ABSTRACT
In this study, the biodegradable and biocompatible gelatin-based film containing natamycin was crosslinked by transglutaminase (TGase). The profiles of micro structure, secondary structure, physical properties, and antifungal activities were evaluated. The results of electron microscope and Fourier transform infrared spectroscopy (FTIR) indicated that the structures of films were significantly changed. In addition, the environment for microbial growth and the mechanical properties were evaluated with different concentrations of TGase. Moreover, the films could inhibit the growth of fungus based on microbiological assays. The findings in this paper would facilitate the production of gelatin-based fungistatic composite films.
Introduction
Foods and biomaterial with long shelf life, high safety, and health benefits attract more and more attention. The demand to naturally preserve food and biomaterial advances the development of packaging, such as composite films.[Citation1] A vast variety of food-based materials as packaging includes polysaccharides,[Citation2] lipids,[Citation3,Citation4] and proteins.[Citation5] Gelatin is recognized as a translucent, colourless, and flavourless compound of polypeptide and protein extracted from collagen protein containing beef, pork, fish sources, and so on. [Citation2] Gelatin-based coating has been reported as an antimicrobial film.[Citation6] In addition, a variety of bacteriostatic agents have been incorporated into edible films, such as oregano essential oil,[Citation7] cinnamon or ginger essential oils,[Citation8] and essential oils of clove, garlic, and origanum.[Citation9] Natamycin was studied due to its bacteriostatic ability as fungicide in the field of medicine.[Citation10,Citation11] It was also reported that food raw materials containing natamycin could prevent from spoilage.[Citation12] The lactonic ring structure of natamycin (in ) could react with sterol compounds in the membrane of fungus and destroy the cytomembrane structure and membrane permeability to inhibit fungal growth.[Citation10] Thus, natamycin is usually sprayed onto the surfaces of fresh fruits to retain the quality of food products.[Citation13] However, to our best knowledge, there has been no work performed in the literature on the use of gelatin as the matrix of natamycin to inhibit fungal growth.
In addition, crosslinker can tailor the structure properties of macromolecules and create covalent bonds between food biopolymers or matrices, for instance, proteins and carbohydrates,[Citation14] by physical (heat, UV, and gamma radiation), chemical (glutaraldehyde hexamethylenediisocyanate, bis-epoxide, and carbodiimide), and enzymatic means as reviewed by.[Citation15] Of note, these treatments result in the generation of strong structures or alter the degradation rate of polymers.[Citation16] However, the physical treatments always have non-determinacy on production parameters and the quality of the final products and long exposure times under harsh conditions.[Citation17] Meanwhile, chemical treatments are generally accompanied by overt cytotoxicity and enhanced mineralization or flintiness of the tissue,[Citation18] and residual chemical reagents, leading to health issues for customers. In comparison to the physical and chemical approaches, the enzymatic crosslinking of polymers is recognized as a non-toxic, efficient, and feasible method for preferable collagen materials. Transglutaminase (TGase, EC 2.3.2.13,c-glutamyl-peptide, aminec-glutamyl transferase) from microorganisms has been widely applied in crosslinking.[Citation19] It catalyses de-amidation, acyl transfer reactions, and crosslinks with protein intra-chain or inter-chain glutamine, to provide acyl peptide residues as acyl donors.[Citation20] TGase can alter food matrices, generating improved texture and stability of food products, such as temperature, emulsifying property, gelation capacity, and other properties.[Citation21] In recent decades, TGase has been applied in varieties of food proteins containing ovalbumin,[Citation22] soybean protein,[Citation23] and β-casein and κ-casein,[Citation24] even used in food-packing materials, especially in edible films.[Citation24,Citation25] Moreover, TGase was used to enhance calcium carbonate gelatin-based films in our previous study.[Citation26,Citation27]
The objective of this study was to investigate the feasibility of the production of gelatin-natamycin edible films crosslinked by different concentrations of TGase. The microstructure and secondary structure were studied by electron microscope and Fourier transform infrared spectroscopy (FTIR), to elaborate the effect of TGase on the structure of films. In addition, mechanical properties, water vapour permeability (WVP), moisture content, and oxygen gas permeability were evaluated. Furthermore, the antifungal properties of gelatin-based films were assessed.
Material and methods
Chemicals
A commercial grade of gelatin (Bloom 220), Natamycin (≥95%, HPLC), was obtained from Shanghai Jianglai Brotech Co. Ltd (Shanghai, China); microbial TGase powder was kindly donated by Yuanye company (Activa TGase-S, Tianjin, China) in a powder form with 100 U/g. Plasticizer (glycerol, molecular biology, ≥99%) and Tris–HCl were from Sigma–Aldrich (Shanghai, China). All commercial chemicals were of analytical grade and used with no further purification.
Microorganisms and culture media
Aspergillus ochraceus, Aspergillus niger (Aspergillus), and Penicillium funlculosu (Penicillium) were provided by institute of microbiology Chinese Academy of Sciences (Beijing, China). The mixed strains by mixing strains from five glass culture dishes containing potato dextrose agar medium (PDA) and without a lid in the laboratory were used for meat production for 1 h, then cultivated with a lid for 3 days at 25°C in a microbiological incubator. PDA (JL0233-12, containing chloroamphenicol) was purchased from Shanghai Jianglai Brotech Co. Ltd (Shanghai, China).
