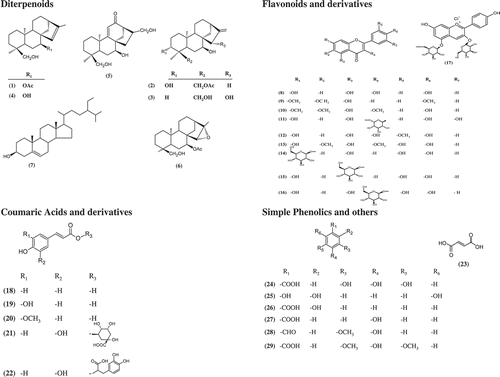
ABSTRACT
Essential oil and phenolic compositions of Sideritis brevibracteata P.H. Davis, which is an endemic species in Turkey and commonly used as a herbal tea for some diseases, were determined by GC-MS and Liquid chromatography–mass spectrometry (LC-MS/MS) techniques. While the main components of the essential oil were determined as caryophyllene, germacrene-D, and α-cadinene, the phenolic compounds quercetagetin-3,6-dimethylether and chlorogenic acid were found to be the main compounds in the extracts of the species. The characteristic diterpenoids of Sideritis species such as siderol, linearol, eubotriol, sideridiol, and athonolone were isolated and their structures were elucidated by 1D and 2D NMR (Nuclear Magnetic Resonance) techniques. Weak inhibitory activity of species against butyryl-cholinesterase was determined. Antioxidant capacity of the acetone and methanol extracts was determined by DPPH free radical scavenging activity, β-carotene linoleic acid assays, and CUPRAC assays.
Introduction
Sideritis L. belongs to the family of Lamiaceae (Labiatae), which is one of the most common and diverse plants in the world, comprising over 150 species.[Citation1] They are mainly found in the Mediterranean area and particularly in Spain and Turkey.[Citation2] Turkey is the 2nd country in the world, having the maximum number of the species of the genus.[Citation3] The Sideritis species are available in two groups, including annual and perennial species in Turkey. While the annual species are included in Hesiodia (Moench) Benth. and Burgsdorfia (Moench) Briq. sections, all of the perennial species are in Empedoclia (Rafin) Benth. section.[Citation3,Citation4] There are 46 Sideritis species (52 taxa) in Turkey and the endemism rate of this genus is as high as almost 80%.[Citation4] As 42 endemic species belong to Empedoclia section, it is not wrong to call Turkey the gene centre of this section.
The therapeutic use of Sideritis species was initially mentioned in the Dioscorides book written in the 1st century, “De Materia Medica”.[Citation2] For centuries, Sideritis species have been used due to their digestive anti-inflammatory, anti-ulcerogenic, and antimicrobial properties as well as to treat common cold, flu, and allergies.[Citation5] Sideritis brevibracteata P.H. Davis, which is an endemic species in Turkey, named “Dağ çayı” or “Alanya adaçayı” in vernacular, grows especially in the Alanya-Antalya region.[Citation6] A large number of studies on members of the genus Sideritis were conducted due to widespread use of its species as folk medicine. Active constituents of the extracts and/or essential oil of the species were identified as terpenes, phenolics, coumarins, and lignans.[Citation7–Citation9]
In previous studies, essential oil, antioxidant, anti-inflammatory, antinociceptive activities, and antimicrobial activities of S. brevibracteata were reported.[Citation6,Citation10,Citation11] Isolation and investigation of the in vitro effects of phenolic compounds, the richest flavonoid content, isolated from the species on bovine kidney Glutathione reductase, are available in the literature.[Citation12] In the present study, we report the biolgical activities, the essential oils, diterpenoids and phenolic profiles of the extracts of S. brevibracteata.
Materials and methods
Plant material
The aerial parts of the species were collected from Antalya: Alanya-Türbelinaz road in July 2014. The species were identified by Dr Tuncay Dirmenci of Balikesir University. Voucher specimens were deposited at the Herbarium of Faculty of Education, Balikesir University, Balikesir, Turkey (Herbarium number EA 5623).
Chemicals
n-Hexane (Merck), acetone (Merck), and methanol (Merck) were used for the preparation of the extracts. The following compounds were used as standards in LC-MS/MS analyses: fumaric acid (99%, Sigma-Aldrich), pyrogallol (98%, Sigma-Aldrich), rutin (94%, Sigma-Aldrich), chlorogenic acid (95%, Sigma-Aldrich), gallic acid (99%, Merck), syringic acid (95%, Sigma-Aldrich), t-ferulic acid (99%, Sigma-Aldrich), caffeic acid (98%, Sigma-Aldrich), pelargonin chloride (98%, Sigma-Aldrich), quercitrin (97%, Sigma-Aldrich), salicylic acid (99%, Sigma-Aldrich), p-coumaric acid (98%, Sigma-Aldrich), luteolin-7-O-glu (99%, AppliChem), rosmarinic acid (96%, Sigma-Aldrich), pyrogallol (98%, Sigma-Aldrich), apigenin (95%, Sigma-Aldrich), kaempferol (96%, Sigma-Aldrich), and isorhamnetin (98%, ExtraSynthese, Genay-France). Siderol (98%), linearol (98%), and eubotriol (96%) were isolated from the species and their purity was determined by quantitaive NMR in TUBITAK, UME. Stock solutions were prepared as 10 mg/L in methanol. Calibration solutions were prepared in methanol in a linear range. Dilutions were performed using automatic pipettes and glass volumetric flasks (A class). In total, 100 mg/L curcumin solution was freshly prepared, from which 50 μL was used as an internal standard (IS) in all experiments.
