Abstract
Macamides are bioactive and marker compounds of maca (Lepidium meyenii). Thirty-five commercial maca products were surveyed for macamide composition and content by HPLC-UV/MS. Significant variations of macamide content were found in these products (69–2738 μg/g). Analysis of the macamide biosynthetic pathway suggests that: (a) glucosinolate catabolism, (b) lipid hydrolysis, and (c) amide formation are key steps controlling macamide accumulation in the tissues during the postharvest drying process. Therefore, we further investigated the effects of sample forms, drying temperatures, and storage times on macamide accumulation. Our results show that (1) powdered maca provided the largest macamide accumulation followed by sliced hypocotyls, while whole roots displayed a significantly reduced amide-generating potential; (2) the ideal temperature for macamide formation is about 30°C; (3) macamide content increases continuously along with storage time; (4) exposure to air results in the percentage of unsaturated macamides decreasing. These findings provide useful insights which can be applied in the industrial manufacture of maca products with higher content of bioactive amides.
Introduction
Maca (Lepidium meyenii) is a Peruvian herbaceous plant of Brassicaceae family, which grows in the central Andes. Its root has been used as both food and traditional medicine for centuries.[Citation1] Biochemical and pharmacological studies have shown that maca has various health-related properties, including beneficial effects on fertility, sexual behavior, energy, memory, osteoporosis, prostate, and skin.[Citation1,Citation2] Because of the above advantages, worldwide demand for maca has increased significantly over the past decade. In 2011, maca powder was authorized by Ministry of Health of the People’s Republic of China to be a new resource food, which legalized maca products in China.
Maca contains a variety of secondary metabolites, such as macamides,[Citation3] glucosinolates,[Citation4] and flavonolignans.[Citation5] Macamides are the bioactive and marker compounds that have only been found in maca so far.[Citation6] In recent years, cannabimimetic action and neuroprotective effects of macamides have been reported by several studies.[Citation7–Citation11] As fatty acid amide hydrolase (FAAH) inhibitors, macamides might act on the central nervous system by modulating the release of neurotransmitters.[Citation7] The positive effect of macamides in Alzheimer’s disease was proved by their protective activity against amyloid β peptide-induced neurotoxicity in B-35 neuroblastoma cells.[Citation11] Besides, Liu et al. reported that macamides showed a positive effect on preventing osteoporosis.[Citation12] Therefore, the investigation of macamides in commercial maca products and the major influence factors have great significance for both edible and medicinal usage.
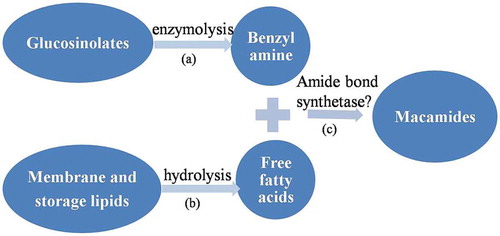
Recently, Esparza et al.[Citation3] discovered that macamides are a result of traditional postharvest drying practices and depicted a putative mechanism of macamide biosynthesis. Macamides are a series of long-chain fatty acid N-benzylamides, which were formed by one benzylamine or 3-methoxybenzylamine and one fatty acid through an amide bond.[Citation7] It was believed that benzylamine and 3-methoxybenzylamine are conversion products formed during degradation of glucosinolate, while free fatty acids are hydrolysis products of membrane and storage lipid.[Citation3] Therefore, we believe that factors at play during postharvest drying have direct impacts on (a) the enzymatic processing of glucosinolates to amines, (b) lipid hydrolysis, and (c) amide bond formation and that all these events affect macamide biosynthesis ().
So far, the reports of macamides in commercial maca products are limited, especially for the products from China. Pan et al. reported that different drying methods significantly affected the content of macamides in maca products.[Citation13] However, research is lacking on the impact that specific factors of maca postharvest processing have on final macamide content. In this study, 35 commercial maca products were surveyed to analyze macamide composition and content by HPLC-UV/MS. Then the influence factors, such as sample forms, drying temperatures, and storage times were further investigated. These results were anticipated to provide information about macamides in commercial maca products and to give guidance on maca industrial processing.
