ABSTRACT
The prebiotic properties of sour curry paste in the upper gut and the gut microbiota were investigated in vivo during digestion. The effect of the addition of garcinia as souring agent in curry paste was studied. Curry paste without garcinia (P1) and curry paste with garcinia (P2) increased the number of beneficial bacteria in the gut microbiota, especially bifidobacteria and lactobacilli, and significantly (p < 0.05) decreased the number of harmful bacteria (Clostridia). Fecal fermentation with P1 resulted in a prebiotic index (PI) of 1.19, whereas fermentation with P2 resulted in a PI of 2.75. The fermented metabolites produced were lactic acid; vitamins; and short-chain fatty acids (SCFAs) such as acetic acid, propionic acid, and butyric acid. P1 produced metabolites including lactic acid, SCFAs, and B vitamins in higher amounts than P2. After a 24 h fermentation period with colonic microbiota, P1 produced vitamins B1 (18.38 ± 0.10 µg/ml) and B2 (45.28 ± 2.02 µg/ml) but not folic acid, whereas P2 produced only vitamin B1 (5.99 ± 0.48 µg/ml).
Introduction
Prebiotics are non-digestible oligosaccharides that beneficially affect the host by stimulating the growth and/or activity of one or a limited number of bacteria in the colon, thus improving host health.[Citation1] The fermentation of carbohydrates and prebiotics by gut microbiota produces acetate, butyrate, ethanol, succinate, lactate, pyruvate, and hydrogen gas.[Citation2,Citation3] However, the production of short-chain fatty acids (SCFAs) by the microflora can promote human health, decrease the risk of colon cancer, and stimulate human immunity.[Citation4]
The prebiotic concept considers that many potential health-promoting microorganisms, such as bifidobacteria and lactobacilli, already reside in the human colon. Prebiotics must be stable in the acidic medium of the stomach until they reach the colon where they are then selectively fermented by beneficial bacteria and must not be absorbed in the small intestine.[Citation5] In vitro models of the gut are often used to screen the effects that prebiotic can exert on the colonic microflora. Prebiotics can be screened using batch cultures to ascertain how they affect the colonic microflora. Glass vessels containing a medium that can support the growth of the colonic microflora are used. The substance to be tested is added just before the addition of a fecal slurry (1% w/w total volume), which is representative of colonic microflora. The vessels are maintained under anaerobic conditions at 37°C and sampled periodically. Static batch cultures are generally involve small volumes and controlled pH, so they are best suited for the initial screening process. Stirred, pH-controlled batch cultures can then be used to obtain more detailed information at a pH that is representative of the distal region of the colon. Ten samples from thirteen fruits and vegetables from southern Thailand were reported to be potential sources of natural prebiotics, including dragon fruit with the highest oligosaccharide content of 9.81% (w/w).[Citation6,Citation7] Benkeblia and Shiomi (2006) reported that shallot and garlic consist of non-digestible carbohydrates (soluble component) that contain 2–6% inulin, 2–6% oligofructose and 9–16% inulin, and 3–6% oligofructose, respectively.[Citation8]
Southern sour curry or Keang-hleung soup is a popular traditional spicy-sour curry consumed particularly in southern Thailand and is a purported healthy food because of its low calorie content, which is due to a low fat but high fiber content in the form of vegetables. Moreover, the ingredients used in the paste are normally turmeric rhizome, garlic, shallot, and chili, which have been reported as sources of antimicrobial and antioxidant compounds.[Citation9–Citation13] When cooking the sour soup, lime juice, tamarind, and any type of sour fruit as well as leaves are added. However, southern Thais generally use dried garcinia fruit as a souring agent in many types of sour soups. In addition, our previous study reported that the total phenolic contents of the basic paste without garcinia, the garcinia Keang-hleung paste, and the garcinia Keang-hleung paste without salt decreased with increasing storage time. Moreover, the DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging activity and ferric reducing power (FRAP) of the basic paste without garcinia decreased as the storage time increased.[Citation14] The addition of dried garcinia fruit to curry paste may not only increase the antioxidant property and extend the shelf life but also alter other qualities, particularly the prebiotic property, of the paste. The findings of this study further the knowledge of the interaction between the dietary nutrients in curry paste and the human gut microbiota. This study aimed to investigate the prebiotic effect and microbiota interactions resulting from the addition of garcinia to sour curry paste.
