ABSTRACT
Tara gum (Mw = 1519 × 103 g/mol) was degraded by hydrogen peroxide in the presence/absence of ascorbic acid, and properties of the products were investigated using gel permeation chromatography, Fourier transform infrared spectroscopy, atomic force microscope, and rheometer methods. Results showed that molecular weight of tara gum decreased with increasing concentration of hydrogen peroxide. The addition of ascorbic acid could sharply increase the degradation rate and degree of tara gum by hydrogen peroxide, which is also confirmed by atomic force microscope. The degradation products kept the structure characteristics of galactomannan. However, they performed different rheological properties. At 1% (w/v) of concentration, DP1 (941 × 103 g/mol) and DP2 (335 × 103 g/mol) prepared by H2O2 exhibited non-Newtonian behavior, while DP3 (35.1 × 103 g/mol) and DP4 (14.2 × 103 g/mol) obtained by hydrogen peroxide plus ascorbic acid displayed Newtonian property. These results may promote the application of tara gum in diverse fields.
Chemical compounds studied in this article
Galactomannan (PubChem CID: 439336); Mannose (PubChem CID: 18950); Galactose (PubChem CID: 6036)
Introduction
Tara gum (TG) is a commercial galactomannan derived from seed endosperms of the Caesalpinia spinose tree.[Citation1,Citation2] The ratio of mannose to galactose in TG is 3:1, it is 2:1 and 4:1 in guar gum and locust bean gum, respectively. Due to the similar functional properties, TG has been considered as an important alternative to relieve supply shortage of the two common galactomannans. Generally, TGs are used as thickener and stabilizer in food and chemical industries.[Citation3] As some positive physiological effects and other advantages of galactomannans, such as easy availability, non-toxicity, biocompatibility and biodegradability, have been reported,[Citation4–Citation6] TG has shown the potential value in terms of food supplement, biomaterial, and biomedicine.[Citation7–Citation10]
However, the application range of TG is greatly limited by its rheological properties which are dependent on the weight-average molecular weight (Mw) of polysaccharide.[Citation1] TG with large Mw presents high viscosity even at low concentration. Like guar gum, it fails to achieve physiologically effective concentrations as a food supplement and might cause throat choke in case of partial hydration.[Citation11,Citation12] Moreover, its high viscosity negatively affects chemical modifications of TG, often leading to poor modifying effects and low solid content of products.[Citation13] Given the above, it is necessary to decrease its viscosity and prepare tara galactomannans with various molecular weights for meeting diverse processing demands in different industries, while the relevant research has rarely been conducted.
Compared with other degradation methods, oxidative degradation by hydrogen peroxide (H2O2) is an economic, non-toxic, and environment-friendly way for polysaccharide depolymerization. In related studies, polysaccharides depolymerization in Fenton-type reaction which based on catalytic decomposition of H2O2 by transition metals have been extensively studied.[Citation14–Citation16] Although it was reported that H2O2 reacting with ascorbic acid (VC) could also cause degradation of mucin,[Citation17] this method has not attracted more attention. Given that VC is deemed safe for human consumption, we degraded TG by using H2O2 in the presence or absence of ascorbic acid in this work. And an investigation was carried out to determine the role of VC in degradation system and to understand the structural features and rheological properties of degradation products using gel permeation chromatography (GPC), Fourier transform infrared (FT-IR) spectroscopy, rheometer, and atomic force microscope (AFM).
Materials and methods
Materials
TG (food grade) was provided in dried form by Shanghai Anmei Co, China. Hydrogen peroxide (30 wt% solution in water), ascorbic acid, sodium nitrate, and other chemicals were of analytical grade and purchased from Chengdu Kelong Chemicals, Ltd., China.