Materials and methods
Reagents and plant materials
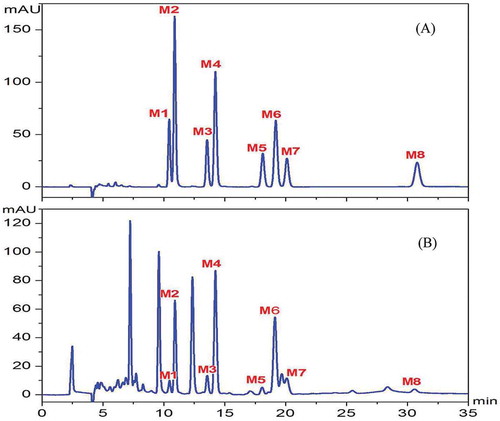
Eight macamide reference substances, including (M1) N-(m-methoxybenzyl)-9Z, 12Z, 15Z-octadecatrienamide, (M2) N-benzyl-9Z, 12Z, 15Z-octadecatrienamide, (M3) N-(m-methoxybenzyl)-9Z, 12Z-octadecadienamide, (M4) N-benzyl-9Z, 12Z-octadecadienamide, (M5) N-(m-methoxybenzyl)-hexadecanamide, (M6) N-benzyl-hexadecanamide, (M7) N-benzyl-9Z-octadecenamide, and (M8) N-benzyl-octadecanamide, were prepared by our lab (; Supplementary data A-B). HPLC-grade acetonitrile was obtained from Fisher Chemicals (USA). HPLC-grade water was purchased from Hangzhou Wahaha Group Co., Ltd. Other chemicals used in this study were of analytical grade and bought from Beijing Chemical Works.
Table 1. Macamides examined in this study.
Thirty-five maca products, including 8 fresh roots, 12 dried slices, and 15 essential tablets, were collected from different places or vendors during 2012–2014 (). Fresh maca roots for further investigation were obtained from Wenbi mountain of Lijiang, Southwest China (100°25′E, 26°86′N) at 3200 masl in January 2014.
Table 2. Information of maca products examined in this study.
Sample preparation
These samples were identified and authenticated by Prof Bing Zhao, Institute of Process Engineering, Chinese Academy of Sciences, and further confirmed using DNA-based barcoding of ITS sequence.[Citation14] To extract macamides sufficiently, the collected fresh roots were cut into slices and dried under some certain conditions. All collected maca samples were ground and passed through a 40-mesh sieve before extraction.
Extraction of macamides was prepared as follows. A 1.00 g portion of sample powder was extracted ultrasonically (KQ5200DB, Kunshan, China) with 40 ml petroleum ether (60–90°C) for 15 min under 200 W at 50°C. The mixture was centrifuged at 5000 rpm for 10 min. Then the supernatant was concentrated to dryness at 50°C by a rotary evaporator (RE52AA, YaRong, Shanghai, China). The residue was made up to exactly 5 ml with acetonitrile using a volumetric flask. The sample was filtered through a 0.22 μm filter before HPLC analysis.
HPLC-UV/MS analysis
The HPLC-UV analysis of macamides was carried out on a Shimadzu LC-20AT consisting of an auto-sampler and binary pump system (Shimadzu, Kyoto, Japan) coupled with a diode array detector. A 10 μL aliquot of each sample solution was injected and analyzed using a Waters XTerra C18 reversed-phase column (250 mm × 4.6 mm, 5 μm; Waters, Milford, MA, USA) with an isocratic elution (acetonitrile: water:formic acid, 90:9.98:0.02) for 35 min. The flow rate was set at 0.6 mL/min, and the column temperature was 30°C. HPLC chromatograms of macamide reference substances and sample extraction recorded at 210 nm are shown in . The HPLC-MS analysis for macamide composition was carried out on an Agilent Q-TOF 6540 (Angilent Technologies, Palo Alto, CA, USA). The spectrums are shown in Supplementary data C.