Preparation of films
Based on some preliminary experiments and the previous study,[Citation28] the mixtures of gelatin, glycerol, and distilled water (5:1:100 weight ratio) were prepared, and a required amount of natamycin with the method as the literature reported finally in concentration of 0.027 g natamycin/ 100 g slurry (or 9.25 mg natamycin/dm2 of film) was added.[Citation29,Citation30] The mixture was heated in a water bath until 45°C. Then TGase in 0.05 M of Tris–HCl (pH 7.4) was introduced into the slurry with a concentration gradient of 0, 2, 4, 6, and 8 U/g, respectively. Subsequently, the reaction media was degassed and incubated at the 45°C for 2 h while stirring with a glass rod. The stock solution was carefully dropped onto polyacrylic plates (15 cm × 15 cm) immediately, followed by oven-dried treatment in a drying oven at 35°C. Afterwards, films were peeled off from the polyacrylic plates and stored at 25°C, in a humidistat with 51% relative humidity (RH) (saturated magnesium nitrate solution) for 2 days before evaluating the film properties. For each film, two replicates were prepared for measurements.
Structural characterization
Fourier transform infrared spectroscopy
The FTIR spectra of the films were scanned using a Thermo Nicolet Avatar 370 Fourier Transform Infrared spectrometer equipped with a DTGASES KBr detector (Thermo, Shanghai, China) and evaluated as mentioned in the literature.[Citation26] The test wavelength was from 400 to 4000 cm−1. For each sample spectrum, there were 16 scans at a resolution of 1.9 cm−1. The FTIR spectra were analysed by Omnic software (OMNIC 8.2.).
Scanning electron microscope
The morphologies of the films surface and cross section were characterized using a scanning electron microscope (SEM) (SU1510, Hitachi, Japan) as reported in the literature[Citation31] with minor modifications. Prior to observation, the films were dried in an incubator at 60°C for 48 h and preserved in a desiccator with anhydrous calcium chloride for further tests. The films were immersed in liquid nitrogen for 3–5 min to embrittle and detect the cross section. The samples were immobilized to the stage with double-sided conductive adhesive and then sputtered with a layer of gold. Then the films were placed into the cold stage of a SEM chamber for observation. The surface and cross section were observed with acceleration voltage in 20 kV and magnified 1000- and 2000-fold.
Physical properties
Film thickness
The thickness of the films was measured by a thickness gauge (0–10 × 30, Japan). Five areas were randomly selected and averaged as the thickness.
Water vapour permeability
Water vapour permeability (WVP) of the films was measured using a reported method.[Citation32] The film was cut to 3 cm in diameter, and the film was covered close to a testing cup with anhydrous calcium sulphate. The cup was placed in humidistat within sodium chloride saturated solution. It was equilibrated for 1 h and then the weight of the whole cup was recorded as the initial weight. Subsequently, the mixture was weighted after 24 h. The detection of each sample was triplicated and calculated as follows:
where W = increase in cup weight (g), L = thickness of film (mm), t = measuring time (s), A = measuring area (m2), and
P = difference in pressure between outside of the cup and inside of the cup (Pa).
Moisture content
Films were clipped at 2 × 7 cm and placed in a beaker. Then, they were oven dried at 105°C in an oven until obtaining a consistent weight.
Oxygen gas permeability
Oxygen permeability was measured according to a method described in the literature,[Citation32] and the data were also calculated according to the cited paper.
Mechanical properties
Mechanical properties including ultimate tensile strengths (TS, MPa) and elongations at break (EAB,%) of the films were evaluated using Texture Analyzer (Stable Micro Systems Ltd, UK). The mechanical properties of the films were estimated according to a published paper[Citation33] with minor modifications. The films were trimmed to 70 × 20 mm, and the cross-head speed was set at 20 mm/s to break of the films. The elongation and ultimate TS can be given by the following equations:
where Ft is the maximum load (N), T is the thickness (mm) of the films, W is the width (mm) of the samples, ΔL is the elongation length (mm) of samples, and L0 is the initial grip length (mm) of the samples.
Antifungal activities of edible films
The antifungal activities of films were determined by the disc diffusion assay according to Aldana’s report[Citation34] with minor modifications. The 90-mm-diameter plates were sterilized by high pressure containing PDA, and then they were dried at 25°C. Culture (1.5 × 108 CFU/mL, Aspergillus ochraceus, Aspergillus niger, Penicillium funiculosum, respectively) in saline physiological solution was dispersed by the spread plate technique. The edible films discs (20 mm diameter) sterilized by ultraviolet light for 30 min were placed into the previously inoculated agar plates. The zones of inhibitions’ (mm) diameters were measured three times at different sites after incubation at 37°C for 48 h.
Statistical analysis
For each experiment, the sample was tested in triplicate. Data was expressed as the mean ± standard deviation using analysis of variance (ANOVA), and the mean comparisons were done by Duncan’s multiple range tests. Statistical analysis was performed using the SPSS17.0 software (SPSS Inc., Chicago, IL, USA), and a probability value of p < 0.05 was considered significant.