Isolation of essential oil
The aerial parts of S. brevibracteata, which were air-dried in shade, were chopped into small pieces and subjected to hydro distillation with water for 4 h, using a Clevenger-type apparatus to produce the essential oil (0.1 g from 100 g of the dried aerial parts, yield 0.1% w/w).
Extraction and isolation
The shade-dried powdered plant (600 g) was extracted sequentially with n-hexane, acetone, and methanol at room temperature. The extracts were dried in vacuum at 40 °C, which gave 5.8 g n-hexane (yield: 0.96%), 17 g acetone (yield: 2.83%), and 35.6 g methanol (yield: 5.93%) extracts. Each extract was fractionated on a column chromatography packed with silica gel (500 g, 8x 100 cm), eluting with n-hexane and gradient of chloroform and acetone and methanol. Fractions showing similar spots on TLC were combined to yield seven main fractions (1–7). While fraction 1 possessed mainly fatty contents, fraction 7 gave water-soluble substances, which were not studied further. For fractions 2–6 similar column chromatography (300 g of silica gel, 4 x 60 cm), eluting with n-hexane, and gradient of CHCl3 and acetone were applied, followed by MeOH. Diterpenoids were best eluted with the solvent systems n-hexane:CHCl3 (30–70; v/v) and CHCl3:acetone (70:30; v/v). The fractions were controlled by TLC. For purification of the isolated diterpenoids, preparative TLC on pre-coated silica gel F254 aluminium plate was applied. From the acetone and methanol extracts, siderol (ent-7α-acetyl-18-hydroxykaur-15-ene) (1) was isolated and purified by preparative TLC, using CHCl3: acetone (90:10; 95:5; v/v) solvent system. Linearol (ent-3β,7α-dihydroxy-18-acetoxykaur-16-ene) (2) and eubotriol (ent-7α,15β,18-trihydroxykaur-16-ene) (3) were isolated from n-hexane extract and purified by preparative TLC using CHCl3: acetone (80:20; v/v) and CHCl3 systems, respectively. From the acetone extract, sideridiol (ent-7α, 18β-dihydroxykaur-15-ene) (4) and athonolone (ent-7α,17,18-trihydroxy-9,11-(ene)-12-on) (5) were isolated, applying the CHCl3: acetone (80:20; v/v) system.
For LC-MS/MS studies, 100 g of the shade-dried and powdered plant was extracted with n-hexane for 15 days (named SB 1, 0.25 g). After filtration and evaporation, the residuary plant was extracted with acetone (named SB 2; 3.2 g) and then methanol (named SB 3; 18.02 g). Additionally, the products were extracted directly from 100 g of the plant using acetone and methanol as solvents, named SB 4 (5.1 g) and SB 5 (19.2 g), respectively.
Preparation of the test solution for LC-MS/MS
To 50 mg of each extract (n-hexane, acetone, and methanol) in a round-bottomed flask was added 4 mL of ethanol–water mixture (50:50 v/v). To obtain a good solubility, the flask was refluxed on a water bath until a clear solution was obtained. They were then transferred into a 5 mL volumetric flask and diluted to volume. A portion of 1 mL of this stock solution was transferred into a 5 mL volumetric flask, and 50 μL of curcumin solution was added as IS and diluted to the volume with methanol and mixed. The solution was filtered through a 0.45 µm Millipore Millex-HV filter and the final solution (1 mL) was transferred into a capped auto sampler vial and 10 μL of the sample was injected into LC for each run. The samples in the auto sampler were kept at 15 °C during the experiment.
Instruments and chromatographic conditions
1H- and 13C-NMR spectra were obtained in CDCl3 at 600 and 150 MHz, respectively, using a Varian 600 NMR. Heteronuclear Multiple Quantum Coherence (HMQC) and Heteronuclear Multiple Bond Correlation (HMBC) experiments were recorded on the same spectrometer, using the standard pulse sequence programs. LC-MS/MS experiments were performed by a Zivak® HPLC and Zivak® Tandem Gold Triple quadrupole (Istanbul, Turkey) mass spectrometry, equipped with a Synergy Max C18 column (250 x 2 mm i.d., 5-μm particle size). The mobile phase was composed of water (A, 0.1% formic acid) and methanol (B, 0.1% formic acid), the gradient programme of which was 0–1.00 min 55% A and 45% B, 1.01–20.00 min 100% B, and finally 20.01–23.00 55% A and 45% B. The flow rate of the mobile phase was 0.25 mL/min, and the column temperature was set to 30 οC. The injection volume was kept at 10 μL.