Statistical analysis
Values are presented as means ± SD. All measurements were compared statistically by the IBM SPSS statistics 19.[Citation15] Differences with p < 0.05 were considered significant.
Results
Composition and content of macamides in commercial maca products
A total of 35 maca products, including 8 fresh roots, 12 dried slices, and 15 essential tablets, were collected, most of them originating from Yunnan province, the main growing region in China. The composition and content of macamides in these samples were detected and analyzed ().
Figure 3. Macamide composition (A) and total content (B) in 35 maca products of three different types. (C) is the Box-plot of total macamide content in three types of maca products. Median values are the horizontal lines in the box. Different letters on the top of each column showed the statistical differences (p < 0.05).
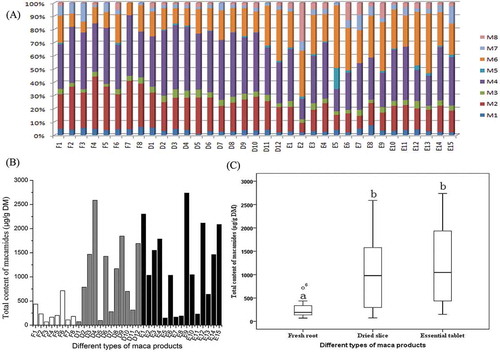
Macamide compositions in those products are shown in . Macamides (M2) N-benzyl-9Z, 12Z, 15Z-octadecatrienamide, (M4) N-benzyl-9Z, 12Z-octadecadienamide, and (M6) N-benzyl-hexadecanamide are the major components, which account for 70–92% of total content. Moreover, an interesting phenomenon was observed that fresh root had slightly higher content of unsaturated macamides (M1–M4 and M7) than dried slice and essential tablet. In fresh root, they took up 72–92% of total content, with an average of 84%. While in dried slice, it was 58–86%, mean of 77%, and in essential tablet, the ratio was 34–74% with a mean value of 60%.
Macamide contents in those products are shown in and C and Supplementary data D, which varied dramatically within and among product types. The total contents of macamides in 8 fresh maca products ranged from 69 to 714 μg/g DM, while the macamide contents were from 74 to 2588 μg/g DM in dried slices, and 148 to 2738 μg/g DM in essential tablets. Big differences can be observed among different maca products. The total content of macamides in fresh root is significantly lower than those in dried slice and essential tablet evaluated by statistical analysis, while no statistical difference was found between dried slice and essential tablet (Fig. 3C). Esparza et al. reported that macamides are absent from fresh maca roots.[Citation3] Therefore, the low contents of macmaides in these 8 fresh maca products are likely the product of bruising of the hypocotyls during harvest and transport. Moreover, the contents of glucosinolates were determined and list in Supplementary data D. A significant negative correlation (r = −0.397, p < 0.05; Supplementary data E) was found between the contents of macamides and glucosinolates, which further supported that glucosinolate catabolism is the key step affecting macamide accumulation.
Effect of sample forms
A batch of maca root was collected from the same place at the same time and made into three forms: whole root, slice, and powder. After one year and three months of storage at room temperature, the composition and content of macamides were analyzed (). The compositions of macamides in different forms of maca sample are shown in . Similar compositions were found in the three forms of maca. Macamides (M2) N-benzyl-9Z, 12Z, 15Z-octadecatrienamide, (M4) N-benzyl-9Z, 12Z-octadecadienamide, and (M6) N-benzyl-hexadecanamide are still the major components. Maca powder exhibited slightly higher content of (M6) N-benzyl-hexadecanamide, while whole root showed a slightly higher content of (M4) N-benzyl-9Z, 12Z-octadecadienamide. The percentage of unsaturated macamides in whole root (67%) was higher than those in slice (61%) and powder (61%).
Figure 4. The composition (A) and total content (B) of macamides affected by different sample forms. Different letters on the top of each column showed the statistical differences (p < 0.05).