Results and discussion
Structural characterization of the natamycin gelatin-based films by TGase-induced
Fourier transform infrared spectroscopy
FTIR is usually used for secondary structure analysis, since the spectrum of FTIR could sensitively reveal the structural changes in the food matrix at the molecular level when the functional groups were transformed.[Citation35] To illustrate the changes in the films, FTIR spectra of the films in different concentrations of TGase were investigated ().
Figure 2. FTIR spectra of natamycin gelatin-based fungistatic edible films were treated with different concentrations of TGase.
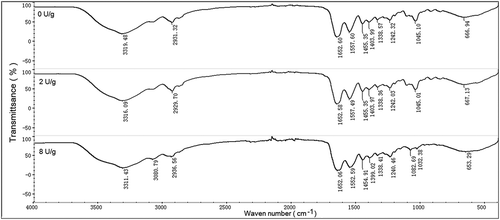
FTIR spectra for acylamino distributed at 1700–1600 cm−1 (acylamino I) and 1590–1500 cm−1 (acylamino II). Simultaneously, acylamino I arising from C=O stretching or hydrogen bonding linked with COO, and 1300–1000 cm−1 was attributed to C-O stretching vibration and acylamino II corresponded to the bending vibration of N-H groups and the stretching vibrations of C-N stretching[Citation26,Citation36] and.[Citation37] The absorption peak of O-H was in accordance with the wavelength at 3500–3000 cm−1.[Citation38]
Although some changes occurred, the spectra of natamycin gelatin-based fungistatic edible films treated with different concentrations of TGase displayed similar patterns. As TGase with a low concentration at 2 U/g was added into the films, compared with the gelatin film in the absence of TGase, the spectrogram of FTIR appeared approximately at 1100 cm−1 corresponding to the C-O stretching vibration of ketone bending vibration inside. When the concentration of TGase increased at 8 U/g, the films’ spectrogram of FTIR around 1100 cm−1 disappeared and the stretching vibration of the O-H identical peak approximately at 3080 cm−1 appeared significantly. The peak appearing at 3080 cm−1 was related to polar interactions between films and unbounded water present in the composite films.[Citation39] The crosslinking using TGase would promote growth in polymer intermolecular hydrogen bonding, which is consistent with the increase in moisture content with the content of TGase increasing. For the signals of acylamino I and acylamino II, the intensities increased, indicating the formation of transamination and molecule aggregation caused by TGase. The spectrum of the crosslinked film with the high concentration at 8 U/g, N–H bonds in the amide II band region (1300–1000 cm−1) moved to lower wave numbers compared to the film without TGase treatment. According to Staroszczyk et al, it may be explained that the film formed by cross-linked gelatin required gelatin conformational changes and this change cost a decrease energy of interaction among intramolecular involving the group of TGase added with a high concentration. And this may explained why the film formed by crosslinked gelatin required gelatin conformational changes.[Citation40] However, the amino and carbonyl groups on the cyclic annular ring of natamycin vibrated around 920 cm−1, whose peaks appeared at 0U/g and 2U/g but disappeared at 8U/g, suggesting that amino and carbonyl groups in natamycin might intertwine with gelatin. In summary, TGase caused the changes of functional groups and secondary structure of gelatin, to some degree, by crosslinking gelatin.
Scanning electron microscopy
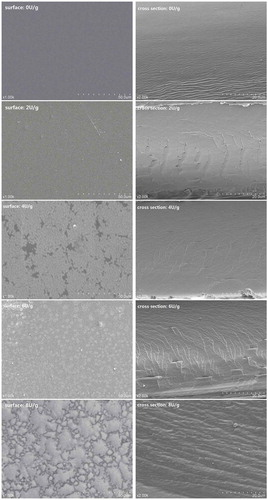
The internal morphological structures of films were determined by SEM. The surface and cross-section images of the films by TGase-induced are shown in . Compared with the film in the absence of TGase, TGase-induced films displayed different morphological properties. Aggregation intensity of the films was enhanced with the increase in the concentration of TGase. For the surfaces of the films, a few white spots were visualized in TGase-induced films, and the areas of white spots increased as the concentrations of TGase increased. The surface structures of the films with different concentrations of TGase tend to depend on the degree of crosslinking. Compared with the gelatin–calcium carbonate composite films crosslinked by TGase and the casein–gelatin mixture modified by TGase,[Citation41] the films in this study were smoother and orderly regardless of the internal and external structures. It might be due to the fact that natamycin is more hydrophilic, enabling it to homogeneously mix with gelatin molecules.[Citation26] Compared with the gelatin film crosslinked by UV and the gelatin-based polymeric biocomposite film prepared by poly (AAm-co-Ac), the gelatin-natamycin films have smooth surfaces.[Citation42] As indicated in , the cross sections displayed a high extent of roughness in the films with the concentration of TGase increased, probably since the interactions among gelatin molecules enhanced and led to the polymerization of macromolecules in gelatin substantially, in agreement with that of chitosan/gelatin-based films using proanthocyanidin as a crosslinking agent .[Citation43] Therefore, it was concluded that the concentrations of TGase in the films were positively proportional to the degree of roughness in the surface and cross sections of the TGase-induced films containing natamycin.
