental, A.; Sharon, I.; Engelberg-Kulka, H. Two Programmed Cell Death Systems in Escherichia coli: An Apoptotic-like Death is Inhibited by the mazEF-mediated Death Pathway. PLoS Biology 2012, 10, e1001281.
- Erental, A.; Kalderon, Z.; Saada, A.; Smith, Y.; Engelberg-Kulka, H. Apoptosis-like Death, An Extreme SOS Response in Escherichia coli. MBio 2014, 5, e01426–e01414.
- Xu, P.; Zh, X.W.; Chen, J.H. Study on Component of Essential Oil Extracted from Sonchus brachyotus DC. by GC-MS. Bulletin Science and Technology 2010, 26 (3), 374–376.
- Xia, D.Z.; Yu, X.F.; Zh,Y.; Z, Zh.D. Antioxidant and Antibacterial Activity of Six Edible Wild Plants (Sonchus spp.) in China. Natural Product Research 2011, 25 (20), 1893–1901.
- Wang, X.; Wu, Y.C.; Xia, S.P.; Gao, Y.; Li, C.X. Inquiring into the Method of Counting Live Germ with MTT. Journal of Luzhou Medicine College 2002, 25, 291–293.
- Yun, D.G.; Lee, D.G. Antibacterial Activity of Curcumin via Apoptosis-like Response in Escherichia coli. Applied Microbiology and Biotechnology 2016, 100 (12), 5505–5514.
- Choi, H.; Hwang, J.S.; Lee, D.G. Coprisin Exerts Antibacterial Effects by Inducing Apoptosis-like Death in Escherichia coli. IUBMB Life 2015, 68 (1), 72–78.
- Li, X.M.; Luo, X.G.; Si, Ch.L., Wang, N.; Zhou, H.; Li, K.; Ma, N.; Zhang, T.C. The Extract of Hypericum ascyron L. Induces Bacterial Cell Death Through Apoptosis Pathway. Journal of Ethnopharmacology 2015a, 166, 205–210.
- Li, X.M.; Luo, X.G.; Si, Ch. L.; Wang, N.; Zhou, H.; He, J.F.; Zhang, T.C. Antibacterial Active Compounds from Hypericum ascyron L. Induce Bacterial Cell Death Through Apoptosis Pathway. European Journal of Medicinal Chemistry 2015b, 96, 436–444.
- Nikaido, H. Outer Membrane in Escherichia Coli and Salmonella. In Cellular and Molecular Biology; Neidhardt, F.C., Ed.; ASM Press: Washington, DC, 1996; 29–47.
- Yerra, R.; Gupta, M.; Mazumder, U. In Vitro Lipid Peroxidation and Antimicrobial Activity of Mucuna pruriens Seeds. Iran Journal of Pharmacology Therapy 2005, 4, 32–35.
- Kuete, V.; Nguemeving, J.R.; Beng, V.P.; Azebaze, A.G.B.; Etoa, F.-X.; Meyer, M.; Bodo, B.; Nkengfack, A.E. Antimicrobial Activity of the Methanolic Extracts and Compounds from Vismia laurentii De Wild (Guttiferae). Journal of Ethnopharmacology 2007, 109, 372–379.
- Evans, M.D.; Dizdaroglu, M.; Cooke, M.S. Oxidative DNA Damage and Disease-induction, Repair and Significance. Mutation Research 2004, 567, 1–61.
- Ninganagouda, S.; Rathod, V.; Singh, D.; Hiremath, J.; Singh, A.K.; Mathew, J.; ul-Haq, M. Growth kinetics and Mechanistic Action of Reactive Oxygen Species Released by Silver Nanoparticles from Aspergillus niger on Escherichia coli. Biomed Research International 2014, 1–9.
- Jung, J.I.; Lim, S.S.; Choi, H.J.; Cho, H.J.; Shin, H.K.; Kim, E.J.; Chung, W.Y.; Park, K.K.; Park, J.H. Isoliquiritigenin Induces Apoptosis by Depolarizing Mitochondrial Membranes in Prostate Cancer Cells. Journal of Nutrition Biochemistry 2006, 17, 689–696.
- Dwyer, D.J.; Kohanski, M.A.; Hayete, B.; Collins, J.J. Gyrase Inhibitors Induce an Oxidative Damage Cellular Death Pathway in Escherichia coli. Molecular Systems Biology 2007, 3, 91.