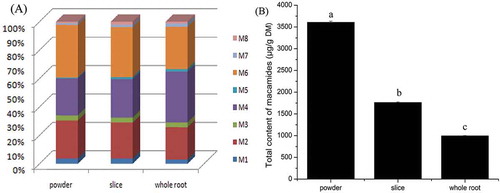
Significant statistical differences can be observed in the three different forms of maca, though the samples were of the same origin and dried under the same conditions (). The highest content of macamides (3607 μg/g DM) was observed in maca powder, followed by maca slice (1762 μg/g DM) and whole root (995 μg/g DM).
Effect of drying temperatures
Fresh maca roots were chopped into pieces and dried at 4, 20, 30, 40, 60, and 80°C for one week. shows the composition of macamides in these samples. Macamides (M2) N-benzyl-9Z, 12Z, 15Z-octadecatrienamide, (M4) N-benzyl-9Z, 12Z-octadecadienamide, and (M6) N-benzyl-hexadecanamide are the major components, which account for more than 85% of total content. Due to the low content, (M5) N-(m-methoxybenzyl)-hexadecanamide and (M8) N-benzyl-octadecanamide can not be detected in these samples. The percentage of unsaturated macamides increased from 47% to 85% as the drying temperature increased from 4 °C to 60°C. But for the sample dried at 80°C, unsaturated macamides took up 74% of total.
Figure 5. Composition (A) and total content (B) of macamides in maca affected by different drying temperatures. Different letters on the top of each column showed the statistical differences (p < 0.05).
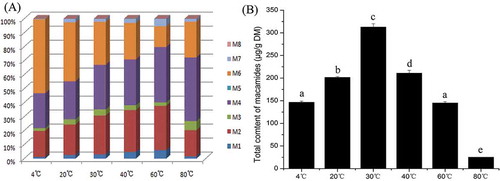
The drying temperature showed a significant impact on macamide content (). The highest content of total macamides was found in maca root slices dried at 30°C (313 μg/g DM), followed by sample dried at 40°C (211 μg/g DM) and 20°C (202 μg/g DM). There was no significant difference of macamide content between maca dried at 4°C (147 μg/g DM) and 60°C (145 μg/g DM). For the root silce dried at 80°C, only trace amount of macamides was observed (25 μg/g DM).
Effect of storage times
Fresh maca root slices were dried at room temperature for one week. After being ground and passed through a 40-mesh sieve, the powder was put into a plastic ziplock bag and kept at 4°C. The HPLC chromatograms, composition, and content of macamides in this sample were checked every 30 days for 180 days (). is the HPLC chromatogram of macamide extracts at 0, 60, 120, and 180 days of storage. Macamide peaks (M1–M8) became taller with longer storage times. At the same time, significant decreases of peak p1 and p2 can be observed obviously. The changes of these peaks illustrated that the contents of free acids, linolenic acid (p1) and linoleic acid (p2), progressively reduced as the contents of macamides progressively increased, which confirmed that free fatty acids were biosynthesis precursor of macamides.
Figure 6. The HPLC chromatograms of macamides in maca samples after storage of 0, 60, 120, and 180 days (A). The composition (B) and total content (C) of macamides affected by different storage times. Different letters on the top of each column showed the statistical differences (p < 0.05).
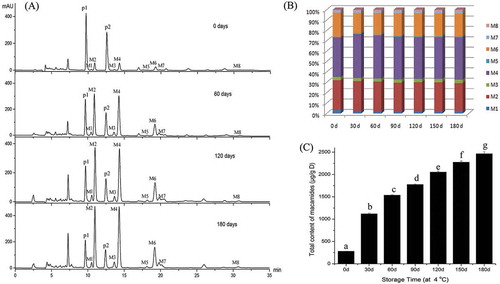
The composition of macamides in this powder is similar to that of other samples. Macamides (M2) N-benzyl-9Z, 12Z, 15Z-octadecatrienamide, (M4) N-benzyl-9Z, 12Z-octadecadienamide, and (M6) N-benzyl-hexadecanamide are the major components and accounted for about 30%, 40%, and 20% of total content, respectively. The percentage of each macamide remained consistent in this sample for 180-day storage.