Physical properties
Film thickness
As depicted in , the increase in the concentration of TGase facilitated the improvement of films thickness. The trend was reported for the protein films in the presence of cell-free supernatant of Lactobacillus rhamnosus.[Citation44] Compared to gelatin, the natamycin gelatin-based films with a low concentration of TGase had no statistically significant difference (p>0.05), but the high concentration of TGase could result in significant statistical differences (p<0.05). Among the groups with TGase, they were significantly different (p<0.05). The films thickness increased due to molecule aggregation and polymerization produced by TGase crosslinking. It is in agreement with the results.[Citation20] As observed in the SEM pictures in , the degree of aggregation of films was fundamentally aggravated by TGase, and this may contribute to the increase of the films’ thickness.
Table 1. Physical properties of the natamycin gelatin-based films crosslinked by different concentrations of TGase.
Water vapour permeability
The WVP of the edible coating film is highly associated with the shelf life of packaging materials for products. The low WVP could benefit the storage of food products.[Citation45] indicates that WVP was reduced with the increasing content of TGase. The TGase-induced gelatin films containing natamycin at a low concentration (2 U/g) had no significant change (p > 0.05). When the concentration of TGase was increased to 4 U/g, a significant decrease was observed (p > 0.05) compared to the control. The hydrophilicity of gelatin molecules resulted in the poor moisture barrier properties of the gelatin-based film.[Citation46] When TGase was incorporated into the gelatin network, it allowed for the formation of tight twines in the gelatin network and decreased the permeation of moisture through the film. Galus et al. illustrated that the water sorption isotherms for all films consisting of sodium alginate and pectin were affected by the composition of the film.[Citation47] It was postulated that natamycin and gelatin were combined and interlaced tightly by crosslinking, filling in the gaps in the molecular layer next to their three-dimensional structure or allow for the formation of tortuous paths between interspaces at the molecular layer by intermolecular assemblage. This result that the ability of TGase at higher concentration would bring in a better barrier to water vapor for the films was in accordance with salmon and pollock films crosslinked by glutaraldehyde.[Citation48]
Moisture content
The moisture content of films could be greatly related to the physical properties and microbial growth,[Citation49] especially for gelatin-based films because gelatin has a high concentration of hydrophilic amino acids, leading to protein dissociation. Therefore, they are highly sensitive to moisture when exposed to water (Liu, Majeed et al. 2016). As shown in , the moisture content was increased by the increased concentration of TGase in the films. A similar trend was reported by Liu et al. for the TGase-crosslinked gelatin films at 35°C.[Citation50] Moisture content was increased apparently with the increasing concentration of TGase (p < 0.05), and at the low content of TGase (2 U/g) it had no significance (p > 0.05). For the concentrations of TGase (>2 U/g), the films exhibited significant differences (p < 0.05) with the control due to the improved interaction between water molecules and gelatin. Of note, the hydrogen bonds were considered as the main interaction between water molecules and gelation in the films.[Citation51] The interaction of hydrogen bonding in the film was elevated in the presence of TGase, demonstrated by the vibrating peak of the hydroxyl that appeared in the spectrum of FTIR.
Oxygen gas permeability
Oxygen gas permeability can be an important parameter, especially for fungistatic films in the packing field. The value of oxygen gas permeability is demonstrated in . No significant differences (p > 0.05) between 0 U/g and 2 U/g were found. However, it was significantly decreased with the improvement of the content of TGase (p < 0.05) ranging from 4 U/g to 8 U/g. The decrease in the oxygen transfer may be related to the degree of polymerization and may be explained by the TGase-induced gelatin–gelatin interaction to form compact networks, enabling the production of the tighter matrix, thereby possessing a more compact structure with less pores or cracks. Internal protein–protein interaction in gelatin acts as barriers to prevent oxygen permeability, resulting in the decrease in the tortuous path for traveling through the film.[Citation32] In addition, the TGase-induced polymerization in the crosslinked films could lead to a lower free volume and a higher cohesive energy density among the polymerized chains. The volume was regarded as the vacant space without the microstructure of the polymer molecule and acted as pores or cracks for gas molecules to get through.[Citation52]
Mechanical properties
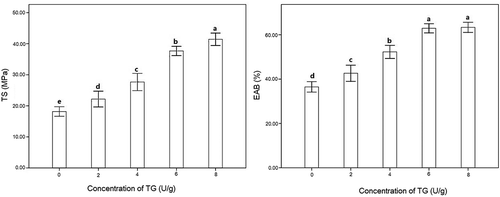
Previous studies showed that since the pure gelatin films are brittle, chemical and physical crosslinking methods were applied and could produce improved mechanical properties.[Citation53] Mechanical properties were estimated by TS and EAB. TS and EBA of TGase-induced gelatin-based films containing natamycin are illustrated in . The TS of composite films significantly (p < 0.05) increased to 41.10 ± 0.81 MPa in the concentration of TGase at 8 U/g due to the interaction between gelatin and natamycin by TGase-induced crosslinking, forming a stable three-dimensional polymeric matrix and resulting in excellent mechanical properties in TS.[Citation54] In addition, EBA increased by the concentration of TGase at 6 U/g significantly (p < 0.05); however, no significant difference between 6 U/g and 8 U/g was detected. The change in EAB was caused by the polymeric assembly of the gelatin molecule. In this paper, the trends of mechanical properties (TS and EAB) are in agreement with the modifications of the mechanical properties of TGase-induced films mentioned in a previous report.[Citation26] Meanwhile, the degrees of change in TS and EAB reported in this paper were greater than those of the gelatin-calcium carbonate film since inorganic nano-calcium carbonates may produce small gaps in gelatin, generating roughness and embrittlement. When genipin was used as a crosslinking agent, the mechanical properties of the films could not be enhanced all the time with the content of genipin increased, revealing that genipin might destroy the gelatin molecule in polymerization at a high concentration.[Citation20]
Fungistasis action
Gelatin films could be readily attacked by microorganism, especially for fungus.[Citation55] Thus, natamycin was selected as a fungistat and added into the gelatin films. The antifungal profiles of the gelatin-natamycin films crosslinked by TGase at different contents for certain fungus are described in . The trends of inhibition diameter for Aspergillus ochraceus, Aspergillus niger, and Penicillium funiculosum were identical from 0 U/g to 8 U/g, following the order: Aspergillus ochraceus < Aspergillus niger < Penicillium funiculosum at their corresponding values. The inhibition ability of fungal growth was enhanced at the initial phase (2 U/g) but gradually decreased at the latter phase (from 4 U/g to 8 U/g).