GC/MS conditions
GC-MS was conducted on Thermo Electron Trace 2000 GC model gas chromatography and Thermo Scientific TSQ GC-MS/MS. A Phenomenex DB5 fused silica column (30 m x 0.32 mm, with 0.25 µm film thickness) was used with helium as a carrier gas at 1 mL/min flow rate (20 psi). The GC oven temperature was kept at 60 °C for 10 min and programmed to 220 °C at a rate of 4 °C/min increment and then kept constant at 220 °C for 15 min. The split ratio was adjusted to 1:20, the injection volume was 0.1 mL, and EI/MS was recorded at 70 eV ionization energy. Mass range was m/z 35–500 amu. A homologous series of n-alkanes was used as reference in the calculation of Kovats Indices (KIs). Identification of the compounds was based on the comparison of their retention times and mass spectra with those obtained from authentic samples and/or the NIST and Wiley spectra as well as the literature data.[Citation13] GC-FID was performed using a Thermo Electron Trace GC-FID detector and the same GC program stated above was used.
Optimization of HPLC methods and LC-MS/MS procedure
As described in our previous studies,[Citation14,Citation15] the best mobile phase solution was determined to be a gradient of acidified methanol and water system, which was found to be satisfactory for ionization abundance and separation of the compounds. The good ionization of small and relatively polar antioxidants was obtained by the Electrospray ionization (ESI) source .[Citation15] The optimum ESI parameters were determined as 2.40 mTorr CID gas pressure, 5000.00 V Electrospray ionization (ESI) needle voltage, 600.00 V ESI shield voltage, 300.00 °C drying gas temperature, 50.00 °C API housing temperature, 55 psi nebulizer gas pressure, and 40.00 psi drying gas pressure. Detailed information on the experiment parameters is given in Appendix A, Supplementary data.
Validation of experiments and uncertainty evaluation
The validation parameters were determined to be linearity, repeatability, limit of detection (LOD), and limit of quantification (LOQ) experiments. The linearity of each compound was determined by analysing standard solutions; the ranges of each compound are given in Appendix A, Supplementary data. The correlation coefficients (r2) were found to be ≥0.99. Linear regression equations of the reported compounds are also presented in Appendix A. Detailed procedures of uncertainty evaluation are available in the literature.[Citation14,Citation16,Citation17]
Biological activity
The inhibitory activities of acetyl- and butyryl-cholinesterase were measured by the slightly modified spectrophotometric method, developed by Ellman, Courtney, Andres and Featherston.[Citation16] Acetylthiocholine iodide and butyrylthiocholine iodide were used as substrates of the reaction, and the DTNB method was applied for the measurement of anticholinesterase activity.[Citation18,Citation19] The antioxidant activities were measured based on 2,2-Diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging activity, β-carotene linoleic acid assays, and cupric (Cu2+) ion-reducing power assay (CUPRAC).[Citation19–Citation25] The procedures for determination of the activities are given in Appendix A.
Results and discussion
Essential oil
Sideritis species have a wide range of biological activities due to their varying main chemical compositions (monoterpene, sesquiterpene, diterpene, phenolics), which could be considered as chemotaxonomic markers for Sideritis genus.[Citation5,Citation7,Citation26–Citation29] Sideritis taxa from Turkey is classified into six groups, depending on their main essential oil constituents, which are monoterpene hydrocarbon-rich, oxygenated monoterpene-rich, sesquiterpene hydrocarbon-rich, oxygenated sesquiterpene-rich, diterpene-rich, and others.[Citation10] Most of the Anatolian Sideritis were reported to be poor in essential oil and contained monoterpene hydrocarbons as the major constituents, among which α-pinene, β-pinene, β-phellandrene, sabinene, and myrcene were reported to be in higher amounts.[Citation10,Citation30–Citation31]
GC-FID/GC-MS analyses of the oil, representing 98.6 of the components, resulted in 28 compounds (). The content of the essential oil was found to be rich in sesquiterpenes, while the percentage of the oxygenated sesquiterpenes was low. Although the previous studies reported that the bicyclic sesquiterpene natural product β-caryophyllene (43.3%) is the main compound of S. brevibracteata oil,[Citation10] it was determined to be the main component of S. brevibracteata oil, which was followed by germacrene-D (10.6%) and α-cadinene (10.3%). The essential oil of S. brevibracteata, which is rich in sesquiterpenes and belongs to a sesquiterpenes-rich group, was found to be consistent with the previous reports.[Citation10] β-Caryophyllene (43.3%) was determined as the main component, and this study demonstrated the presence of S. brevibracteata in caryophyllene chemotype, which is known for its anti-inflammatory, local anaesthetic, antimicrobial, and non-allergenic activities.[Citation32–Citation34] Moreover, germacrene D (10.6%) and α-cadinene (10.3%) were analysed to be in high amounts, the antimicrobial activities of which have already been reported.[Citation35]
Diterpenoids
The extracts of S. brevibracteata yielded five known diterpenoids. Their structures were identified to be siderol (ent-7α-acetyl-18-hydroxykaur-15-ene) (1) (22.2 mg), linearol (ent-3β,7α-dihydroxy-18-acetoxykaur-16-ene) (2) (4.3 mg), eubotriol (ent-7α,15β,18-trihydroxykaur-16-ene) (3) (3.2 mg), sideridiol (ent-7α, 18β-dihydroxy kaur-15-ene) (4) (7.4 mg), and athanolone (ent-7α,17,18-trihydroxy-9,11-(ene)-12-on) (5) (3.4 mg) through spectroscopic methods (1H-NMR, 13C-NMR, COSY, HMQC, and HMBC) and comparison with the literature data ().[Citation9,Citation36,Citation37] Structures of the isolated compounds are given in . Additionally, a steroid, stigmasterol (7) (10.4 mg), is fairly present in all Lamiaceae species.