Total content of macamides in this powder increased with the extension of storage time. Macamides cannot be detected in fresh maca root. However, after 1-week drying, the total content of macamides in root slices increased to 281 μg/g DM. Being ground and stored at 4°C for 30 days, the total content of macamides significantly increased from 281 to 1118 μg/g DM and kept a sustained growth. However, the total content of macamides showed a progressively smaller increment during the 180 days of storage, as the increment was 837 for the first month storage and 117 μg/g DM for the sixth month storage.
Discussion
Macamides have been suggested as a quality indicator for maca products due to their high bioactivities.[Citation7–Citation12] In the present study, we found the significant variations of macamides in commercial maca products and analyzed the influence factors for macamide biosynthesis. As shown in , (a) glucosinolate catabolism, (b) lipid hydrolysis, and (c) amide formation are considered to be the key steps for macamide biosynthesis.
Sample forms showed high influences on macamide accumulation (). For the total content, powder (3607 μg/g DM) > slice (1762 μg/g DM) > whole root (995 μg/g DM). For the composition, the percentage of unsaturated macamides in powder was 61%, slice 61%, and whole root 67%. The essential differences among powder, slice, and whole root were the degree of cell breakdown. It is well known that myrosinase and glucosinolates are in different compartment in intact cells. Cell damage favors glucosinolate breakdown by myrosinase.[Citation16,Citation17] Yábar et al. reported an important decrease of glucosinolates due to cell breakdown was caused by fluctuation in temperatures during the 30–45 days period of postharvest field drying process.[Citation4] Therefore, cell damage will be beneficial for the step (a) glucosinolate catabolism, and then more benzylamine will be generated. On the other hand, cell damage will also accelerate the step (b) lipid hydrolysis, which results in higher release of free fatty acids.[Citation18] Previous study had reported the release of free fatty acids due to cell damage caused by freeze-thaw cycles in maca.[Citation3] Furthermore, cell damage will also make the contact between free fatty acids and benzylamine more sufficiently, which might benefit the step (c) amide bond formation. At the same time, cell breakdown results in exposure to air. As unsaturated bonds are easily oxidized by lipoxygenase, maca powder and slice exposed to air more fully than whole root, which resulted in higher oxidation level and lower percentage of unsaturated macamides.
Drying temperatures had significant effects on compositon and content of macamides (). The highest content was found in samples dried at 30°C, followed by 40, 20, 4, 60, and 80°C. The percentages of unsaturated macamides in maca rose with temperature increasing, except for 80°C. Previous studies indicated that the activity of myrosinase was stable up to 40°C and reduced 90% after thermal treatment at 75°C for 10 min.[Citation19,Citation20] So high temperature is harmful to glucosinolate catabolism, reduced the activity of myrosinase and the content of benzylamine, and further affected the content of macamides. Myrosinase exhibits similar activity after thermal treatments at 20, 30, and 40°C, respectively.[Citation20] But the highest content of macamides was observed in maca dried at 30°C. The results implied that the drying temperature might also have impact on (c) amide bond formation, which offered a clue that there might be a sort of amide bond synthetase. As to the macamide composition, the major components were consistent with previous reports.[Citation3,Citation13,Citation21] However, the percentages of unsaturated macamides in samples dried at different temperatures were quite different. This result implies that drying temperature might also influence lipid hydrolysis and amide bond formation though we cannot explain the mechanism.