Materials and methods
Materials
Turmeric rhizomes (Curcuma longa), garlic (Allium sativum), dried finger chili (Capsicum annuum), shallot (Allium ascalonicum), and dried garcinia (Garcinia atroviridis) were purchased from a local market in Hat Yai, Songkhla, Thailand.
Chemicals
All chemical reagents and enzymes were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Hydrochloric acid, sodium acetate, sodium carbonate (Na2CO3), and absolute ethanol were purchased from Merck (Darmstadt, Germany).
Keang-Hleung paste preparation
All spices were sorted, trimmed, and thoroughly washed to remove dust and dirt and then soaked in 150 ppm and 10 ppm chlorine solutions, respectively, for 1 min, after which they were drained and weighed according to a basic recipe before adding 20% salt. Thereafter, the samples were divided into two groups: basic Keang-hleung paste (without garcinia, P1) and garcinia Keang paste (with 15% garcinia, P2). The details of the paste preparation were published elsewhere.[Citation14] Based on calculations, the compositions of each paste were differentiated as shown in . All mentioned ingredients were then ground in a blender (Moulinex, TYPE 276, France) to obtain a fine paste (20–40 mesh).
Table 1. The ingredient compositions in curry paste formula (P1 and P2).
Preparation of Keang-Hleung paste under simulated gastrointestinal conditions
In vitro digestion was studied by simulating the conditions that occur within the upper gastrointestinal tract of humans, which consists of digestion in the mouth, stomach, and small intestine. The enzyme activity was determined using the Sigma quality control test procedures for α-amylase. The enzyme was prepared in solution using cold distilled water (30 ml) and mixed with an artificial saliva solution (270 ml) to obtain a final concentration of 2 unit/ml α-amylase, pH 6.8. The artificial human saliva contained (g/l) NaCl, 1.60; NH4NO3, 0.33; NH2PO4, 0.64; KCl, 0.20; K3C6H5O.7H2O, 0.31; C5H3N4O3Na, 0.02; H2NCONH2, 1.98; and C3H3O3Na, 0.15 and 15 ml of porcine mucin.[Citation15] The paste sample (100 g) was mixed with artificial saliva (270 ml) and human salivary α-amylase (30 ml) and then incubated in a shaking water bath at 85 rpm for rigorous mixing at a controlled temperature of 37 ± 1°C for 2 min. Samples (0.5 ml) were taken every 15 s to determine the reducing sugar and total sugar contents. The percentage hydrolysis was calculated based on the amount of reducing sugar liberated and the total sugar.[Citation16]
Samples were dissolved in reverse osmosis (RO) water to give a 1% (w/v) solution. Artificial human gastric juice was mimicked using a hydrochloric acid buffer containing (g/l): NaCl, 8; KCl, 0.2; Na2HPO4·2H2O, 8.25; NaHPO4, 14.35; CaCl2·2H2O, 0.1; and MgCl2·6H2O, 0.18. The pH of the buffer was adjusted to 1, 2, 3, 4, and 5 using 5 M HCl.[Citation17] HCl buffer (5 ml) at each pH was added to the sample solution (5 ml), and the reaction mixture was incubated in a shaking water bath at a controlled temperature of 37 ± 1°C for 4 h. Samples (1 ml) were taken periodically at 0, 0.5, 1, 2, 4, and 6 h. The reducing sugar content in the sample was determined using the DNS method, and the total sugar content was determined using the phenol–sulfuric acid method.[Citation18,Citation19] The percentage hydrolysis of the samples was calculated based on the amount of reducing sugar liberated and the total sugar content of the sample.[Citation16]
After the sample solution (pH 2) was incubated for 30 min, the pH was adjusted to 6.9 to mimic the conditions of digestion in the small intestine. Then, 0.75 unit/ml human pancreatic α-amylase enzyme was added, and the sample was incubated at 37 ± 1°C for 6 h. Samples (0.5 ml) were taken at 1 h intervals to determine the reducing sugar and total sugar contents. The percentage hydrolysis was calculated based on the amount of reducing sugar liberated and the total sugar content. Each sample was cooled by immersion in an ice bath and precipitated by 95% ethanol (final concentration of 80%). The mixture was left overnight at 4°C. The sample was precipitated and re-precipitated twice to separate the sugar molecules from the solution. The precipitated sample was evaporated on a rotary evaporator under low pressure and dried on a freeze drier. The dried powder samples were stored at –20°C until use.[Citation16]