The diterpenoid contents of the extracts were investigated applying LC-MS/MS techniques. Four kaurene diterpenoids, siderol (1), linearol (2), eubotriol (3), and 7-acetyl sideroxol (6), which were reported in our previous study, [Citation5,Citation27–Citation29,Citation36–Citation40] were used as standards in this method. In the extracts, except for compound 6, the rest of the compounds were determined by the LC-MS/MS technique.
According to Fraga, the Mediterranean Sideritis species were divided into four chemotaxonomic groups, considering their content in diterpenes and triterpenes. The Turkish species, belonging to the third group, mainly possess tetracyclic diterpenes of the ent-kaurene type.[Citation41] All of the reported diterpenoids from S. brevibracteata P.H. Davis herein have ent-kaurene skeleton such as other Anatolian Sideritis species.[Citation5,Citation9,Citation27–Citation29,Citation36–Citation40]
Phenolics
Total 22 compounds, composed of 13 phenolic acids, eight flavonoids, and one flavonoid glycoside, were determined in the S. brevibracteata extracts (). In terms of phenolic contents, the acetone extract was determined as the richest extract as expected. The rare 6-OH flavonoids, penduletin, previously isolated from Anatolian Sideritis caesarea,[Citation42] and penduletin and quercetagetin-3,6-dimethylether from S. brevibracteata, were also observed in this study. The amount of quercetagetin-3,6-dimethylether was found to be higher than the other compounds in nonpolar extracts SB 1 (138.17 mg/kg, 59% of the total phenolic profile in the SB1 extract). For the polar extracts, SB 2, SB 3, SB 4, and SB 5, chlorogenic acid was determined to be the main compound (1309.84, 2141.89, 1132.58, and 1787.33 mg/kg, respectively, 60%, 80%, 68%, and 66% of the total phenolic profile).
Table 1. Chemical composition of the essential oil of S. brevibracteata.
Table 2. LC-MS/MS parameters of selected compounds and concentrations (mg/kg) in SB extracts.
Previously, seven phenolic compounds, 7-glycosides of 8-OH-flavones were isolated (hypolaetin, isoscutellarein, and their methyl ethers and verbascoside) from the butanol fraction of the methanol extract of S. brevibracteata.[Citation6,Citation12] The Sideritis species were characterized by the accumulation of 7-glucosides of 8-hydroxyflavones (hypolaetine, 8-hydroxy luteolin), while the Hesiodia section had 7-glycosides of common flavones, such as apigenin, luteolin, and chrysoeriol.[Citation43,Citation44]
Biological activity
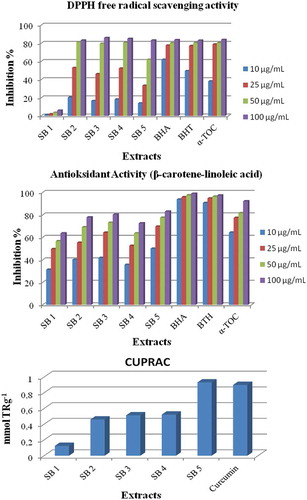
The antioxidant activities of n-hexane, acetone, and methanol extracts were determined in vitro using antioxidant assays (DPPH free radical scavenging activity, β-carotene linoleic acid, and CUPRAC assays). DPPH free radical scavenging effect and β-carotene bleaching were determined at 10, 25, 50, and 100 μg/mL and the results are summarized in . Butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), and α-tocopherol were used as standard compounds in each antioxidant assay. In DPPH free radical scavenging activity assay, all the extracts at 50 ve 100 µg/mL concentrations showed the best activity, except the n-hexane extract. At 100 µg/mL, all the extracts showed higher inhibition activity of 83.12%, 85.92%, 85.06%, and 83.16% in SB 2, SB 3, SB 4, and SB 5, respectively, while the standards had 83.66%, 83.05%, and 83.92% inhibition of BHA, BHT, and α-toc, respectively. In β-carotene linoleic acid assays, all the extracts performed moderate activities. In CUPRAC assays, the positive control curcumin was 0.900 mmol TR g−1. While the methanol extracts, SB 3 and SB 5, showed the best activities, the directly prepared methanol extract was determined to be 0.931 mmol TR g−1. The extracts obtained through the solvent sequence of n-hexane, acetone, and methanol and the extract obtained directly using acetone indicated almost the same scavenging effects.
