ABSTRACT
The antioxidant potential of five tuberous root leaves was evaluated. High performance liquid chromatography (HPLC/DAD) showed the presence of phenolic compounds in leaves, and the predominant ones were chlorogenic acids in carrot (458.79 mg.L−1± 0.03): rosmarinic acid in sweet potato (222.05 mg.L−1± 0.01) and quercetin in sweet potato (292.42 mg.L−1± 0.01). Radish leaves showed a higher total in vitro antioxidant activity for all methods used in this study. The results indicated that these leaves were natural sources of antioxidants and, therefore, could be included in the health beneficial diet.
KEYWORDS:
Introduction
Vegetables are among the main sources of natural antioxidants, and the potential of these biologically active substances has been intensively investigated due to their protective ability against the action of free radicals. The excessive formation of reactive oxygen and nitrogen species (ROS and RNS) results in oxidative stress and increases the risk of developing chronic non-communicable diseases (NCDs) such as cardiovascular, neurological, and several types of cancer.[Citation1–Citation4]
Phenolic compounds are secondary metabolites synthesized in plants in response to environmental stresses, such as the attack of pathogens and insects, UV radiation, and injuries.[Citation5–Citation7] These phytochemicals have the ability to scavenge free radicals, inhibit lipid peroxidation, and chelate metals, besides playing a vital role in the stability of food products, as well as in defense mechanisms of biological systems.[Citation4,Citation8]
Plant foods are the largest sources of natural phenolic compounds in the human diet, and high concentrations of these compounds can be found in their leaves, shell, and peel.[Citation9] However, these parts are discarded, generating a large amount of waste and, therefore, the waste of nutrients. Several studies reported the potential of tuberous root leaves as nutraceutical food. The leaves of carrot (Daucus carota L.) are natural sources of omega 3 and 6[Citation10] and are used in salads, soups, and in the preparation of food products;[Citation10–Citation12] beet (Beta vulgaris L.) leaves are used in food formulations to increase their nutritional value[Citation13]; the leaves of sweet potato (Ipomea batatas L.) have a high content of soluble fiber, polyphenols, and minerals, especially iron and vitamin E;[Citation14] yam (Colocasia esculenta L.) leaves are consumed in sauces, purées, stews, and soups and are used in the treatment of scarring;[Citation15] and radish (Raphanus sativus L.) leaves present a broad spectrum of antibacterial activity against foodborne pathogens.[Citation16]
Although several studies elucidated the antioxidant potential of most of these leaves, few analyze the content of phenolic compounds. In this context, the objective of this study was to identify and quantify twelve phenolic compounds by high-performance liquid chromatography (HPLC/DAD) and evaluate the antioxidant potential of this waste.
Material and methods
Identification and quantification of phenolic compounds by high performance liquid chromatography (HPLC)
For the obention of extracts, 1 g of the powder of each leaf was used with 50 mL of 50% methanol under reflux without vacuum, on a hot plate at 80°C, boiling for 15 min, in three replications. Subsequently, the extract was filtered through filter paper and collected. The extraction residue was again subjected to the extraction process twice. The combined filtrates were evaporated on the hot plate up to 25 mL.[Citation17]
The analyses were performed on a high performance liquid chromatograph (HPLC Shimadzu, LC- 6AD), equipped with two high pressure pumps (LC-20AD), a diode array (DAD), a detector (SPD-M20A), an automatic sampler (SIL-M20A), and a microwave (CTO-20AC). Separations were performed using a packed Shim-pack VP-ODS column (250 mm 4.6 mm) with spherical particles of 5 mm connected to a Shim-pack VP-ODS pre-column (5.0 cm 4.0 mm 5 mm - Shimadzu). Samples were eluted using two mobile phases: the first (A) consisted of water and acetonitrile (2%) and the second (B) comprised methanol, water, and acetonitrile in the proportions 70:28:2 v/v, respectively. Samples were eluted under the following gradient: 0–25 min (0–40%B); 25 to 43 min (40–45%B); 43–50 min (45–80%B); 50–55 min (80–0%B); and 55–65 min (0%B). The absorbance was measured at 280 nm, the flow rate was 1 mL.min−Citation1 at a temperature of 35°C, and the injected volume was 20 μL. The standards used for the chromatographic analysis were gallic acid, catechin, chlorogenic acid, caffeic acid, vanillin, p-coumaric acid, ferulic acid, m-coumaric acid, o-coumaric acid, rosmarinic acid, quercetin, and trans-cinnamic acid. All standards were purchased from Sigma-Aldrich. All solvents employed for chromatography were of analytical HPLC grade: methanol (Merck), glacial acetic acid (JT Baker), and ultrapure water obtained from a Milli-Q system.