Oxidative degradation of TG
TG was degraded by H2O2 with or without ascorbic acid (VC), respectively. The optimal conditions for two degradation systems (H2O2: 60°C, 4.0 h, pH = 4.5; H2O2–VC: 60°C, 15 min, pH = 4.5, mole ratio = 2:1), including reaction time, temperature, pH, and the ratio of hydrogen peroxide and ascorbic acid, were determined by pre-experiments and employed in subsequent oxidation reactions. An amount of TG (1.00 g) was solubilized in 200 mL of distilled water (Milli-Q, Millipore, Bedford, USA), and stirred at 30°C for 1.0 h, then stood for 10 h at room temperature to ensure complete hydration. Next, degradation agents (H2O2, H2O2+VC) were, respectively, added into the completely hydrated solution, and the reaction was run at 60°C in thermostatic water bath oscillators (250 rpm/min), the pH of solution was maintained at 7.5 by NaOH solution (1 mol/L), then H2O2 system and H2O2+VC system were kept for 4.0 h and 15 min, respectively. The concentrations of H2O2 were 0.4, 0.6, 0.8, 1.0, 50, and 100 mM. Besides, the molar ratio of H2O2 to VC was 2:1, and the concentrations of VC were 0.2, 0.3, 0.4, 0.5, and 5 mM. After reaction, the solution was concentrated to one-third of initial volume using a rotary evaporator (IKA, RV10, Germany) and mixed with triple volume of 95% ethanol under slow stirring, and then stood for 10 h at 4.0°C. Finally, the degradation products (DPs) were obtained by filtration, freeze drying, grinding, and sieving through 120-mesh sieve.
Infrared spectral analysis
TG (2.00 mg) and its DPs (2.00 mg) were dispersed in KBr powders (200 mg), respectively. The FT-IR spectra of samples were obtained using KBr pellet on Fourier transform infrared spectrometer (Nicolet IS10, Thermo Scientific, USA). Thirty-two scans in the region of 4000 and 400 cm−1 were recorded with a resolution of 4 cm−1 for each sample.
Gel permeation chromatography measurement
Gel permeation chromatography (Malvern 270 max GPC, Malvern Instruments, UK) equipped with a TSK gel GMPWXL (7.8 mm×300 mm, Tosoh Bioscience, Japan) chromatographic column, refractive index detector, and light scattering detector with orientations of 7° (LALS) and 90° (RALS) was used to determine the molecular weight of TG and its DPs. Sample solution (2.5 mg/mL, 10 mL) was filtered through a 0.22 μm pore membrane to eliminate dust particles. The injection volume of sample was 100 μL. The eluent was 0.1 mol/L NaNO3 at a flow rate of 0.8 mL/min under 40°C elution temperature. The specific refractive index increment (dn/dc) adopted is 0.145.[Citation18] Before measurements, the apparatus was calibrated using pullulan P-50 standard which was purchased from Sigma-Aldrich Co. Weight-average molecular weight (Mw), number average molecular weight (Mn), and polydispersity (Mw/Mn) of samples were calculated by OmniSEC software, version 4.7.
Rheological measurements
The rheological measurements of all samples were performed using a rotational rheometer (MARS III, HAKKE, Germany) equipped with a temperature-controlled system and a cone-and-plate geometry (35 mm or 60 mm diameter, cone angle 1°, 0.05 mm gap). In order to relax the samples before the rheological measurements, all samples were loaded and left on the platen at 25°C for 5 min. All experimental data were recorded and analyzed by using Rheowin Job and Data Manager software (version 4.30, HAAKE, Germany).
For steady tests, samples were sheared continuously at the rate ranging from 0.01 to 100 s−1 at 25°C. For dynamic viscoelastic test, oscillatory stress sweeps between 0.1 and 10 Pa were carried out at a frequency of 1 Hz first to establish a linear viscoelastic range. And then frequency sweeps from 0.01 to 100 Hz were performed at a fixed shear stress (1.1 Pa) which was in the linear viscoelastic range to obtain the storage modulus (G′), loss modulus (G″), and complex viscosity (η*). The dynamic tests were conducted at 25°C.
AFM measurement
Atomic force microscopy (AFM) was used to observe the morphologies of TG and its DPs. The aqueous solution of TG/DP was stirred at 30°C for 1.0 h, and kept overnight at room temperature, then followed with a centrifugation at 4000 rpm for 30 min at 25°C. The supernatant was collected and diluted 10 times. About 3 μL of the diluted solution was carefully spread onto a new mica using a micropipette. The spread sample was dried in air for 24 h at room temperature before test. The AFM observations were performed on a Dimension 3100 Nanoscope IV equipped with Silicon TESP cantilevers (SPM-9600, SHIMADZU Co., Japan) in tapping mode. Each sample was analyzed repeatedly to confirm the consistency of observed morphologies.
Statistical analysis
All GPC and rheological tests were conducted in triplicate, and relevant data were presented as mean value ± standard deviation (SD). Differences were considered to be statistically significant at P < 0.05. Statistical analysis was performed with software Origin 8.0 (OriginLab, Northampton, MA).