Preparation of fecal slurry
A human fecal slurry with a concentration of 10% (w/w) was prepared by dilution in PBS in a stirred, pH-controlled batch culture. A fresh stool sample was weighed in a stomacher bag (inside a pre-weighed container), after which PBS was added to obtain the desired concentration of the fecal slurry. Each sample was blended in a stomacher at a normal speed for 120 s.[Citation20]
Stirred, pH-controlled batch culture fermentation
One hundred grams of Keang-hleung paste was measured for both P1 and P2, with moisture contents of 56% and 58%, respectively, and then digested under stimulated conditions similar to the mouth, stomach and small intestine. Then, the samples were freeze dried, and 9.8 g and 9.2 g of dried powder were obtained for P1 and P2, respectively. To determine the prebiotic index (PI), a sterile glass fermenter (300 ml capacity) was filled with sterile basal medium (100 ml) and purged with oxygen-free nitrogen gas to obtain stable conditions. Fecal slurries (100 ml) were added to each fermenter, and the system was maintained under a head space of oxygen-free nitrogen gas. Fermentation was carried out at 37°C for 24 h, with magnetic stirring and culturing. The pH was automatically controlled at 6.8 ± 0.1 by the addition of 0.5 N NaOH or HCl during fermentation using a pH controller (Fermac 260, Electrolab, UK). Dried sample powder (8 g) of the Keang-hleung paste was added into a glass vessel. Samples (5 ml) were taken at 0, 6, 12, and 24 h for the enumeration of bacteria using fluorescence in situ hybridization (FISH). SCFAs and vitamins were analyzed by HPLC.[Citation20] The PI was used as an indicator of prebiotic characteristics. The PI of the samples was calculated according to an equation given by Palframan and coworkers.[Citation21]
Fluorescence in situ hybridization
A sample (375 µl) was taken from the batch culture and added to 1.125 ml of a filtered 4% (w/v) paraformaldehyde solution (pH 7.2), which was mixed and stored at 4°C overnight to fix the bacterial cells. The fixed cells were washed twice with filtered PBS (pH 7) and resuspended in 150 µl of filtered PBS. Ethanol (150 µl) was added, and the sample was mixed and stored at -20°C for at least 1 h or until needed, no longer than 3 months.
The fixed cells (20 µl) were spread on a slide (Teflon/poly-L-lysine), which was placed on a slide warmer at 45°C for 10–12 min until the sample had dried. The slide was dipped in 50, 80, and 96% (v/v) ethanol. For the hybridization of lactobacillus, 20 µl of lysozyme was dropped into the solution, after which the sample was left for 15 min and then washed with distilled water before dipping in ethanol. The purpose of the dip at each concentration for 3 min was to break the cell walls, thereby enabling the DNA probes to penetrate and bind to specific bacterial DNAs. Next, the slide was dried on the slide warmer.
Pre-warmed hybridization buffer was mixed with 5 µl of sample and 45 µl of a DNA probe solution. Each sample was specific to each bacterial species and incubated in a hybridization oven ().[Citation22] The samples were then left for 4 h and washed with washing buffer (50 ml) by soaking for 15 min. Next, the samples were washed with 50 ml of chilled distilled water twice; the slide was dried; and antifade (5 µl) was added immediately into each hole, after which the slides were covered with cover slips. A fluorescence microscope was used to count the bacterial cells. A minimum of 15 fields, each containing 10–100 cells, was counted for each slide.[Citation16] The equation for the PI calculation is as follows:
Table 2. Probe reference and hybridization temperature.