Collection of materials and preparation of extracts for colorimetric analysis
Leaves of carrot (Daucus carota L.), beet (Beta vulgaris L.), sweet potato (Ipomea batatas L.), yam (Colocasia esculenta L.), and radish (Raphanus sativus L.) were acquired at a local market in the city of Lavras, Minas Gerais, and processed on the same day. The leaves were rinsed with distilled water and lyophilized. The dried samples were ground and hermetically stored at -80°C until analysis. For the preparation of the extracts, 3 grams of the powder from each leaf was weighed and 40 mL of 50% methanol was added. The extracts were homogenized and left at rest for 60 min at room temperature. Each extract was centrifuged at 15,000 rpm for 15 min, and the supernatants were transferred to 100-mL volumetric flasks. About 40 mL of 70% acetone was added to the first extraction residue with subsequent homogenization and left at rest for 60 min at room temperature. The extracts were again centrifuged at 15,000 rpm for 15 min, and the supernatants were transferred to volumetric flasks containing the first supernatant. The volume of each extract was completed to 100 mL with distilled water.
Determination of total phenolic content
The content of total phenolics was determined by the method proposed by,[Citation18] and the Folin–Ciocalteu reagent was used. A 0.5-mL aliquot of the extract from each sample was added to tubes containing 2.5 mL of 10% Folin–Ciocalteu solution (v/v); 2 mL of 4% sodium carbonate solution (v/v) was then added. The tubes were vortexed and left at rest for 120 min, protected from light. The blue color produced by the reduction of the Folin–Ciocalteu reagent by the phenolics was measured spectrophotometrically, at a range of 750 nm. The calculation of the phenolic content was based on the equation of the line obtained from the gallic acid standard curve. The results were expressed in mg of gallic acid equivalent per gram of sample (mgGAE.g−Citation1).
Scavenging activity of free radicals (DPPH)
The total antioxidant activity was evaluated by the DPPH method (1,1-diphenyl-1,2-picrylhydrazyl) proposed by.[Citation19] A 0.1-mL aliquot of each dilution of the extracts was transferred into test tubes with 3.9 mL of the DPPH radical and homogenized. Methyl alcohol was used as a blank in order to calibrate the spectrophotometer. Readings were taken after 30 min in a spectrophotometer at 515 nm, and the results were expressed as IC50 (μg/g).
Total antioxidant activity by the iron reduction method (FRAP)
The antioxidant activity (FRAP) was determined according to the method proposed by.[Citation20] A 90 µL aliquot of each dilution of the extract was transferred to test tubes with 270 µL of distilled water and 2.7 mL of the FRAP reagent. The tubes were homogenized in a shaker and incubated in a water bath at 37 °C. The tubes were left at rest protected from light, at room temperature for 30 min. Readings were taken at 595 nm, and the FRAP reagent was used as a blank to calibrate the spectrophotometer.
Determination of the total antioxidant activity by the β-carotene/linoleic acid co-oxidation method
The antioxidant activity by the β-carotene/linoleic acid co-oxidation method was determined according to the method proposed by.[Citation21] About 0.4 mL of each dilution of the extracts was mixed with 5 mL of the solution system. The tubes were then homogenized and placed in a water bath at 40 °C. Readings were taken at the times 2 min and 120 min in a spectrophotometer, at 470 nm. The results were expressed as IC50 (mg/L).