Figure 2. Antioxidant activities of the S. brevibracteata extracts; inhibition (%) of lipid peroxidation, DPPH free radical scavenging activity of S. brevibracteata extracts and α-Toc, BHA, and BHT and Cu2+ reducing power (CUPRAC) assay of S. brevibracteata extracts and curcumin.
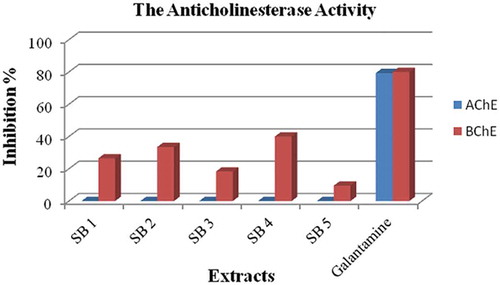
The acetyl-cholinesterase (AChE) and butyryl-cholinesterase (BChE) activities of all the extracts were determined at 200 μg/mL concentrations, for which galanthamine was used as a standard compound. All the extracts did not inhibit AChE and had a slight inhibition of BChE. The acetone extract prepared after n-hexane extraction and the directly prepared acetone extract (SB 2 and SB 4) had relatively weak butyryl-cholinesterase activities ().
Plant phenolics were considered to have antioxidant activities due to their reducing agents, hydrogen-donating properties, and singlet oxygen-quenching acts.[Citation45] Therefore, identifying the phenolic profile of the plant is important. We observed that chlorogenic acid (1309.84, 2141.89, 1132.58, and 1787.33 mg/kg in SB 2, SB 3, SB 4, and SB 5, respectively) is the most abundant phenolic compound in S. brevibracteata extracts. LC-MS/MS results indicated the presence of the highest total phenolic content in methanolic extract of S. brevibracteata (4760.78 mg/kg) (SB 5), which is an important factor in the antioxidant capacities of S. brevibracteata. Phenolic compounds may also act as chemical messengers, physiological regulators, and cell-cycle inhibitors.[Citation46]
All the studied extracts (SB 1, SB 2, SB 3, SB 4, and SB 5) consisted of penduletin, quercetagetin-3,6-dimethylether, caffeic acid, chlorogenic acid, rosmarinic acid, and gallic acid as dominant compounds. The effective antioxidant and anticholinesterase activities of S. brevibracteata extracts might be due to the high level of those phenolic compounds. According to recent reports, chlorogenic acid, caffeic acid, ferulic acid, and quercetin had antioxidant, anticancer, anticarcinogenic, and antimutagenic activities.[Citation47–Citation49] The antioxidant activity of phenolic compounds is an issue of their chemical structures, and the structure–activity relationship of various types of phenolic compounds has been studied.[Citation50–Citation52] These compounds have an aromatic ring containing one or more hydroxyl groups, which are synthesized in plants, from the products of the shikimic acid pathway in plants. These phenolic compounds are believed to intercept the free radical chain of oxidation and donate hydrogen from their phenolic hydroxyl groups.[Citation46] In general, free radical scavenging and antioxidant activities of the phenolic compounds depend on the number of hydroxyl groups (-OH) and their positions on the aromatic rings. For example, flavonol aglycones (quercetin, myricetin, and kaempferol), containing multiple hydroxyl groups, have higher antioxidant activity compared with their glycoside analogues (rutin, myricitrin, and astragalin).[Citation47] Such compounds, having the hydroxyl groups in the meta-position to the carboxyl groups (-COOH), demonstrate better activities. On the other hand, meta-positions of the methoxy groups do not provide such a property, they rather lower the activities, which may explain the importance of the H-donating ability of the hydroxyl groups.[Citation46]
Conclusion
In this study, as a chemotaxonomic marker of Sideritis species, six common diterpenoids were isolated and identified. Linearol (2) and eubotriol (3) were found to be the most abundant diterpenoids in S. brevibracteata. Moreover, 10 flavonoids, 12 phenolic acids, and steroid were determined in apolar and polar extracts. 6-OH derivatives of the flavonoids, i.e. quarcetagenin-3,6-dimethyl ether and penduletin, were determined as the major flavonoids. All of the studied extracts were found to be a rich source of phenolics, while chlorogenic acid was found to be the major unit of the composition. Considering the antioxidant capacity determination assays, such as DPPH, β-carotene linoleic acid, and CUPRAC methods, there is a good relationship between the antioxidant capacity and polyphenolic composition of the extracts of the species. Thus, S. brevibracteata is a promising source of natural antioxidant as used in folkloric.