PI = α + β-γ–δ
α = (Bif24/Bif0)/Total
β = (Lac24/Lac0)/Total
γ = (Bac24/Bac0)/Total
δ = (Clos24/Clos0)/Total
Total = Eub24/Eub0
Eub0 and Eub24 are the Eubacterium numbers or total bacterial numbers at 0 and 24 h
Bif0 and Bif24 are the Bifidobacterium numbers at 0 and 24 h
Lac0 and Lac24 are the Lactobacillus numbers at 0 and 24 h
Bac0 and Bac24 are the Bacteroides numbers at 0 and 24 h
Clos0 and Clos24 are the Clostridium numbers at 0 and 24 h
The equation assumes that an increase in the populations of bifidobacteria and/or lactobacilli has a positive prebiotic effect, in contrast to an increase in Bacteroides and Clostridium, which has negative prebiotic effect.[Citation21]
Analysis of short-chain fatty acids and lactic acid
Samples (1.5 ml) were centrifuged (13,000 × g) at 28°C for 15 min to remove particulate materials and cells. Then, the supernatants were filtered through a 0.2 µm nylon filter. Samples (20 µl) were injected into an HPLC system equipped with a UV detector, monitoring at 214 nm. A Bio-Rad Aminex HPX-87H ion exclusion column (Bio-Rad, California, USA) was maintained at 50°C with a column heater. The eluent, 0.005 M sulfuric acid in HPLC-grade water, was pumped through the column at flow rate of 0.6 ml/min. Data were integrated using HPLC software (Agilent Technologies, California, USA), and external calibration curves for lactate, acetate, propionate, and butyrate were used to quantify the concentrations in the samples.[Citation23,Citation24]
Analysis of vitamins B1 and B2 and folic acid
Samples (1.5 ml) were centrifuged (13,000 × g) at 28°C for 15 min to remove particulate materials and cells. Thereafter, the supernatants were filtered through a 0.2 µm nylon filter. Samples (20 µl) were injected into an Agilent 1200 HPLC system equipped with a UV detector set at 254 nm. An Inertsil Diol column was maintained at 40°C with a column heater. The eluent, acetonitrile:water:trifluoroacetic acid (CN3CN:H2O:TFA) at a ratio of 90:10:0.1, was pumped through the column at flow rate of 1 ml/min. Data were integrated using HPLC software (Agilent Technologies, California, USA). Vitamins B1 and B2 and folic acid were quantified in the sample using external calibration curves.[Citation23,Citation24]
Statistical analysis
Bacterial counts at 0 and 24 h of the batch culture fermentations were tested for significance using paired t-tests, assuming equal variance and considering both sides of the distribution (two-tailed distribution). The difference was considered at the 95% significance level if p < 0.05. Analyses were conducted using SPSS for Windows software version 16 (SPSS Inc., Chicago, USA).
Results and discussion
Digestibility of Keang-Hleung paste under simulated gastrointestinal conditions
The digestion of basic Keang-hleung paste without garcinia (P1) and with garcinia (P2) by human salivary α-amylase at pH 6.8 and 37°C for 120 s showed that the degree of hydrolysis increased rapidly within 5 s and slightly increased until 60 s, after which it gradually decreased. The maximum hydrolysis of P1 and P2 were 3.38% and 3.41%, respectively, after 60 s of incubation (). The reducing sugar content increased from 144.82 µmole/ml to 282.32 µmole/ml.
Figure 1. The hydrolysis of basic Keang-hleung paste without garcinia (P1) and garcinia Keang-hleung paste (P2) with (A) human salivary α-amylase at pH 6.8, 37°C; (B) artificial human gastric juice (pH 2); (C) human pancreatic α-amylase digestion.
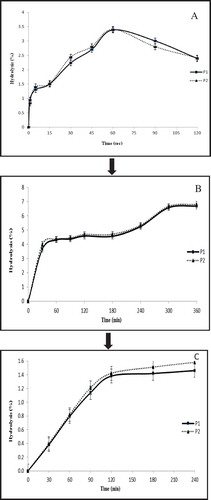
After hydrolysis by human salivary α-amylase, the mixed Keang-hleung paste was further hydrolyzed by artificial human gastric juice (pH 2) for 240 min. The percentage of hydrolysis increased with increasing incubation time until 120 min and then remained approximately unchanged (). The percentage of hydrolysis of P1 and P2 were 1.46% and 1.58% at 120 min, respectively. The reducing sugar increased from 190.69 µmole/ml to 214.60 µmole/ml.
The maximum hydrolysis of basic Keang-hleung paste without garcinia (P1) and with garcinia (P2) was investigated after hydrolysis with human pancreatic α-amylase. The degree of hydrolysis increased rapidly within 30 min and then slightly increased until 6 h of incubation. The percentage of hydrolysis of P1 and P2 were 6.69% and 6.80%, respectively, after 6 h of incubation (Fig. 1C). The reducing sugar increased from 180.01 µmole/ml to 282.06 µmole/ml. The composition of Thai red curry powder reportedly consists of crude protein, crude fat, and crude fiber.[Citation25] However, based on this result, approximately 88% of both Keang-hleung pastes (P1 and P2) would reach the colon if consumed, since some of the pastes were hydrolyzed by salivary α-amylase (3.38% and 3.41%), stomach acid (1.46% and 1.58%), and human pancreatic α-amylase (6.69% and 6.80%). Therefore, the totally and partially hydrolyzed material in the mouth, stomach, and small intestine was approximately 12%, indicating that the curry paste (P1 and P2) may contain non-digestible components other than carbohydrates. Typically, carbohydrates are mostly digested in the small intestine where brush border enzymes, that is, isomaltase, glucoamylase, maltase, sucrose, and lactase, hydrolyze α-1,4- and α-1,6-linked glucosaccharides to yield monosaccharides as end products.[Citation26] Using both the ileostomy patient model and the incubation model, 88% of inulin and oligofructose was determined to reach the colon.[Citation27] Therefore, the basic Keang-hleung paste without garcinia (P1) and with garcinia (P2) in this experiment appeared to be partially resistance to digestion, up to 88%, under stimulated gastrointestinal conditions.