Statistical analysis
All tests were carried out in triplicate, and the results were presented as means ± standard deviation (SD). The colorimetric variables were subjected to analysis of variance (ANOVA) and compared by the Scott-Knott test at 5% probability using the statistical program SISVAR.[Citation22] The relationships between variables were assessed by Pearson’s correlation, and value of p < 0.05 was considered statistically significant.
Results and discussion
Identification and quantification of twelve phenolic compounds by HPLC-DAD
The quantification of phenolic compounds (gallic acid, catechin, chlorogenic acid, caffeic acid, vanillin, p-coumaric acid, ferulic acid, m-coumaric acid, o-coumaric acid, rosmarinic acid, quercetin, and trans-cinnamic acid) by HPLC-DAD was performed by peak integration, using the external standard method and the chromatographic profile of phenolic compounds in the standard solutions and in the different leaves studied (4 x 10−Citation5 mol L−Citation1), and are shown in . The identification and quantification of phenolic compounds in the leaf extracts of carrot, beet, sweet potato, yam, and radish are presented in .
Figure 1. Chromatogram of the standard solution of phenolic compounds (1) Gallic acid, (2) Catechin, (3) Chlorogenic acid, (4) Caffeic acid, (5) Vanillin, (6) p-coumaric acid, (7) Ferulic acid, (8) m-coumaric acid, (9) o-coumaric acid, (10) Rosmarinic acid, (11) Quercetin (12) Trans-cinnamic acid (4 x 10-5 mol L-1) (A) and methanol extracts of leaves of carrot (B), beet (C), sweet potato (D), yam (E), radish (F). Identified peaks: (B): 1) Gallic acid, (2) Catechin, (3) Chlorogenic acid, (4) Caffeic acid, (5) Vanillin, (6) p-coumaric acid, (7) Ferulic acid, (9) o-coumaric acid, (10) Rosmarinic acid, (11) Quercetin, (12) Trans-cinnamic acid. (C): 1) Gallic acid, (2) Catechin, (3) Chlorogenic acid, (4) Caffeic acid, (5) Vanillin, (6) p-coumaric acid, (7) Ferulic acid, (10) Rosmarinic acid, (11) Quercetin (12) Trans-cinnamic acid. (D): (1) Gallic acid, (2) Catechin, (3) Chlorogenic acid, (4) Caffeic acid, (5) Vanillin, (6) p-coumaric acid, (7) Ferulic acid (9) o-coumaric acid, (10) Rosmarinic acid, (11) Quercetin (12) Trans-cinnamic acid. (E): (1) Gallic acid, (2) Catechin, (3) Chlorogenic acid, (4) Caffeic acid, (5) Vanillin, (8) m-coumaric acid, (9) o-coumaric acid, (12) Trans-cinnamic acid. (F): 1) Gallic acid, (4) Caffeic acid, (3) Chlorogenic acid, (5) Vanillin, (6) p-coumaric acid, (7) Ferulic acid, (10) Rosmarinic acid, (11) Quercetin (12) Trans-cinnamic acid.
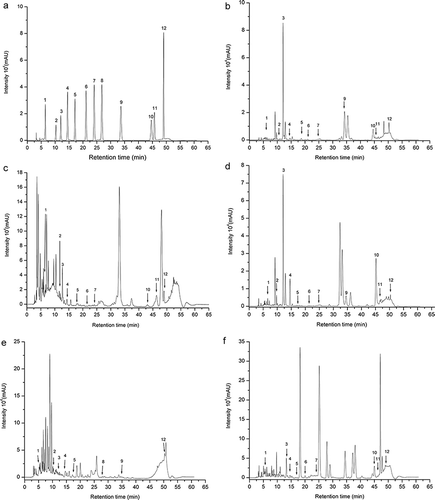
Table 1. Identification and quantification of phenolic compounds in leaf extracts of carrot, beet, sweet potato, yam, and radish.