Figure 5. Inhibition diameters of natamycin gelatin-based films were crosslinked by TGase induced at different contents for certain fungus. Values with the same lowercase letters in a line are not significantly different (p>0.05).
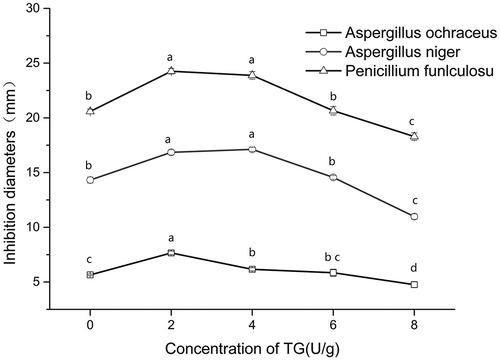
The cyclic structure in natamycin is highly related with its antifungal activities by destroying the fungus cell membrane.[Citation56] Compared with the sample without TGase treatment, the antifungal activities for Aspergillus niger and Penicillium funiculosum of the films were significantly improved (p < 0.05) with increasing TGase content (0–4 U/g). Subsequently, the antifungal activities of the films prepared with TGase contents above 4 U/g decreased markedly (p < 0.05). As depicted in , for Aspergillus ochraceus, in situ TGase at lower concentration (2 U/g) significantly increased the antifungal activities. However, when the concentrations increased to 4 U/g or higher, the presence of TGase significantly decreased the antifungal activities of the films (p < 0.05). In terms of the SEM images shown in , the polymerization of gelatin was enhanced as TGase increased gradually. This may lead to the phenomenon that the cyclic structure in natamycin, which is related to fungistatic property, is exposed or enveloped in the films. It was speculated that the gelatin matrix crosslinked by TGase at low concentration (2 U/g or 4 U/g) may concentrate and expose the cyclic structure in natamycin. When the high concentration of TGase was added, the extent of intermolecular aggregation was distinctly intensified and enveloped the cyclic structure of natamycin in the aggregation, resulting in the reduction of the antifungal profiles of natamycin. Fajardo et al. studied the antimicrobial profiles of chitosan containing natamycin on cheese. They concluded that the chitosan coating with natamycin could retard the growth of mould/yeast and mesophilic bacteria after 27 days of storage.[Citation57] Isolated soy protein (SPI)-poly(lactic acid) film as a carrier of natamycin could inhibit the growth of mould and yeast at 0.33% w/w of SPI with a small inhibition zone (~2 cm in diameter).[Citation58]
Additionally, the potential for phenotypic variation in protein as a barrier may change the sensitivity of target site(s) and the number of fungistat.[Citation59] The ability of fungistasis action, is greatly associated with the degree of gelatin polymerized, and the degree of films aggregation changed by different content of TGase. Therefore, another reason for the reduction of the antifungal profiles at the high concentration of natamycin is that the high degree of molecule aggregation between natamysin and gelatin or gelatin-gelatin by TGase-crosslinking resulted in a decrease in the concentration of natamycin, causing the decrease of the fungistasis capability.
Conclusion
In conclusion, natamycin was successfully incorporated into gelatin film crosslinked by TGase for the production of gelatin-based fungistatic edible films. The microstructures, functional groups, and physical properties in gelatin films were significantly impacted by the presence of the high concentration of TGase. Natamycin successfully retarded the growth of Aspergillus ochraceus, Aspergillus niger, and Penicillium funlculosu. Therefore, the present work demonstrates that natamycin could be incorporated into the gelatin film by enzyme in the absence of the use of chemical compatibility agents and adhesives. The results obtained from this study would contribute to the development of active food-packaging materials.
Funding
This work was supported by the Foundation of National 863 Plan (grant numbers 2013AA102204) and Ministry of Agriculture of China (grant numbers 201303082-3).
Additional information
Funding
References
- Gould, G.W. Methods for Preservation and Extension of Shelf Life. International Journal of Food Microbiology 1996, 33, 51–64.
- Mu, C.; Guo, J.; Li, X.; Lin, W.; Li, D. Preparation and Properties of Dialdehyde Carboxymethyl Cellulose Crosslinked Gelatin Edible Films. Food Hydrocolloids. 2012, 27, 22–29.