Microbial population changes and prebiotic index calculation
The fermentability of the basic Keang-hleung paste without garcinia (P1) and with garcinia (P2) by a fecal slurry was tested in stirred, pH-controlled batch cultures. The changes in microbial populations were determined by FISH in accordance with the specific DNA probes used for five bacterial genera. In the basic Keang-hleung paste without garcinia (P1) (), the number of bifidobacteria significantly (p < 0.05) increased from 7.96 Log cell/ml to 8.13 Log cell/ml; the number of lactobacilli increased, but not significantly (p > 0.05), from 7.66 Log cell/ml to 7.76 Log cell/ml; the number of Bacteroides significantly (p > 0.05) decreased from 7.74 Log cell/ml to 7.48 Log cell/ml; and the number of Clostridia significantly (p < 0.05) decreased from 9.04 Log cell/ml to 8.63 Log cell/ml.
Figure 2. Change in bacterial populations enumerated using fluorescent in situ hybridization in stirred pH-controlled batch culture fermentation with (A) basic Keang-hleung paste without garcinia (P1); (B) garcinia with Keang-hleung paste (P2).
Bif: Bifidobacteria; Lab: Lactobacillus; Bac: Bacteroides; Chis: Clostridia; Eub: Eubacteria
Different letters within same genus mean significant difference (p < 0.05).
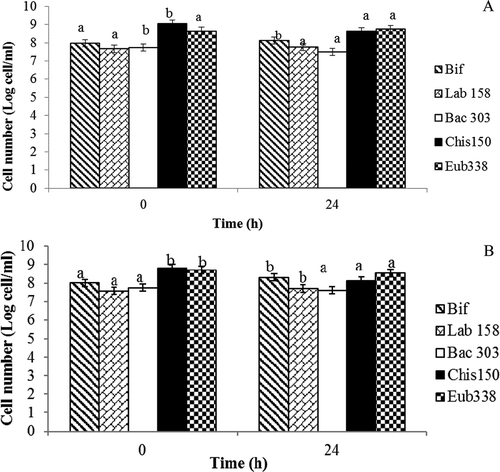
In Keang-hleung paste with garcinia (P2) (), the number of bifidobacteria significantly (p < 0.05) increased from 8.00 Log cell/ml to 8.30 Log cell/ml; the number of lactobacilli also significantly (p < 0.05) increased from 7.56 Log cell/ml to 7.71 Log cell/ml; the number of eubacteria significantly (p < 0.05) decreased from 8.71 Log cell/ml to 8.53 Log cell/ml; the number of Bacteroides decreased, but not significantly (p > 0.05), from 7.75 Log cell/ml to 7.61 Log cell/ml; and the number of Clostridia significantly (p < 0.05) decreased from 8.81 Log cell/ml to 8.12 Log cell/ml.
The PIs of basic Keang-hleung paste without garcinia (P1) and with garcinia (P2) in the batch culture were calculated according to their bacterial change, and these results are summarized in . Keang-hleung paste with garcinia (P2) had a PI of 2.75, which was higher than the PI of the basic Keang-hleung paste without garcinia (P1) of 1.19, probably because garcinia plays an important role in the inhibition of microbial growth. For example, a decrease in the number of Clostridia () may be due to the function of hydroxy acids and other weak acids, which are mainly derived from garcinia. Both pastes stimulated the growth of bifidobacteria and lactobacilli, particularly Keang-hleung paste with garcinia. Benkeblia and Shiomi (2006) reported that shallot and garlic consist of soluble non-digestible carbohydrates including 2–6% inulin, 2–6% oligofructose and 9–16% inulin, and 3–6% oligofructose, respectively.[Citation8] In addition, the total dietary fiber content of Keang-hleung paste with garcinia (P2) was higher than that of basic Keang-hleung paste without garcinia (P1). To summarize, P2 had a higher total dietary fiber content and PI than P1.