Appendix_A-revised.docx
Download MS Word (122.2 KB)Acknowledgments
The authors thank TÜBİTAK for supporting this study as a part of the project 113Z710.
References
- Obon de Castro, C.; Rivera-Nunez, D. A Taxonomic Revision of the Section Sideritis Genus Sideritis) (Labiatae); Cramer, J; Eds.; Berlin-Stuttgart, 1994, 86 pp.
- Gonzalez-Burgos, E.; Carretero, M.E.; Gomez-Serranillos, M.P. Sideritis spp.: Uses, Chemical Composition and Pharmacological Activities—A Review. Journal of Ethnopharmacol 2011, 135, 209–225.
- Duman, H.; Kırımer, N.; Ünal, F.; Güvenç, A.; Şahin, P. Türkiye Sideritis L. Türlerinin Revizyonu. Proje No: TÜBİTAK-TBAG-1853 (199T090), 2005 (in Turkish).
- Güvenç, A.; Duman, H. Morphological and Anatomical Studies of Annual Taxa of Sideritis L. (Lamiaceae), with Notes on Chorology in Turkey. Turkish Journal of Botany 2010, 34, 83–104.
- Ertaş, A.; Öztürk, M.; Boga, M.; Topçu, G. Antioxidant and Anticholinesterase Activity Evaluation of Ent-kaurane Diterpenoids from Sideritis arguta. Journal of Natural Products 2009, 72, 500–502.
- Güvenç, A.; Okada, Y.; Akkol, E.K.; Duman, H.; Okuyama, T.; Çalış, I. Investigations of Anti-Inflammatory, Antinociceptive, Antioxidant and Aldose Reductase Inhibitory Activities of Phenolic Compounds from Sideritis brevibracteata. Food Chemistry 2010, 118, 686–692.
- Zengin, G.; Sarıkürkçü, C.; Aktümsek, A.; Ceylan, R. Antioxidant Potential and Inhibition of Key Enzymes Linked to Alzheimer’s Diseases and Diabetes Mellitus by Monoterpene-Rich Essential Oil from Sideritis galatica Bornm. Endemic to Turkey. Records of Natural Products 2016, 10, 195–206.
- Topçu, G.; Gören, A.C. Biological Activity of Diterpenoids Isolated from Anatolian Lamiaceae Plants. Records of Natural Products 2007, 1 (1), 1–16.
- Topçu, G.; Gören, A. C.; Yıldız, Y. K.; Tümen, G. Ent-Kaurene Diterpenes from Sideritis athoa”. Natural Products Letter 1999, 14 (2), 123–129.
- Kırımer, N.; Başer, K.H.C.; Demirci, B.; Duman, H. Essential Oils of Sideritis Species of Turkey Belonging to the Section Empedoclia. Chemistry of Natural Compounds 2004, 40, 19–23.
- Dulger, B.; Gonuz, A.; Bıcan, T. Antimicrobial Studies on Three Endemic Species of Sideritis from Turkey. Acta Bıiologica Cracoviensia Botanica 2005, 47, 153–156.
- Tandoğan, B.; Güvenç, A.; Çalış, I.; Ulusu, N.N. In Vitro Effects of Compound Isolated from Sideritis brevibracteata on Bovine Kidney Cortex Glutathione Reductase. Acta Biochimica Polonica 2011, 58, 471–475.
- Goren, A.C.; Piozzi, F.; Akcicek, E.; Kılıç, T.; Çarıkçı, S.; Mozioğlu, E.; Setzer, W.N. Essential Oil Composition of Twenty-Two Stachys Species (mountain tea) and Their Biological Activities. Phytochemistry Letters 2011, 4, 448–453.
- Gülçin, İ.; Bursal, E.; Şehitoğlu, H.M.; Bilsel, M.; Gören, A.C. Polyphenol Contents and Antioxidant Activity of Lyophilized Aqueous Extract of Propolis from Erzurum Turkey. Food and Chemical Toxicology 2010, 48, 2227–2238.
- Kalin, P.; Gulcin, I.; Goren, A.C. Antioxidant Activity and Polyphenol Content of Cranberries (Vaccinium macrocarpon). Records of Natural Products 2015, 9, 496–502.
- Gören, A.C.; Çıkrıkçı, S.; Çergel, M.; Bilsel, G. Rapid Quantitation of Curcumin in Turmeric via NMR and LC-tandem Mass Spectrometry. Food Chemistry 2009, 113, 1239–1242.
- Goren, A.C.; Bilsel, G.; Bilsel, M. Rapid and Simultaneous Determination of 25-OH-vitamin D2 and D3 in Human Serum by LC/MS/MS: Validation and Uncertainty Assesment. Journal of Chemical Metrology 2007, 1, 1–10.
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherston, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochemical Pharmacology 1961, 7, 88–95.