The predominant phenolic compounds were chlorogenic acid in leaves of carrot and sweet potato, rosmarinic acid in leaves of beet and sweet potato, and quercetin in leaves of beet and sweet potato. A variety of biological activities have been described for rosmarinic acid, and the main ones are the antioxidant, anti-inflammatory, antiviral, and antibacterial.[Citation23,Citation24] However, the most important activity is its antioxidant capacity, which is assigned to the four hydroxyl groups present in the molecule, acting as free radical scavengers.[Citation24,Citation25] Other studies have demonstrated the antioxidant potential of rosmarinic acid in the absorption and neutralization of free radicals, in the removal of singlet and triplet oxygen atoms and also in the decomposition of peroxides.[Citation24,Citation26]
Chlorogenic acids are found in most plant species, and several studies have elucidated the beneficial pathophysiological effects of this acid, as well as the effect against hypertension and hyperglycemia, in the prevention of colon cancer, in the inhibition of cell proliferation of tumors from different origins and anti-inflammatory action.[Citation24,Citation27,Citation28] Quercetin is a major flavonoid present in the human diet and, since it has several therapeutic properties, it has been extensively studied in recent decades. It has antioxidant and anticarcinogenic potential, as well as protective effects on kidney, liver, and cardiovascular systems. In a study on the evaluation of the effect of quercetin supplementation on indicators of oxidative stress and inflammation in sarcoidosis,[Citation29] Boots et al. concluded that quercetin supplementation in the diet increased the body defense against inflammatory processes and oxidative damage. When investigating the neuroprotective effect of quercetin against H2O2-induced apoptosis in SH-SY5Y neuronal cells in humans, [Citation30] Suematsu et al. inferred that quercetin can potentially serve as an agent for the prevention of neurodegenerative diseases caused by oxidative stress and apoptosis. The results in this study indicated the potential of the studied leaves to be used as natural sources of polyphenols.
Total phenolic compounds and total antioxidant activity
presents the results of phenolic compounds and total antioxidant activity. The content of total phenolics was higher in radish leaves, followed by yam, beet, sweet potato, and carrot, respectively. A high content of total phenolics in methanol (86.16 ± 4.51 mg catechin/g) and acetone (78.8 ± 5.3 mg catechin/g) extracts was also reported for radish leaves by[Citation16]. Results lower than those found in this study were reported by[Citation14], for the methanol extract of carrot (9.3 mg GAE/g), beet (6.1 mg GAE/g), and sweet potato (17.3 mg GAE/g) leaves. The difference between the reported values was possibly due to the use of only one type of solvent, which may have compromised the extraction efficiency of phenolic compounds.
Table 2. Iron reducing antioxidant power (FRAP), total compounds, phenolics, β-carotene, scavenging activity of free radicals (DPPH/IC50).
Another reason for the difference between the results described is the low specificity of the colorimetric method, which not only indicates the presence of polyphenols, but also of other compounds, including inorganic and non-phenolic organic substances that can be oxidized by the Folin–Cicateau reagent[Citation31,Citation32] The highest free radical scavenging capacity (DPPH/IC50) was observed in radish leaves, followed by beet, yam, sweet potato, and carrot. A strong negative correlation between the content of total phenolic compounds and scavenging free radical activity was observed (DPPH/IC50) (), indicating that these compounds are highly responsible for the antioxidant activity of the studied samples. These results are in agreement with those of[Citation4] and[Citation33]. However, some studies indicate that it is not always possible to correlate total phenolics and antioxidant capacity.[Citation24,Citation34,Citation35] In this study, it was possible to observe that total polyphenols did not correspond to the lowest values of DPPH/IC50 for leaves of beet, yam, and sweet potato, as described for radish and carrot leaves. This can be explained by many factors, including the presence of different active compounds in the leaves, which can change the antioxidant capacity, such as the synergistic effect of different compounds, experimental conditions, and the mechanism of action of the methods used in the evaluation of the antioxidant activity.[Citation24,Citation36] There are also compounds which react strongly with DPPH, and others which have a slower reaction rate.[Citation24,Citation37]
Table 3. Correlation coefficient (p value) between the analysis and the antioxidant capacity of the studied leaves.