Results and discussion
Molecular weight parameters
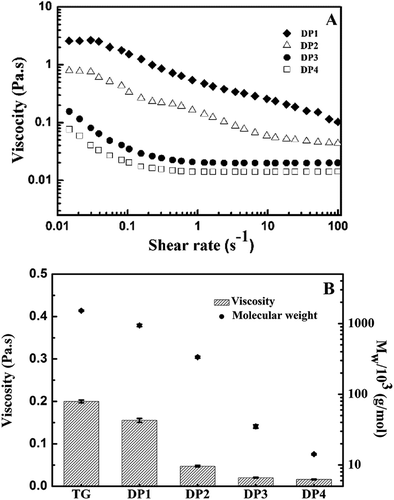
The molecular weights (Mw and Mn) and polydispersities (Mw/Mn) of TG and its DPs oxidized by different dosages of degradation agents are presented in . It was found that the native TG had high Mw with narrow molecular weight distribution (MWD) (Mw/Mn = 1.154). After oxidative treatments, the molecular weight of TG decreased with the increasing concentration of degradation agents (H2O2; H2O2+VC), and the Mw/Mn of DPs had no obvious changes compared with that of TG, which indicated that the components of DP were relatively homogeneous.[Citation19]
Table 1. Molecular weight of TG and degradation products.
In general, the degradation of TG by H2O2 was predominantly attributed to free radical reactions. H2O2 is a very weak acid with a pKa of 11.6, it reacts with the perhydroxyl anion to form highly reactive hydroxyl radical, which results in the scission of polysaccharide chains.[Citation20] The DPs maintained relatively high Mw (above 1000 × 103 Da) when low concentration of H2O2, ranging from 0.4 to 1.0 mM, was used. However, obvious decreases in Mw occurred after TG reacting with high concentrations of H2O2 (from 10 to 100 mM) for 4 h, the lowest Mw of DP was 62.4 × 103 g/mol. It implied that single addition of H2O2 could lead to the degradation of TG, and the degradation degree was quite dependent on H2O2 concentration.
In contrast with H2O2 system, H2O2+VC system caused the degradation of TG, with resulting reduction of Mw from 1519 × 103 to 35.1 × 103 g/mol during 15 min even at low concentration of degradation agent (0.4 mM H2O2 and 0.2 mM VC). It was proved that VC could facilitate the generation of H2O2 from oxygen and increase the •OH levels by catalyzing decomposition of H2O2.[Citation17,Citation21–Citation23] Thus, the presence of VC had a great impact on the degradation degree of TG, and the degradation rate in H2O2+VC system was much faster than that in H2O2 system. The above results suggested that the various Mw of TG galactomannans with narrow MWD could be prepared via the selection of degradation system (H2O2, H2O2+VC) and dosage control of degradation reagents.
FT-IR spectroscopy
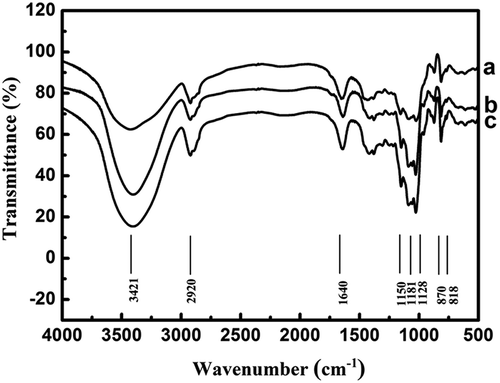
TG and DPs prepared by H2O2 and H2O2+VC system were analyzed by infrared spectroscopy that is employed as an effective tool to estimate changes in polymer structure. As shown in , the FT-IR spectra of DPs were similar to that of TG, they presented common characteristic absorption peaks of galactomannan. Among of all characteristic absorption peaks, the peaks at 818 and 870 cm–1 show the existence of α-d-galactopyranose units and β-d-mannopyranose units, respectively.[Citation8,Citation24] The peak at 1150 cm–1, which shows the bending vibrational modes of C–O, results from the presence of pyranose ring. The spectrum range of 1150–950 cm−1 reported in literature is a characteristic contribution of the C–OH bending vibration. The broad bands between 2800 and 3000 cm–1 and 3100 and 3500 cm–1 represent the C–H and O–H stretching vibration of polysaccharide, respectively.[Citation25] Besides, the intensity of hydroxyl absorption peak (3421 cm–1) was strengthened with an increasing of degradation degree, which indicated the formation of a large number of hydroxyls due to the cleavage of glycoside bonds. These results further confirmed that the oxidations induced by H2O2 in absence or presence of VC were effective methods to degrade TG without changing basic structure of galactomannan, and the addition of VC could substantially increase the extent of degradation of TG.