- Kanmani, P.; Lim, S.T. Development and Characterization of Novel Probiotic-Residing Pullulan/Starch Edible Films. Food Chemistry 2013, 141, 1041–1049.
- Santacruz, S.; Rivadeneira, C.; Castro, M. Edible Films Based on Starch and Chitosan. Effect of Starch Source and Concentration, Plasticizer, Surfactant’s Hydrophobic Tail and Mechanical Treatment. Food Hydrocolloids 2015, 49, 89–94.
- Ramos, ÓL.; Reinas, I.; Silva, S.I.; Fernandes, J.C.; Cerqueira, M.A.; Pereira, R.N.; Vicente, A.A.; Poças, M.F.; Pintado, M.E.; Malcata, F.X. Effect of Whey Protein Purity and Glycerol Content upon Physical Properties of Edible Films Manufactured Therefrom. Food Hydrocolloids 2013, 30, 110–122.
- Hanani, Z.N.; Beatty, E.; Roos, Y.; Morris, M.; Kerry, J. Manufacture and Characterization of Gelatin Films Derived from Beef, Pork and Fish Sources Using Twin Screw Extrusion. Journal of Food Engineering 2012, 113, 606–614.
- Benavides, S.; Villalobos-Carvajal, R.; Reyes, J. Physical, Mechanical and Antibacterial Properties of Alginate Film: Effect of the Crosslinking Degree and Oregano Essential Oil Concentration. Journal of Food Engineering 2012, 110, 232–239.
- Atarés, L.; De Jesús, C.; Talens, P.; Chiralt, A. Characterization of SPI-Based Edible Films Incorporated with Cinnamon or Ginger Essential Oils. Journal of Food Engineering 2010, 99, 384–391.
- Teixeira, B.; Marques, A.; Pires, C.; Ramos, C.; Batista, I.; Saraiva, J.A.; Nunes, M.L. Characterization of Fish Protein Films Incorporated with Essential Oils of Clove, Garlic and Origanum: Physical, Antioxidant and Antibacterial Properties. LWT-Food Science and Technology 2014, 59, 533–539.
- Pedersen, J.C. Natamycin as a Fungicide in Agar Media. Applied and Environmental Microbiology 1992, 58, 1064–1066.
- Jones, D.B.; Forster, R.K.; Rebell, G. Fusarium solani Keratitis Treated with Natamycin (pimaricin): Eighteen Consecutive Cases. Archives of Ophthalmology 1972, 88, 147–154.
- Bierhalz, A.C.K.; Da Silva, M.A.; Kieckbusch, T.G. Natamycin Release from Alginate/Pectin Films for Food Packaging Applications. Journal of Food Engineering 2012, 110, 18–25.
- Duran, M.; Aday, M.S.; Zorba, N.N.D.; Temizkan, R.; Büyükcan, M.B.; Caner, C. Potential of Antimicrobial Active Packaging ‘Containing Natamycin, Nisin, Pomegranate and Grape Seed Extract in Chitosan Coating to Extend Shelf Life of Fresh Strawberry. Food and Bioproducts Processing 2016, 98, 354–363
- Buchert, J.; Ercili Cura, D.; Ma, H.; Gasparetti, C.; Monogioudi, E.; Faccio, G.; Mattinen, M.; Boer, H.; Partanen, R.; Selinheimo, E. Crosslinking Food Proteins for Improved Functionality. Annual Review of Food Science and Technology 2010, 1, 113–138.
- Singh, H. Modification of Food Proteins by Covalent Crosslinking. Trends in Food Science & Technology. 1991, 2, 196–200.
- Mitra, T.; Sailakshmi, G.; Gnanamani, A.; Mandal, A.B. Cross-Linking with Acid Chlorides Improves Thermal and Mechanical Properties of Collagen Based Biopolymer Material. Thermochimica Acta 2011, 525, 50–55.
- Stachel, I.; Schwarzenbolz, U.; Henle, T.; Meyer, M. Cross-Linking of Type I Collagen with Microbial Transglutaminase: Identification of Cross-Linking Sites. Biomacromolecules 2010, 11, 698–705.
- Chvapil, M.; Speer, D.; Mora, W.; Eskelson, C. Effect of Tanning Agent on Tissue Reaction to Tissue Implanted Collagen Sponge. Journal of Surgical Research 1983, 35, 402–409.
- Yang, M.; Shi, Y.; Liang, Q. Effect of Microbial Transglutaminase Crosslinking on the Functional Properties of Yak Caseins: A Comparison with Cow Caseins. Dairy Science and Technology 2016, 96, 39–51.
- Gaspar, A.L.C.; de Góes-Favoni, S.P. Action of Microbial Transglutaminase (MTGase) in the Modification of Food Proteins: A Review. Food Chemistry 2015, 171, 315–322.
- Ali, N.A.; Ahmed, S.H.; Mohamed, E.A.; Ahmed, I.A.M. Effect of Transglutaminase Cross Linking on the Functional Properties as a Function of NaCl Concentration of Legumes Protein Isolate. International Journal of Biological and Life Sciences 2011, 7(1), 8–12.