The highest values for the antioxidant power of iron reduction were observed for radish, followed by yam, beet, sweet potato, and carrot. Data are positively correlated with total phenolics (), in accordance with[Citation16,Citation38] and negatively correlated with DPPH/IC50 () as a result obtained by[Citation39,Citation40]. The greatest protective effect of β-carotene/IC50 against co-oxidation by radicals derived from the oxygenation of linoleic acid was determined for beet and radish leaves, followed by yam, sweet potato, and carrot leaves. These data are negatively correlated with FRAP and total phenolics (), which shows the effectiveness of the method for the evaluation of the antioxidant activity. In general, all methods used to measure antioxidant activity in foods have some limitations; for this reason, a combination of several methods is necessary. However, the results presented in this study indicate that total phenolics and FRAP are highly correlated with other methods, and, therefore, one can be selected as an indicator of the antioxidant activity in vegetable residues used in this study.
Conclusion
HPLC/DAD analysis showed the presence of high concentrations of quercetin for beet and sweet potato leaves; chlorogenic acids for carrots and sweet potato; and rosmarinic acid for beet and sweet potato leaves. Radish leaves showed the highest antioxidant activity for the studied methods. These results show the great potential of these leaves in the prevention of damage from reactive species. Thus, the likely inclusion of these leaves in human diet will contribute to increase the intake of phenolic compounds, and in vivo studies are necessary to confirm the antioxidant power in the body.
Acknowledgments
The authors would like to thank Universidade Federal de Lavras (UFLA) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
References
- Shah A.M.; Channon K.M. Free Radicals and Redox Signalling in Cardiovascular Disease. Heart 2004, 90, 486–487.
- Moreira, P.I.’ Santos, M.S.; Oliveira, C.R.; Shenk, J.C.; Nunomura, A.; Smith, M.A.; Zhu, X.; Perry, G. Alzheimer Disease and the Role of Free Radicals in the Pathogenesis of the Disease. CNS & Neurological Disorders - Drug Targets 2008, 7, 3–10.
- Valko, M.; Rhodes, C. J.; Moncol, J.; Izakovic, M.; Mazur, M. Free Radicals, Metals and Antioxidants in Oxidative Stress-induced Cancer. Chemico-Biological Interactions 2006, 160, 1–40.
- Morales, P.; Carvalho, A.M.; Sánchez-Mata, M.C.; Molina, M.; Ferreira, I.C.F.R. Tocopherol Composition and Antioxidant Activity of Sapanish Wild Vegetables. Genetic Resources and Crop Evolution 2012, 59, 851–863.
- Tomas-Barberan, F.A.; Ferreres, F.; Gil, M.I. Antioxidant Phenolic Metabolites from Fruit and Vegetables and Changes During Postharvest Storage and Processing. In Bioactive Natural Products (Part D); Rahman, A.; Ed.; Elsevier: Amsterdam, 2000; 739–795.
- Lapornik, B.; Prosek, M.; Golc, Wondra. A. Comparison of Extracts Prepared from Plant by-products using Different Solvents and Extraction Time. Journal of Food Engineering 2005, 71, 214–222.
- Ignat, I.; Volf, I.; Popa, V.I. A Critical Review of Methods for Characterisation of Polyphenolic Compounds in Fruits and Vegetables. Food Chemistry 2011, 126, 1821–1835.
- Nijveldt, R.; Nood, E.; Hoorn, D.; Boelens, P.; Norren, K.; Leeuwen, P. Flavonoids: A Review of Probable Mechanisms of Action and Potential Applications. The American Journal of Clinical Nutrition 2011, 74, 418–425.
- Moon, J-K.; Shibamoto, T. Antioxidant Assays for Plant and Food Components. Journal of Agricultural and Food Chemistry 2009, 57, 1655–1666.
- Leite, C.W.; Boroski, M.; Boeing, J.S.; Aguiar, A.C.; França, P.B.; Souza, N.E.; Visentainer, J.V. Chemical Characterization of Leaves of Organically Grown Carrot (Dacus carota L.) in Various Stages of Development for Use as Food. Ciência e Tecnologia de Alimentos 2011, 31, 735–738.