AFM observation
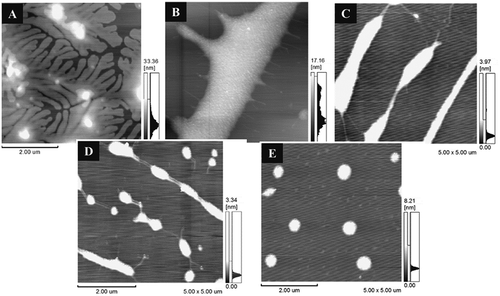
Polysaccharide is an important biological macromolecule showing potential application value in food and biomedicine due to its physicochemical and biological features.[Citation26] These functional properties are predominantly affected by their chemical structures and chain conformations in solution. Therefore, the molecular morphologies of DPs were investigated by AFM for a better understanding of their own properties. The polysaccharide chains of TG can form a network through intertwining each other, making the solution display certain viscosity.[Citation27] As shown in AFM images, TG in aqueous solution displayed branchy polymers after complete swelling (). After being degraded by 10 mM H2O2, its side-branch structures were destroyed (DP1, ), but DP1 kept the main backbone structure. Thus, its Mw was still up to 941 × 103 g/mol. As the amount of H2O2 increased to 50 mM, the molecular chains of TG suffered severe destruction by oxidation and performed long rod-like morphology (DP2, Fig. 2C). Therefore, the Mw of DP2 (335 × 103 g/mol) was lower than that of DP1. Compared with the H2O2 system, TG directly degraded to short stick-like aggregates with weak linkages (DP3, Fig. 2D) using H2O2 (0.4 mM) and VC (0.2 mM), its Mw was sharply decreased to 35.1 × 103 g/mol. A continuing increase in the concentration of degradation agent (1.0 mM H2O2, 0.5 mM VC) resulted in a mild decrease in molecule size, and DP4 exhibited granular shape () and its Mw was slightly reduced to 14.2 × 103 g/mol. As discussed above, AFM effectively observed the variation of microcosmic morphology of tara galactomannans. The observations were consistent with the changes of molecular weight of TG under the action of degradation agents.
Steady flow behavior
Rheological study is essential to understand structural features of polysaccharide and to determine its potential functionality in various fields. Thus, the flow behaviors of four DPs were recorded using a rotational rheometer. As shown in , the viscosities of DP1 and DP2 gradually decreased with the increase of shear rate at the tested concentration (0.5%, w/v) due to the unfolding of entangled polysaccharide chain. This indicated that DP1 and DP2 exhibited a characteristic of pseudoplastic fluid.[Citation1] Their shear-thinning nature is similar with native TG (in the online supplementary information (SI) file 1). Nevertheless, the viscosity of DP3 and DP4 remained steady at a shear rate ranging from 0.1 to 100 s−1, which could be regarded as Newtonian region after the rapid and slight decline of viscosity that occurred at a low shear rate range (0.01–0.1 s−1). The rheological characteristic of tara galactomannans manifested a shift from typical non-Newtonian behavior to Newtonian behavior with the decrease in Mw on account of a more complete depolymerization of polysaccharide. Furthermore, shows that the apparent viscosity of tested samples decreased with the lowering of Mw at the shear rate of 50 s–1. H2O2 and VC reacted to cause a drastic reduction in apparent viscosity of TG from 200 to 19 mPa·s, but the viscosity gap between degradation products became narrowed when their Mw was less than 35.1 × 103 g/mol. These different viscous features of DPs will contribute to increase the diversity of functional properties and broaden the scope of applications for TG galactomannans.