Table 3. Prebiotic index of basic Keang-Hleung paste without garcinia (P1) and garcinia Keang-Hleung paste (P2).
Production of short-chain fatty acids and lactic acid
The SCFAs produced by fermentation of the basic Keang-hleung paste without garcinia (P1) and with garcinia (P2) in a batch culture were determined by HPLC. Fermentation of the basic Keang-hleung paste without garcinia produced 120.81 µg/ml lactic acid within 24 h of fermentation (). A high concentration of acetic acid was produced and significantly (p < 0.05) increased with the increase in fermentation time, and the maximum concentration (26.43 µg/ml) was detected at 24 h. Propionic acid was produced throughout the fermentation, with a maximum concentration of 14.56 µg/ml detected at 6 h. Unfortunately, butyric acid was produced at lower concentrations (9.18–11.81 µg/ml).
Table 4. Short-chain fatty acid (SCFA) production of basic Keang-Hleung paste without (P1) and with garcinia (P2).
Fermentation of the Keang-hleung paste with garcinia (P2) produced lower concentrations of lactic acid (72.41–82.97 µg/ml) compared to the Keang-hleung paste without garcinia (P1). Acetic acid was produced, and its concentration increased as the fermentation time increased, with the maximum concentration (23.55 µg/ml) detected at 24 h. Propionic acid was generated in large quantities at 6 and 12 h of fermentation, with the maximum concentration of 9.80 µg/ml detected at 12 h. Butyric acid slightly decreased as the fermentation time increased, from 10.65 µg/ml to 9.18 µg/ml at 6 and 12 h of fermentation, respectively.
Comparing the basic Keang-hleung paste without garcinia (P1) to that with garcinia (P2) revealed that the concentration of lactic acid produced from P1 was higher than from P2 at all fermentation times, which was probably due to the apparently higher number of lactobacilli in the fermentation of P1 (7.76 Log cell/ml) than in P2 (7.56 Log cell/ml). In general, the lactic acid detected in the fermentation of plant materials is mainly produced by lactobacilli.[Citation28] In addition, the concentration of total SCFAs produced by the fermentation of P1 was higher than that produced by P2. Importantly, the products of fermentation depending on the species and the type and availability of substrates can be converted to various end products.[Citation28]
Production of vitamins B1 and B2 and folic acid
Vitamins B1 and B2 and folic acid produced by the fermentation of the basic Keang-hleung paste without garcinia (P1) and with garcinia (P2) in a batch culture were determined by HPLC, and the results are shown in . The basic Keang-hleung paste without garcinia (P1) produced higher concentrations of vitamin B1 as the fermentation time increased, from 12.82 µg/ml to 18.38 µg/ml at 6 and 24 h, respectively. Vitamin B2 significantly (p < 0.05) increased from 34.42 µg/ml to 45.28 µg/ml at 6 and 12 h, respectively. Folic acid was not detected throughout the fermentation. The vitamin B1 in Keang-hleung paste with garcinia (P2) sharply decreased from 21.65 µg/ml to 5.17 µg/ml and then slightly increased to 5.99 µg/ml at 24 h. Unfortunately, in P2, vitamin B2 was not detected at 6, 12, or 24 h, and folic acid was not detected throughout the fermentation. Fecal fermentation of P1 resulted in a higher production of lactic acid and SCFAs than the fermentation of P2, which may have been due to compounds such as hydroxycitric acid (HCA) in garcinia that can inhibit the growth of lactic acid-producing bacteria. Fecal fermentation of P1 resulted in a higher production of vitamins B1 and B2 than the fermentation of P2 because P1 did not inhibit the growth of lactic acid-producing bacteria. In addition, lactic acid-producing bacteria could produce vitamins B1 and B2, and compounds in garcinia might inhibit the growth of bacteria that produce lactic acid and vitamins B1 and B1. Folic acid can be produced by different genera, and sour curry paste did not support the growth of these bacteria. Gibson et al. (1998) identified various end products of fecal fermentation depending on the species, type, and availability of substrates in the sample.[Citation28] Nudler and Mironov (2004) reported that vitamin B1 was synthesized by bacilli and vitamin B2 was synthesized by B. subtilis, E. coli and R. etli.[Citation29] Therefore, the basic Keang-Hleung paste without garcinia (P1) could be used as a food source of vitamins B1 and B2 produced in the human gut. In general, these vitamins are as beneficial to gut health as flavoproteins, which are essential for the metabolism of amino acids, energy production and the activation of folate and pyridoxine to their respective coenzyme forms.