- Boroski, M.; Aguiar, A.C.; Boeing, J.S.; Rotta, E.M.; Wibby C.L.; Bonafé, E. G., Souza N.E.; Visentainer, J.V. Enhancement of Pasta Antioxidant Activity with Oregano and Carrot Leaf. Food Chemistry 2011, 125, 696–700.
- Sousa, C.M.M.; Silva, H.R.; Vieira-Jr, G.M.; Ayres, M.C.C.; Costa, C.L.S.; Araújo, D.S.; Calvalcante, L.C.D.; Barros, E.D.S.; Araújo, P.B.M.; Braandão, M.S.; Chaves, M.H. Fenóis totais e atividade antioxidante de cinco plantas medicinais. Química Nova 2007, 30, 351–355.
- Storck, C.R.; Nunes, G. L.; Oliveira, B.B; Basso, C. Folhas, talos, cascas e sementes de vegetais: composição nutricional, aproveitamento na alimentação e análise sensorial de preparações. Ciência Rural 2013, 43, 537–543.
- Roy, L.G.; Urooj, A. Antioxidant Potency, ph and Heat Stability of Selected Plant Extracts. Journal of Food Biochemistry 2013, 37, 336–342.
- Gonçalves, R.F.; Silva, A.M.S.S.; Silva, A.M.; Valentão, P.; Ferreres, F.; Gil-Izquierdo, A.; Silva, J.B.; Santos, D.; Andrade, P.B; Influence of taro (Colocasia esculenta L. Shott) Growth Conditions on the Phenolic Composition and Biological Properties. Food Chemistry 2013, 141, 3480–3485.
- Beevi, S.S.; Narasu, M.L.; Gowda, B.B. Polyphenolics Profile, Antioxidant and Radical Scavenging Activity of Leaves and Stem of Raphanus sativus L. Plant Foods for Human Nutrition 2010, 65, 8–17.
- ASSOCIATION OF OFFICIAL ANALYTICAL CHEMISTRY (AOAC). Official Methods of Analysis. 19th edition, AOAC International: Gaithersburg, 2012; 3000p.
- Waterhouse, A.L. Polyphenolics Determination of Total Phenolics. In Current Protocols in Food Analytical Chemistry; Wrolstad, R.E,J.; Ed.; Wiley: New York, 2002; 11–18.
- Rufino, M.S.M.; Alves, R.E.; Brito, E.S.; Morais, S.M.; Sampaio, C.G.; Pérez-Jiménez, J.; Saura-Calixto, F.D. Metodologia científica: Determinação da atividade antioxidante total em frutas pela captura do radical livre DPPH. Comunicado Técnico On Line 2007, 127, 1–4.
- Rufino, M.S.M.; Alves, R.E.; Brito, E.S.; Morais, S.M.; Sampaio, C.G.; Pérez-Jiménez, J.; Saura-Calixto, F.D. Metodologia científica: Determinação da atividade antioxidante total em frutas pelo método de redução do ferro (FRAP). Comunicado Técnico On Line 2007, 125, 1–4.
- Rufino, M.S.M.; Alves, R.E.; Brito, E.S.; Filho, E.S.; Moreira, A.V.B. Metodologia científica: Determinação da atividade antioxidante total em frutas no sistema β-caroteno/ácido linoleico. Comunicado Técnico On Line 2007, 126, 1–4.
- Ferreira, D.F. Sisvar: A computer statistical analysis system. Ciência e Agrotecnologia 2011, 35 1039–1042.
- Petersen, M.; Simmonds, M.S.J. Rosmarinic acid. Phytochemistry 2003, 62, 121–125.
- Brum, T.F.; Zadra, M.; Piana, M.; Boligon, A.A.; Fröhlich, J.K.; Freitas, R.B.F.; Stefanello, S.T.; Froeder, A.L.F.; Belke, B.V.; Nunes, L.T.; Jesus, R.S.; Machado, M.M.; Rocha, J.B.T.; Félix Soares, A.A.; Athayde, M.L.; HPLC Analysis of Phenolics Compounds and Antioxidant Capacity of Leaves of Vitex megapotamica (Sprengel) Moldenke. Molecules 2013, 18, 8342–8357.