Viscoelastic behavior
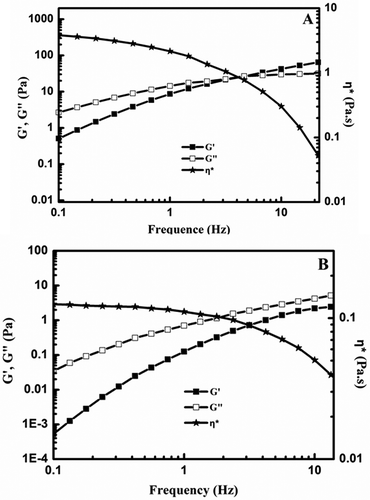
The frequency sweep experiments were done at the constant stress of 1.1 Pa, where was the linear viscoelastic region. The mechanical spectra of DP1 and DP2 (1%, w/v) are shown in . Both of them show the typical shape of macromolecular solutions and the self-consistent shear-thinning character at the same concentration. The complex viscosity (η*) of DP1 and DP2 decreased as the frequency increased. As for DP1, the loss modulus (G″) was higher than the storage modulus (G′) at low frequencies, which exhibited the viscous property as a liquid. Whereas, the G′ was predominant until a crossover point appeared at 4.95 Hz (), which behaved elastic property of solid-like rather than viscous one due to the formation of entanglement network.[Citation28] However, shows that the crossover frequency for DP2 was never observed in spectra where the G″ was always superior to the G′ at tested range (0.01–100 Hz), indicating that the DP2 at 1% concentration displayed viscoelastic characteristics rather than gel properties. In comparison, the elastic feature of native TG (1%, w/v) with the crossover frequency of 2.8 Hz (G′ > G″ at higher frequencies), as shown in SI file 2, was more remarkable than that of DP1 and DP2,[Citation29] which means the gum solutions have more viscous behavior as the extent of degradation increases.
Conclusion
The treatment of H2O2 in the presence or absence of VC could cause the degradation of TG at different extents by free radicals. The addition of VC not only accelerated degradation rate of TG in H2O2 but also increased its degradation degree. The prepared products maintained the structural features of galactomannan, while their molecular morphology changed a lot, which visually confirmed the reduction in molecular weight and viscosity of TG. Among the DPs, DP1 and DP2 prepared by H2O2 system performed in non-Newtonian, shear-thinning properties and possessed different viscoelasticity. DP3 and DP4 prepared by H2O2+VC system at 1% (w/v) concentration showed the Newtonian behavior with very low viscosity. The present work may enlarge the application of TG in diverse fields.
Supplement_material_2__Wu_et_al._.tif
Download TIFF Image (7.8 MB)Supplement_material_1__Wu_et_al._.tif
Download TIFF Image (7.8 MB)Funding
This work is financially supported by National Natural Science Foundation of China (31171656) and Sichuan Youth Sci-tech Fund (2010JQ0009).
Supplemental data
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Sittikijyothin, W.; Torres, D.; Gonçalves, M.P. Modelling the Rheological Behaviour of Galactomannan Aqueous Solutions. Carbohydrate Polymers 2005, 59(3), 339–350.
- Borzelleca, J.F.; Ladu, B.N.; Senti, F.R.; Egle, J.L. Evaluation of the Safety of TG as a Food Ingredient: A Review of the Literature. International Journal of Toxicology 1993, 12(1), 81–89.
- Srivastava, M.; Kapoor, V.P. Seed Galactomannans: An Overview. Chemistry & Biodiversity 2005, 2(3), 295–317.
- Flammang, A.M.; Kendall, D.M.; Baumgartner, C.J.; Slagle, T.D.; Choe, Y.S. Effect of a Viscous Fiber Bar on Postprandial Glycemia in Subjects with Type 2 Diabetes. Journal of the American College of Nutrition 2006, 25(5), 409–414.
- Grabitske, H.A.; Slavin, J.L. Gastrointestinal Effects of Low-Digestible Carbohydrates. Food Science and Nutrition 2009, 49(4), 327–360.
- Slavin, J.L.; Greenberg, N.A. Partially Hydrolyzed Guar Gum: Clinical Nutrition Uses. Nutrition 2003, 19(6), 549–552.
- Avachat, A.M.; Dash, R.R.; Shrotriya, S.N. Recent Investigations of Plant Based Natural Gums, Mucilages and Resins in Novel Drug Delivery Systems. Indian Journal of Pharmaceutical Education and Research 2011, 45(1), 86–99.
- Figueiró, S.D.; Góes, J.C.; Moreira, R.A.; Sombra, A.S.B. On the Physicochemical and Dielectric Properties of gluTGldehyde Crosslinked Galactomannan-collagen Films. Carbohydrate Polymers 2004, 56(3), 313–320.
- Gennadios, A. U.S. Patent No. 6,214,376. Washington, DC: U.S. Patent and Trademark Office. 2001.
- Slavin, J.L.; Greenberg, N.A. Partially Hydrolyzed Guar Gum: Clinical Nutrition Uses. Nutrition 2003, 19(6), 549–552.
- Vanderbeek, P.B.; Fasano, C.; O’Malley, G.; Hornstein, J. Esophageal obstruction from a hygroscopic pharmacobezoar containing glucomannan. Chinese Food Additives. Clinical Toxicology 2007, 45(1), 80–82.