Table 5. Vitamin B1, B2, and folic acid production of basic Keang-Hleung paste without (P1) and with garcinia (P2).
Conclusion
The basic Keang-hleung paste without garcinia (P1) and with garcinia (P2) in this experiment appeared to be somewhat resistant, at approximately 88%, under stimulated gastrointestinal conditions. After the remaining components had been fermented by fecal flora in a pH-controlled batch culture, the numbers of bifidobacteria and lactobacilli slightly increased, while the number of Bacteroides and Clostridia significantly decreased. Keang-hleung paste with garcinia (P2) had a higher PI than the basic Keang-hleung paste without garcinia (P1). However, the concentrations of SCFAs produced by the human fecal fermentation of basic Keang-hleung paste without garcinia (P1) were higher than that produced by fermentation of the paste with garcinia (P2) for all SCFAs. The basic Keang-hleung paste without garcinia (P1) could produce vitamins B1 and B2 but not folic acid. The Keang-hleung paste with garcinia (P2) produced vitamin B1 but not vitamin B2 or folic acid. Therefore, the addition of garcinia to Keang-hleung paste appears to increase the prebiotic effect of the paste by lowering the numbers of harmful bacteria. The interaction of the gut microbiota with sour curry paste will be further confirmed in animal and clinical studies.
References
- Gibson, G.R.; Roberfroid. M.B. Dietary Modulation of the Human Colonic Microbiota: Introducing the Concept of Prebiotics. Journal of Nutrition 1995, 125, 1401–1412.
- Wichienchot, S.; Chinachoti. P. Prebiotic Oligosaccharides: Origins and Production, Health Benefits and Commercial Applications. In Oligosaccharides: Sources, Properties and Applications, N.S.; Gordon, Ed.; Nova Science Publishers, Inc.: New York. 2011; 59–83.
- Vernazza, C.L.; Rabiu, B.A.; Gibson, G.R. Human Colonic Microbiology and the Role of Dietary Intervention: Introduction to Prebiotics. In Prebiotics: Development and Application; G.R.; Gibson, R.A.; Rastall, Eds.; John Wiley & Sons: Chichester. 2006; 1–28.
- Macfarlane, G.T.; Macfarlane, S.; Gibson, G.R. Validation of a Three-stage Compound Continuous Culture System for Investigating the Effect of Retention Time on the Ecology and Metabolism of Bacteria in the Human Colon. Microbial Ecology 1998, 35, 180–187.
- Roberfroid, M.B. Prebiotics: Preferential Substrates for Specific Germ? The American Journal of Clinical Nutrition 2001, 73, 406–409.
- Thammarutwasik, P.; Hongpattarakere, T.; Chantachum, S.; Kijroongrojana, K.; Itharat, A.; Reanmongkol, W. Prebiotics: A Review. Songklanakarin Journal of Science and Technology 2009, 31(4), 401–408.
- Wichienchot, S.; Jatupornpipat, M.; Rastall, R.A. Oligosaccharides of Pitaya (dragon fruit) Flesh and their Prebiotic Properties. Food Chemistry 2010, 120(3), 850–857.
- Benkeblia, N.; Shiomi, N. Fructooligosaccharides of Edible Alliums: Occurrence, Chemistry and Health Benefits. Current Nutrition and Food Science 2006, 2, 181–191.
- Ruby, A.J.; Kuttan, G.; Baru, K.D.; Rajasekharan, K.N.; Kuttan, R. Anti-Tumour and Antioxidant Activity of Natural Curcuminiods. Cancer Letters 1995, 94, 79–83.
- Ahsan, H.; Parveen, N.; Nizam, U.K.; Hadi, S.M. Pro-Oxidant, Anti-oxidant and Cleavage Activities on DNA of Curcumin and Its Derivatives Demethoxycurcumin and Bisdemethoxycurcumin. Chemico-Biological Interactions 1999, 121, 161–175.
- Cousins, M.; Adelberg, J.; Chen, F.; Rieck, J. Antioxidant Capacity of Fresh and Dried Rhizomes from Four Clones of Turmeric (Curcuma longa L.) Grown in vitro. Industrial Crops and Products 2007, 25(2), 129–135.