Storage time is another important factor for macamide formation. Esparza et al. reported that the increase of macamide content was sustainable by the open-air drying for 63 days with sufficient free fatty acids and benzylamine. And they believed that macamide synthesis was occurring during the traditional postharvest drying process.[Citation3] However, in the present study, we observed a continuous increase of macamide content in a dried sample for 180 days. The sample was kept in a plastic ziplock bag and stored in at 4°C. All these results indicated that macamide biosynthesis is a long and sustaining reaction, which started from the drying process and kept on during the storage. In addition, the generation of macamides is slow even though the biosynthetic precursors, free fatty acids, and benzylamine are sufficient. Esparza et al. reported a 9-fold molar ratio of amine to fatty acid that develops during the drying process.[Citation3] The result () in this research shows the existence of free fatty acids from the beginning to the end. This indicated that amide bond formation was probably the rate-limiting step for macamide biosynthesis. As to the percentage of unsaturated macamides in this sample, no significant change happened during 180 days of storage. It can be explained that the sample was stored in a plastic ziplock bag, which kept the sample away from air well, or a drop in lipoxygenase activity under those conditions.
Based on the analysis above, it is easier to understand the differences of macamides in the 35 commercial maca products, which were classified into three types, fresh root, dried slice, and essential tablets. Due to the short time of the drying process, fresh root had significant lower content of macamides than dried slice and essential tablets. Low content of macamides were also observed in some dried slices and essential tablets, such as in D1, D5, E5, and E7. To our knowledge, in order to dry the maca sample more efficiently, high temperature (≥60°C) laundry drier or curing barn methods were used for drying process, which resulted in very low content of macamides. In addition, lower percentage of unsaturated macamides was found in essential tablets (60%), which were compressed with powder. Higher percentages of unsaturated macamides were observed in dried slices (77%) and fresh roots (84%). These findings provide important guidelines for the industrial manufacture of maca products with high levels of bioactive macamides.
Conclusion
In this study, similar compositions of macamides were found in all 35 commercial maca products. (M2) N-benzyl-9Z, 12Z, 15Z-octadecatrienamide, (M4) N-benzyl-9Z, 12Z-octadecadienamide, and (M6) N-benzyl-hexadecanamide are the major components. However, significant differences of macamide content were observed in these products, which ranged from 69 μg/g DM to 2738 μg/g DM. We further investigated the great influences of sample forms, drying temperatures, and storage times on macamide formation and indicated that those factors might act on the steps (a) glucosinolate catabolism, (b) lipid hydrolysis, and (c) amide bond formation in macamide biosynthetic pathway. These findings have important guidance for industrial manufacture of maca products with high bioactive macamides.
Supplementary_data_A-E.doc
Download MS Word (14.3 MB)Acknowledgments
The authors also wish to thank Anders Thygesen and Ayobami Matthew Olajuyin for improving the English writing.
Funding
This work was supported by China’s high-tech research program – 863 Program (Grant NO. 2012AA021702-4).
Supplemental material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Gonzales, G.F. Ethnobiology and Ethnopharmacology of Lepidium meyenii (Maca), a Plant from the Peruvian Highlands. Evidence-Based Complementary and Alternative Medicine 2012, 2012, 1–10.
- Choi, E.H.; Kang, J.I.; Cho, J.Y. Supplementation of Standardized Lipid-Soluble Extract from Maca (Lepidium meyenii) Increases Swimming Endurance Capacity in Rats. Journal of Functional Foods 2012, 4(2), 568–573.
- Esparza, E.; Hadzich, A.; Kofer, W. Bioactive Maca (Lepidium meyenii) Alkamides are a Result of Traditional Andean Postharvest Drying Practices. Phytochemistry 2015, 323, 138–148.
- Yábar, E.; Pedreschi, R.; Chirinos, R. Glucosinolate Content and Myrosinase Activity Evolution in Three Maca (Lepidium meyenii Walp.) Ecotypes during Preharvest, Harvest and Postharvest Drying. Food Chemistry 2011, 127(4), 1576–1583.
- Bai, N.S.; He, K.; Roller, M. Flavonolignans and Other Constituents from Lepidium meyenii with Activities in Anti-inflammatory and Human Cancer Cell Lines. Journal of Agricultural and Food Chemistry 2015, 63(9), 2458–2463.
- Zhao, J.P.; Avula, B.; Chan, M. Metabolomic Differentiation of Maca (Lepidium meyenii) Accessions Cultivated under Different Conditions Using NMR and Chemometric Analysis. Planta Medica 2012, 78(1), 90–101.