- Roberts, K.T. The Physiological and Rheological Effects of Foods Supplemented with Guar Gum. Food Research International 2011, 44(5), 1109–1114.
- Ellis, P.R.; Dawoud, F.M.; Morris, E.R. Blood Glucose, Plasma Insulin and Sensory Responses to Guar-containing Wheat Breads: Effects of Mw and Particle Size of Guar Gum. British Journal of Nutrition 1991, 66(03), 363–379.
- Haber, F.; Weiss, J. The Catalytic Decomposition of Hydrogen Peroxide by Iron Salts. In Mathematical, Physical and Engineering Sciences; William Pope, F.R.S., Eds.; The Royal Society: London, 1934; 332–351.
- Christensen, B.E.; Myhr, M.H.; Smidsrød, O. Degradation of Double-stranded Xanthan by Hydrogen Peroxide in the Presence of Ferrous Ions: Comparison to Acid Hydrolysis. Carbohydrate Research 1996, 280(1), 85–99.
- Wu, M.; Xu, S.; Zhao, J.; Kang, H.; Ding, H. Free-radical Depolymerization of Glycosaminoglycan from Sea Cucumber Thelenata Ananas by Hydrogen Peroxide and Copper Ions. Carbohydrate Polymers 2010, 80(4), 1116–1124.
- Robertson, W.V.B.; Ropes, M.W.; Bauer, W. The Degradation of Mucins and Polysaccharides by Ascorbic Acid and Hydrogen Peroxide. Biochemical Journal 1941, 35 (8–9), 903.
- Pollard, M.A.; Kelly, R.; Fischer, P.A.; Windhab, E.J.; Eder, B.; Amadò, R. Investigation of Molecular Weight Distribution of LBG Galactomannan for Flours Prepared from Individual Seeds, Mixtures, and Commercial Samples. Food Hydrocolloids 2008, 22(8), 1596–1606.
- Gavrilov, M.; Monteiro, M.J. Derivation of the Mw Distributions from Size Exclusion Chromatography. European Polymer Journal 2015, 65, 191–196.
- Tian, F.; Liu, Y.; Hu, K.; Zhao, B. Study of the Depolymerization Behavior of Chitosan by Hydrogen Peroxide. Carbohydrate Polymers 2004, 57(1), 31–37.
- Deutsch, J.C. Ascorbic Acid Oxidation by Hydrogen Peroxide. Analytical Biochemistry 1998, 255(1), 1–7.
- Isbell, H.S.; Frush, H.L. Oxidation of L-ascorbic Acid by Hydrogen Peroxide: Preparation of L-threonic acid. Carbohydrate Research 1979, 72, 301–304.
- Kivelä, R., Gates, F.; Sontag-Strohm, T. Degradation of Cereal Beta-glucan by Ascorbic Acid Induced Oxygen Radicals. Journal of Cereal Science 2009, 49(1), 1–3.
- Cerqueira, M.A.; Souza, B.W.; Simões, J.; Teixeira, J.A.; Domingues, M.R.M.; Coimbra, M.A.; Vicente, A.A. Structural and Thermal Characterization of Galactomannans from non-Conventional Sources. Carbohydrate Polymers 2011, 83(1), 179–185.
- Yuen, S.N.; Choi, S.M.; Phillips, D.L.; Ma, C.Y. Raman and FTIR Spectroscopic Study of Carboxymethylated Non-starch Polysaccharides. Food Chemistry 2009, 114(3), 1091–1098.
- DeMan, J.M.; Voisey, P.W.; Rasper, V.F.; Stanley, D.W. Rheology and Texture in Food Quality; AVI Publishing Co. Inc: Westport, USA, 1976; 28–78.
- Morris, E.R.; Cutler, A.N.; Ross-Murphy, S.B.; Rees, D.A.; Price, J. Concentration and Shear Rate Dependence of Viscosity in Random Coil Polysaccharide Solutions. Carbohydrate Polymers 1981, 1, 5–21.
- Richardson, R.K.; Ross-Murphy, S.B. Non-linear Viscoelasticity of Polysaccharide Solutions. 1: Guar Galactomannan Solutions. International Journal of Biological Macromolecules 1987, 9(5), 250–256.
- Wu, Y.; Ding, W.; Jia, L.; He, Q. The Rheological Properties of Tara Gum (Caesalpinia spinosa). Food Chemistry 2015, 168(1), 366–371.