- Siripongvutikorn, S.; Thammarutwasik, P.; Huang, Y.A. Antimicrobial and Anti-oxidant Effects of Thai Seasoning, Tom-Yum. LWT-Food Science and Technology 2005, 38, 347–352.
- Jayaprakasha, G.K.; Rao, L.J.; Sakariah, K.K. Antioxidant Activities of Curcumin, Demethoxycurcumin and Bisdemethoxycurcumin. Food Chemistry 2006, 98, 720–724.
- Promjiam, P.; Siripongvutikorn, S.; Usawakesmanee, W.; Wichienchot, S. Effect of Added Garcinia Fruit on Total Phenolic Content, Antioxidant Properties and Quality Changes of the Southern Sour Curry Paste, Keang-hleung, during Storage. Food and Nutrition Sciences 2013, 4, 812–820.
- Sarkar, A.; Sarkar, K.K.T.; Goh, R.P.; Singh, H. Behaviour of an Oil-in-Water Emulsion Stabilized by β-lactoglobulin in an in vitro Gastric Model. Food Hydrocolloids 2009, 23(6), 1563–1569.
- Rueangwatcharin, U.; Wichienchot, S. Digestibility and Fermentation of Tuna Products Added Inulin by Colonic Microflora. International Food Research Journal 2015, 22(5), 2068–2077.
- Korakli, M.; Ganzle, M.G.; Vogel, R.F. Metabolism by Bifidobacteria and Lactic Acid Bacteria of Polysaccharides from Wheat and Rye, and Exopolysaccharides Produced by Lactobacillus sanfranciscensis. Journal of Applied Microbiology 2002, 92, 958–965.
- Robertson, J.A.; Ryden, P.; Botham, R.L.; Reading, L.; Gibson, G.R.; Ring, S.G. Structural Properties of Diet-derived Polysaccharides and Their Influence on Butyrate Production during Fermentation. British Journal of Nutrition 2001, 81, S219–S223.
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Calorimetric Method for Determination of Sugars and Related Substances. Analytical Chemistry 1956, 28, 350–356.
- Phrukwiwattanakul, P.; Wichienchot, S.; Sirivongpaisal. P. Comparative Studies on Physic-chemical Properties of Starches from Jackfruit Seed and Mung Bean. International Journal of Food Properties 2014, 17, 1965–1976.
- Palframan, R.J.; Gibson, G.R.; Rastall, R.A. Development of a Quantitative Tool for the Comparison of the Prebiotic Effect of Dietary Oligosaccharides. Letters in Applied Microbiology 2003, 37, 281–284.
- Rycroft, C.E.; Jones, M.R.; Gibson, G.R.; Rastall, R.A. Fermentation Properties of Gentiooligosaccharides. Letters in Applied Microbiology 2001, 32, 156–161.
- Wichienchot, S.; Prasertsan, P.; Hongpattarakere, T.; Rastall, R.A.; Gibson, G.R. In vitro Three-stage Continuous Fermentation of Gluco-oligosaccharides Produced by Gluconobacter oxydans NCIMB 4943, by the Human Colonic Microflora. Current Issues in Intestinal Microbiology 2006, 7, 13–18.
- Wichienchot, S.; Prasertsan, P.; Hongpattarakere, T.; Rastall, R.A. Manufacture of Gluco-oligosaccharide Prebiotic by Gluconobacter Oxydans NCIMB 4943. Songklanakarin Journal of Science and Technology 2009, 31(6), 597–603.
- Inchuen, S.; Narkrugsa, W.; Pornchaloempong, P. Effect of Drying Method on Chemical Composition, Color and Antioxidant Properties of Thai Red Curry Powder. Kasetsart Journal -Natural Science 2010, 44, 142–151.
- Johnson, C.D.; Schmit, G.D. Mayo Clinic Gastrointestinal Imaging Review; Mayo Clinic Scientific Press: New York, 2005; 60 pp.
- Cummings, J.H.; Macfarlane, G.T.; Englyst, H.N. Prebiotic Digestion and Fermentation. The American Journal of Clinical Nutrition 2001, 73(41), 5–20.
- Gibson, G.R.; Wynne, A.; Bird, A. Microflora of Intestine: Role and Effects. In Encyclopedia of Human Nutrition; M.; Sadler, B.; Caballero, S.; Strain, Eds.; Academic Press, London, 1998; 1282–1289.
- Nudler, E.; Mironov, A.S. The Riboswitch Control of Bacterial Metabolism. Trends in Biochemical Sciences 2004, 29, 11–17.