- Di Pierro, P.; Chico, B.; Villalonga, R.; Mariniello, L.; Masi, P.; Porta, R. Transglutaminase-Catalyzed Preparation of Chitosan–Ovalbumin Films. Enzyme and Microbial Technology 2007, 40, 437–441.
- Song, C.L.; Zhao, X.H. Structure and Property Modification of an Oligochitosan-Glycosylated and Crosslinked Soybean Protein Generated by Microbial Transglutaminase. Food Chemistry 2014, 163, 114–119.
- Ridout, M.J.; Paananen, A.; Mamode, A.; Linder, M.B.; Wilde, P.J. Interaction of Transglutaminase with Adsorbed and Spread Films of β-casein and к-casein. Colloids and Surfaces B: Biointerfaces 2015, 128, 254–260.
- Piotrowska, B.; Sztuka, K.; Kołodziejska, I.; Dobrosielska, E. Influence of Transglutaminase or 1-Ethyl-3-(3-Dimethylaminopropyl) Carbodiimide (EDC) on the Properties of Fish-Skin Gelatin Films. Food Hydrocolloids 2008, 22, 1362–1371.
- Wang, Y.; Liu, A.; Ye, R.; Wang, W.; Li, X. Transglutaminase-Induced Crosslinking of Gelatin–Calcium Carbonate Composite Films. Food Chemistry 2015, 166, 414–422.
- Li, X.; Liu, A.; Ye, R.; Wang, Y.;Wang, W. Fabrication of Gelatin–Laponite Composite Films: Effect of the Concentration of Laponite on Physical Properties and the Freshness of Meat during Storage. Food Hydrocolloids 2015, 44, 390–398.
- He, Q.; Zhang, Y.; Cai, X.- Wang, S. Fabrication of Gelatin-TiO 2 Nanocomposite Film and Its Structural, Antibacterial and Physical Properties. International Journal of Biological Macromolecules 2015, 84, 153–160.
- Usha, R.; Ramasami, T. The Effects of Urea and n-Propanol on Collagen Denaturation: Using DSC, Circular Dichroism and Viscosity. Thermochimica Acta 2004, 409, 201–206.
- Resa, C.P.O.; Gerschenson, L.N.; Jagus, R.J. Natamycin and Nisin Supported on Starch Edible Films for Controlling Mixed Culture Growth on Model Systems and Port Salut Cheese. Food Control 2014, 44, 146–151.
- Acevedo-Fani, A.; Salvia-Trujillo, L.; Rojas-Graü, M.A.; Martín-Belloso, O. Edible Films from Essential-Oil-Loaded Nanoemulsions: Physicochemical Characterization and Antimicrobial Properties. Food Hydrocolloids 2015, 47, 168–177.
- Hanani, Z.N.; O’Mahony, J.A.; Roos, Y.H.; Oliveira, P.M.; Kerry, J. Extrusion of Gelatin-Based Composite Films: Effects of Processing Temperature and pH of Film Forming Solution on Mechanical and Barrier Properties of Manufactured Films. Food Packaging and Shelf Life 2014, 2, 91–101.
- Li, J.; He, J.; Huang, Y.; Li, D.; Chen, X. Improving Surface and Mechanical Properties of Alginate Films by Using Ethanol as a Co-solvent during External Gelation. Carbohydrate Polymers 2015, 123, 208–216.
- Aldana, D.S.; Andrade-Ochoa, S.; Aguilar, C.N.; Contreras-Esquivel, J.C.; Nevárez-Moorillón, G.V. Antibacterial Activity of Pectic-Based Edible Films Incorporated with Mexican Lime Essential Oil. Food Control 2015, 50, 907–912.
- Dymerski, T.; Namieśnik, J.; Leontowicz, H.; Leontowicz, M.; Vearasilp, K.; Martinez-Ayala, A.L.; González-Aguilar, G.A.; Robles-Sánchez, M.; Gorinstein, S. Chemistry and Biological Properties of Berry Volatiles by Two Dimensional Chromatography, Fluorescence and Fourier Transform Infrared Spectroscopy Techniques. Food Research International 2016, 83, 74–86
- Rostamzad, H.; Paighambari, S.Y.; Shabanpour, B.; Ojagh, S.M.; Mousavi, S.M. Improvement of Fish Protein Film with Nanoclay and Transglutaminase for food Packaging. Food Packaging and Shelf Life 2016, 7, 1–7.
- Plepis, A.M.G.; Das-Gupta, D.K.; Goissis, G. Pyroelectric Properties of Anionic Collagen and Anionic Collagen: P (VDF/TRFE) Composites. International Symposium on Electrets, 1999, 233–236.
- Tapsir, Z.; Saidin, S. Synthesis and Characterization of Collagen–Hydroxyapatite Immobilized on Polydopamine Grafted Stainless Steel. Surface and Coatings Technology 2016, 285, 11–16.
- Bierhalz, A.C.K.; Silva, M.A.D.; Braga, M.E.M.; Sousa, H.J.C.; Kieckbusch, T.G., Effect of Calcium and/or Barium Crosslinking on the Physical and Antimicrobial Properties of Natamycin-Loaded Alginate Films. LWT - Food Science and Technology 2014, 57, 494–501.