- Ertaş, A.; Gören, A.C.; Haşimi, N.; Tolan, V.; Kolak, U. Evaluation of Antioxidant, Cholinesterase Inhibitory and Antimicrobial Properties of Mentha longifolia subsp. noeana and Its Secondary Metabolites. Records of Natural Products 2015, 9, 105–115.
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200.
- Miller, H.E. A Simplified Method for the Evaluation Of Antioxidants. Journal of American Oil Chemists’ Society 1971, 45, 91–98.
- Bener, M.; Özyürek, M.; Güçlü, K.; Apak, R. Optimization of Microwave-assisted Extraction of Curcumin from Curcuma longa L. (turmeric) and Evaluation of Antioxidant Activity in Multi-test Systems. Records of Natural Products 2016, 10, 794–799.
- Bener, M.; Shen, Y.; Xu, Z.; Apak, R. Changes of the Anthocyanins and Antioxidant Properties of Concord Grape (Vitis labrusca) Pomace After Acid Hydrolysis. Records of Natural Products 2016, 10, 542–554.
- Özyürek, M.; Bener, M.; Güçlü, K.; Dönmez, A.A.; Selçuk, S.S.; Pırıldar, S.; Meriçli, A.H.; Apak, R. Evaluation of Antioxidant Activity of Crataegus Species Collected from Different Regions of Turkey. Records of Natural Products 2012, 6, 263–277.
- Yılmaz, A.; Boğa, M.; Topçu, G. Novel Terpenoids with Potential Anti-alzheimer Activity from Nepeta obtusicrena. Records of Natural Products 2016, 10, 530–541.
- Kiliç, T.; Yıldız, Y.K.; Gören, A.C.; Tümen, G.; Topçu, G. Phytochemical Analysis of Some Sideritis Species of Turkey. Chemistry of Natural Compounds 2003, 39:5, 453–456.
- Topçu, G.; Gören, A.C.; Kılıç, T.; Yıldız, Y.K.; Tümen, G. Diterpenes from Sideritis trojana. Natural Products Letter 2002, 16, 33–37.
- Topçu, G.; Gören, A.C.; Kılıç, T.; Yıldız, Y.K.; Tümen, G. Diterpenes from Sideritis sipylea and S. dichotoma. Turkish Journal of Chemistry 2002, 26(2), 189–194.
- Topçu,G.; Gören, A.C.; Kiliç, T.; Yildiz, Y.K.; Tümen, G. Diterpenes from Sideritis argyrea. Fitoterapia 2001, 72, 1–4.
- Kılıç, Ö. Essential Oil Composition of Two Sideritis L. Taxa from Turkey: A Chemotaxonomic Approach. Asian Journal of Chemistry 2014, 26, 2466–2470.
- Topçu, G.; Barla, A.; Gören, A.C.; Bilsel, G.; Bilsel, M.; Tümen, G. Analysis of the Essential Oil Composition of Sideritis albiflora using Direct Thermal Desorption and Headspace GC-MS Techniques. Turkish Journal of Chemistry, 2005, 29(5), 525–529.
- Ghelardini, C.; Galeotti, N.; Di Cesare Mannelli, L.; Mazzanti, G.; Bartolini, A. Local Anaesthetic Activity of β-caryophyllene. Il Farmaco 2001, 56, 387–389.
- Sköld, M.; Karlberg, A.; Matura, M.; Börje, A. The Fragrance Chemical β-caryophyllene—Air Oxidation and Skin Sensitization. Food and Chemical Toxicology 2006, 44, 538–545.
- Runyoro, D.; Ngassapa, O.; Vagionas, K.; Aligiannis, N.; Graikou, K.; Chinou, I. Chemical Composition and Antimicrobial Activity of the Essential Oils of Four Ocimum Species Growing in Tanzania. Food Chemistry 2010, 119, 311–316.
- Duarte, M.C.T.; Figueira, G.M.; Sartoratto, A.; Rehder, V.L.G.; Delarmelina, C. Anti-Candida Activity of Brazilian Medicinal Plants. Journal of Ethnopharmacology 2005, 97, 305–311.
- Çarıkçı, S.; Kılıç, T.; Azizoğlu, A.; Topçu, G. Chemical Constituents of Two Endemic Sideritis Species from Turkey with Antioxidant Activity. Records of Natural Products 2012, 6, 101–109.
- Topçu, G.; Ertaş, A.; Özturk, M.; Dinçel, D.; Kılıc, T.; Halfon, B. Ent-kaurane Diterpenoids Isolated from Sideritis congesta. Phytochemistry Letters 2011, 4, 436–439.
- Çarıkçı, S.; Çöl, Ç.; Kılıç, T.; and Azizoğlu, A. Diterpenoids From Sideritis tmolea P.H. Davis. Records of Natural Products 2007, 1/4, 44–50.
- Kılıç, T. A New and Known Diterpenoids From Sideritis stricta Boiss. & Heldr. and Their Biological Activities. Molecules 2006, 11, 257–262.