- Wu, H.; Kelley, C.J.; Pino-Figueroa, A. Macamides and Their Synthetic Analogs: Evaluation of In Vitro FAAH Inhibition. Bioorganic & Medicinal Chemistry 2013, 21(17), 5188–5197.
- Almukadi, H.; Wu, H.; Böhlke, M. The Macamide N-3-Methoxybenzyl-Linoleamide is a Time-Dependent Fatty Acid Amide Hydrolase (FAAH) Inhibitor. Molecular Neurobiology 2013, 48(2), 333–339.
- Hajdu, Z.; Nicolussi, S.; Rau, M. Identification of Endocannabinoid System-Modulating N-Alkylamides from Heliopsis helianthoides var. Scabra and Lepidium meyenii. Journal of Natural Products 2014, 77(7), 1663–1669.
- Lewis, S.; Pino-Figueroa, A. Neuroprotective Effects of Maca Extract and Macamides against Amyloid β Peptide Induced Neurotoxicity in B-35 Neuroblastoma Cells. The FASEB Journal 2013, 27, Suppl. 662.19.
- Alquraini, A.; Waggas, D.; Bӧhlke, M. Neuroprotective Effects of Lepidium meyenii (Maca) and Macamides against Amyloid-Beta (25–35) Induced Toxicity in B-35 Neuroblastoma Cells. The FASEB Journal 2014, 28 (1), Suppl. 657.13.
- Liu, H.; Jin, W.; Fu, C. Discovering Anti-Osteoporosis Constituents of Maca (Lepidium meyenii) by Combined Virtual Screening and Activity Verification. Food Research International 2015, 166, 358–364.
- Pan, Y.; Zhang, J.; Li, H. Simultaneous Analysis of Macamides in Maca (Lepidium meyenii) with Different Drying Process by Liquid Chromatography Tandem Mass Spectrometry. Food Analytical Methods 2016, 9(6), 1686–1695.
- Chen, J.J.; Zhao, Q.S.; Liu, Y.L. Identification of Maca (Lepidium meyenii Walp.) and its Adulterants by a DNA-Barcoding Approach Based on the ITS Sequence. Chinese Journal of Natural Medicines 2015, 13(9), 653–659.
- George, D.; Mallery, P. IBM SPSS Statistics 19 Step by Step: A Simple Guide and Reference. Press: Pearson Schweiz Ag.: Boston, MA, 2011.
- Verkerk, R.; Schreiner, M.; Krumbein, A. Glucosinolates in Brassica Vegetables: The Influence of the Food Supply Chain on Intake, Bioavailability and Human Health. Molecular Nutrition & Food Research 2009, 53 (S2), S219–S265.
- Oliviero, T.; Verkerk, R.; Boekel, MAJSV. Effect of Water Content and Temperature on Inactivation Kinetics of Myrosinase in Broccoli (Brassica oleracea var. Italica). Food Chemistry 2014, 163(2), 197–201.
- Dussert, S.; Davey, M.W.; Laffargue, A. Oxidative Stress, Phospholipid Loss and Lipid Hydrolysis during Drying and Storage of Intermediate Seeds. Physiologia Plantarum 2006, 127(2), 192–204.
- Eylen, D.V.; Oey, I.; Hendrickx, M. Kinetics of the Stability of Broccoli (Brassica oleracea Cv. Italica) Myrosinase and Isothiocyanates in Broccoli Juice during Pressure/Temperature Treatments. Journal of Agricultural & Food Chemistry 2007, 55(6), 2163–2170.
- Eylen, D.V.; Indrawati; Hendrickx, M. Temperature and Pressure Stability of Mustard Seed (Sinapis alba L.) Myrosinase. Food Chemistry 2006, 97(2), 263–271.
- McCollom, M.M.; Villinski, J.R.; McPhail, K.L. Analysis of Macamides in Samples of Maca (Lepidium meyenii) by HPLC-UV-MS/MS. Phytochemical Analysis 2005, 16(6), 463–469.