- Staroszczyk, H.; Pielichowska, J.; Sztuka, K.; Stangret, J.; Kołodziejska, I. Molecular and Structural Characteristics of Cod Gelatin Films Modified with EDC and TGase. Food Chemistry 2012. 130(2), 335–343.
- Chambi, H.; Grosso, C. Edible Films Produced with Gelatin and Casein Cross-Linked with Transglutaminase. Food Research International 2006, 39, 458–466.
- Haroun, A.; El Toumy, S. Effect of Natural Polyphenols on Physicochemical Properties of Crosslinked Gelatin-Based Polymeric Biocomposite. Journal of Applied Polymer Science 2010, 116, 2825–2832.
- Kim, S.; Nimni, M.E.; Yang, Z.; Han, B. Chitosan/Gelatin–Based Films Crosslinked by Proanthocyanidin. Journal of Biomedical Materials Research Part B: Applied Biomaterials 2005, 75, 442–450.
- Beristain-Bauza, S.; Mani-López, E.; Palou, E.; López-Malo, A. Antimicrobial Activity and Physical Properties of Protein Films Added with Cell-free Supernatant of Lactobacillus rhamnosus. Food Control 2016, 62, 44–51.
- Jridi, M.; Souissi, N.; Mbarek, A.; Chadeyron, G.; Kammoun, M.; Nasri, M. Comparative Study of Physico-Mechanical and Antioxidant Properties of Edible Gelatin Films from the Skin of Cuttlefish. International Journal of Biological Macromolecules 2013, 61:17-25.
- Xiao, J.; Wang, W.; Wang, K.; Liu, Y.; Liu, A.; Zhang, S.; Zhao, Y. Impact of Melting Point of Palm Oil on Mechanical and Water Barrier Properties of Gelatin-Palm Oil Emulsion Film. Food Hydrocolloids 2016, 60, 243–251.
- Galus, S.; Lenart, A. Development and Characterization of Composite Edible Films Based on Sodium Alginate and Pectin. Journal of Food Engineering 2013, 115, 459–465.
- Chiou, B.S.; Avena-Bustillos, R.J.; Bechtel, P.J.; Jafri, H.; Narayan, R.; Imam, S.H.; Glenn, G.M.; Orts, W.J. Cold Water Fish Gelatin Films: Effects of Cross-Linking on Thermal, Mechanical, Barrier, and Biodegradation Properties. European Polymer Journal 2008, 44, 3748–3753.
- Cao, N. Fu, Y.; He, J. Preparation and Physical Properties of Soy Protein Isolate and Gelatin Composite Films. Food Hydrocolloids 2007, 21, 1153–1162.
- Liu, F.; Majeed, H.; Antoniou, J.; Li, Y.; Ma, Y.; Yokoyama, W.; Ma, J.; Zhong, F. Tailoring Physical Properties of Transglutaminase-Modified Gelatin Films by Varying Drying Temperature. Food Hydrocolloids 2016, 58, 20–28.
- Cuq, B.; Gontard, N.; Guilbert, S. Thermal Properties of Fish Myofibrillar Protein-Based Films as Affected by Moisture Content. Polymer 1997, 38, 2399–2405.
- Wang, L.Z.; Liu, L.; Holmes, J.; Kerry, J.F. and Kerry, J.P. Assessment of Film-Forming Potential and Properties of Protein and Polysaccharide-Based Biopolymer Films. International Journal of Food Science and Technology 2007, 42, 1128–1138.
- Bigi, A.; Cojazzi, G.; Panzavolta, S.; Roveri, N.; Rubini, K. Stabilization of Gelatin Films by Crosslinking with Genipin. Biomaterials 2002, 23, 4827–4832.
- He, Q.; Zhang, Y.; Cai, X. and Wang, S. Fabrication of gelatin–TiO2 Nanocomposite Film and Its Structural, Antibacterial and Physical Properties. International Journal of Biological Macromolecules 2016, 84, 153–160.
- Wu, J.; Ge, S.; Liu, H.; Wang, S.; Chen, S.; Wang, J.; Li, J.; Zhang, Q. Properties and Antimicrobial Activity of Silver Carp (Hypophthalmichthys molitrix) Skin Gelatin-Chitosan Films Incorporated with Oregano Essential Oil for Fish Preservation. Food Packaging and Shelf Life 2014, 2, 7–16.
- Moatsou, G.; Moschopoulou, E.; Beka, A.; Tsermoula, P.; Pratsis, D. Effect of Natamycin-Containing Coating on the Evolution of Biochemical and Microbiological Parameters during the Ripening and Storage of Ovine Hard-Gruyère-Type Cheese. International Dairy Journal 2015, 50, 1–8.
- Fajardo, P.; Martins, J.; Fuciños, C.; Pastrana, L.; Teixeira, J.; Vicente, A. Evaluation of a Chitosan-Based Edible Film as Carrier of Natamycin to Improve the Storability of Saloio Cheese. Journal of Food Engineering 2010, 101, 349–356.
- González, A.; Igarzabal, C.I.A. Soy Protein–Poly (Lactic Acid) Bilayer Films as Biodegradable Material for Active Food Packaging. Food Hydrocolloids. 2013, 33, 289–296.
- Denyer, S.P. Mechanisms of Action of Antibacterial Biocides. International Biodeterioration and Biodegradation 1995, 36, 227–245.