- Cao, H.; Cheng, W.; Li, C.; Pan, X.; Xie, X.; Li, T. DFT Study on the Antioxidant Activity of Rosmarinic Acid. Journal of Molecular Structure 2005, 719, 177–183.
- Renzulli, C.; Galvano, F.; Pierdomenico, L.; Speroni, E.; Guerra, M.C. Effects of Rosmarinic Acid Against Aflatoxin B1 and Ochratoxin-A-induced Cell Damage in a Human Hepatoma Cell Line (Hep G2). Journal of Applied Toxicology 2004, 24, 289–296.
- Chen, Z.; Peng, C.; Jiao, R.; Wong, Y.M.; Yang, N.; Huang, Y. Antihypertensive Nutraceuticals and Functional Foods. Journal of Agricultural and Food Chemistry 2009, 57, 4485–4499.
- Marrassini, C.; Acevedo, C.; Miño, J.; Gorzalczany, S. Evaluation of Antinoceptive, Anti-inflammatory Activities and Phytochemical Analysis of Aerial Parts of Urtica urens L. Phytotherapy Research 2010, 12, 1807–1812.
- Boots, A.W.; Drent, M.; Boer, V.C.J.; Bast, A.; Haenen, G.R.M.M. Quercetin Reduces Markers of Oxidative Stress and Inflammation in Sarcoidosis. Clinical Nutrition 2011, 30, 506–512.
- Suematsu, N.; Hosoda, M.; Fujimori, Ko. Protective Effects of Quercetin Against Hydrogen Peroxide-induced Apoptosis in Human Neuronal SH-SY5Y Cells. Neuroscience Letters 2011, 504, 223–227.
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. Journal of Agricultural and Food Chemistry 2005, 53, 4290–4302.
- Costa, P.; Gonçalves, S.; Andrade, P.B.; Valentão, P.; Romano, A. Inhibitory Effect of Lavandula Viridison Fe2+ -induced Lipid Peroxidation, Antioxidant and Anti-cholinesterase Properties. Food Chemistry 2011, 126, 1779–1786.
- Liao, W.C.; Lai, Y.C.; Yuan, M.C.; Hsu, Y.L.; Chan, C. F. Antioxidative Activity of Water Extract of Sweet Potato Leaves in Taiwan. Food Chemistry 2011, 127, 1224–1228.
- Kahkonen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant Activity of Plant Extracts Containing Phenolic Compounds. Journal of Agricultural and Food Chemistry 1999, 47, 3954–3962.
- Yu, L.; Haley, S.; Perret, J.; Harris, M.; Wilson, J.; Qian, M. Free Radical Scavenging Properties of Wheat Extracts. Journal of Agricultural and Food Chemistry 2002, 50, 1619–1624.
- Jayaprakasha, G.K.; Patil, B.S. In Vitro Evaluation of the Antioxidant Activities in Fruit Extracts from Citron and Blood Orange. Food Chemistry 2007, 101, 410–418.
- Tsimogiannis, D.I.; Oreopoulou, V. The Contribution of Flavonoids C-ring on the DPPH Free Radical Scavenger Efficiency. A Kinetic Approach for the 30,40-hydroxy Substituted Members. Innovative Food Science & Emerging Technologies 2006, 7, 140–146.
- Yuan, G.; Wang, X.; Guo, R.; Wang Q. Effect of Salt Stress on Phenolic Compounds, Glucosinolates, Myrosinase and Antioxidant Activity in Radish Sprouts. Food Chemistry 2010, 121, 1014–1019.
- Wijngaard, H.H.; Rößle, C.; Brunton, N. A Survey of Irish Fruit and Vegetable Waste and by-products as a Source of Polyphenolic Antioxidants. Food Chemistry, 2009, 116, 202–207.
- Orhan, I.E.; Yilmaz, B.S.; Altun, M.L.; Saltan, G. Radical Quenching Activity, Ferric-Reducing Antioxidant Power, and Ferrous Ion-Chelating Capacity of 16 Ballota. Journal of medicinal food 2010, 13, 1537–1543.