- Kılıç, T.; Carikci, S.; Topcu, G.; Aslan, I.; Goren, A.C. Diterpenoids from Sideritis condensta. Evaulation of Chemotaxonomy of Sideritis Species and Insecticidal Activity. Chemistry of Natural Compounds 2009, 45/6, 918–920.
- Fraga, B.M. Phytochemistry and Chemotaxonomy of Sideritis Species from the Mediterranean Region. Phytochemistry 2012, 76, 7–24.
- Halfon, B.; Çiftçi, E.; Topçu, G. Flavonoid Constituents of Sideritis caesarea. Turkish Journal of Chemistry 2013, 37, 464–472.
- Gil, M.I.; Ferreres, F.; Marrero, A.; Tomas-Lorente, F.; Tomas-Barberan, F.A. Distribution of Flavonoid Aglycons and Glycosides in Sideritis Species from the Canary Islands and Madeira. Phytochemistry 1993, 34, 227–232.
- Venditti, A.; Frezza, C.; Guarcini, L.; Foddai, S.; Serafini, M.; Bianco A. Phytochemical Study of a Species with Ethnopharmacological Interest: Sideritis romana L. European Journal of Medicinal Plants 2016, 12 (3), 1–9.
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant Activity Relationships of Flavonoids and Phenolic Acids. Free Radical Biology & Medicine 1996, 20, 933–956.
- Köksal, E.; Bursal, E.; Gülçin, İ.; Korkmaz, M.; Çağlayan, C.; Gören, A.C.; Alwasel SH. Antioxidant Activity and Polyphenol Content of Turkish Thyme (Thymus vulgaris) Monitored by LCMS/MS. International Journal of Food Properties doi:10.1080/10942912.2016.1168438.
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant Activity and Phenolic Compounds of 112 Traditional Chinese Medicinal Plants Associated with Anticancer. Life Sciences 2004, 74, 2157–2184.
- Yang, C.S.; Landau, J.M.; Huang, M.T.; Newmark, H.L. Inhibition of Carcinogenesis by Dietary Polyphenolic Compounds. Annual Review Nutrition 2001, 21, 381–406.
- Tapiero, H.; Tew, K.D.; Ba, N.; Mathe, G. Polyphenols: Do They Play a Role in the Prevention of Human Pathologies? Biomedicine and Pharmacotherapy 2002, 56, 200–207.
- Gülçin, I. Antioxidant Activity of L-Adrenaline: An Activity-Structure Insight. Chemico-Biological Interactions 2009, 179, 71–80.
- Gülçin, I. Antioxidant Properties of Resveratrol: A Structure-Activity Insight. Innovative Food Science and Emerging Technologies 2010, 11, 210–218.
- Gülçin, I. Antioxidant Activity of Eugenol- A Structure and Activity Relationship Study. Journal of Medicinal Food 2011, 14, 975–985.
- Kremer, D.; Matevski, V.; Dunkić, V.; Bezić, N.; Stabentheiner, E. Essential Oil Contents and Micromorphological Traits of Stachys iva Griseb. and S. horvaticii Micevski (Lamiaceae). Records of Natural Products 2016, 10, 228–239.
- Corrêa, A.L.; França, H.S.; Tietbohl, L.A.C; Luna, B.N.; Santos, M.G.; FáFreitas, M.F.; Oliveira, A.P.; Rocha, L. Volatile Constituents of Three myrsine L. Species From Brazil. Records of Natural Products 2017, 11, 82–87.
- Hajdari, A.; Mustafa, B.; Kaçiku, A.; Mala, X.; Lukas, B.; Ibraliu, A.; Stefkov, G.; Novak, J. Chemical Composition of the Essential Oil, Total Phenolics, Total Flavonoids and Antioxidant Activity of Methanolic Extracts of Satureja montana L. Records of Natural Products 2016, 10, 750–760.
- Hatipoglu, S.D.; Zorlu, N.; Dirmenci, T.; Goren, A.C.; Ozturk, T.; Topcu, G. Determination of Volatile Organic Compounds in Forty Five Salvia Species by Thermal Desorption-GC-MS Technique. Records of Natural Products 2016, 10, 659–700.
- Morocho, V.; Toro, M.L.; Cartuche, L.; Guaya, D.; Valarezo, E.; Malagón, O.; Ramírez, J. Chemical Composition and Antimicrobial Activity of Essential Oil of Lepechinia radula Benth Epling. Records of Natural Products 2017, 11, 57–62.
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; 4th Ed.; Allured Publishing Corp.: Carol Stream, Illinois 2007; 1–804 pp.
- Koutsaviti, A.; Bazos, J.; Milenković, M.; Pavlović-Drobac, M.; Tzakou, O. Antimicrobial Activity and Essential Oil Composition of five Sideritis Taxa of Empedoclia and Hesiodia Sect. from Greece. Records of Natural Products 2013, 7, 6–14.