ABSTRACT
Food protein hydrolysates and peptides are known as a promising functional food ingredient. Encapsulation can be used for improving the bioavailability and organoleptic properties of peptides. In the present study, good entrapment efficiency was obtained when 2.5% sodium alginate, 3% protein concentration, and 2% calcium chloride. Fourier transform infra-red analysis (FTIR) and scanning electron microscope presented that papain hydrolysate was cross-linked in calcium alginate beads. The peptide showed high antioxidant activity, metal ion chelation activity, and reducing power as compared to encapsulated peptide.
Introduction
Ziziphus jujube, usually called jujube, is cosmopolitan in Europe and geographic region. This plant possesses multiple healthful properties like a contraceptive, antimicrobial and inhibitor[Citation1], medicine, immune stimulating[Citation2], anti-diabetic[Citation3], hypoglycaemic[Citation4], sedative and hypnotic [Citation5-Citation7], analgesic [Citation8,Citation9], and agglutination activity[Citation10].
Food protein hydrolysates and peptides are fallen down in the classification of favourable functional food ingredients. The application of protein hydrolysates come up with many issues such as low bioavailability, bitter taste, hygroscopic, and likelihood of interacting with the food matrix. Encapsulation as a delivery mechanism can be used to overcome these challenges for improving the bioavailability and organoleptic properties of the peptides [Citation11].
In the food and beverage industry, alginates remain as one of the most important ingredients in food products [Citation12]. Alginates are used as stabilizers and thickeners in various products that include jelly [Citation13], drinks such as chocolate milk [Citation14], and desserts such as ice cream. [Citation15]. Alginates are of growing importance in the healthcare and pharmaceutical industry, since the first successful encapsulation of islet cells in alginate matrices was extensively employed for cell culture and transplantation [Citation16].
This study deals with the encapsulation of peptides from Z. jujube seeds which were produced using papain. Further, the protein hydrolysate from Z. jujube was subjected for characterisation of antioxidant activity during storage.
Materials and methods
Chemicals
1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2-azino-bis(3- ethylbenzothiazoline-6-sulphonic acid) diammonium salt (ABTS) methanol, ethanol, ABTS, potassium persulphate, ferrous chloride, 3-(2-pyridyl)-5,6-diphenyl-1,2,4-triazine- 4,4-disulphonic acid sodium salt(ferrozine), glutathione (GSH), EDTA, and trolox were purchased from Sigma Chemicals Co. (St. Louis, MO, USA). All other chemicals used were of analytical grade. Papain enzyme gifted by Advanced Enzymes Technologies Pvt Ltd., Mumbai, India.
Sample preparation
Ber (Ziziphus jujube) were procured in a lot from local market of Matunga area of Mumbai, India during the month of April – May 2013 and stored at −20°C Z. jujuba were processed to obtain fruit pulp and seeds. Initially, Z. jujuba fruits were manually cleaned, separated from damaged fruits, and washed with distilled water followed by separation of pulp from the seed. Seeds were dried at 50°C in a tray dryer for 12 h. The grinding of seed was carried out by using laboratory and passed through 40 mesh sieve. The resulting Ber Seed Powder (BSP) was packed into clean airtight self-sealable polyethylene pouches and kept at 4°C until further analysis.
Production of papain hydrolysate peptides from Z. jujuba seeds
BSP (1.5g) of BSP was taken in 20 ml of 50 mM Tris-HCl buffer (pH 7.5). The mixture was stirred for 2 h. The mixture was then centrifuged at 8000 g for 25 min at 4°C to remove the solid particles. The supernatant was precipitated with ammonium sulphate at 85% saturation at 4°C. After complete dissolvation of ammonium sulphate salt; the mixture was again centrifuged at 8000 g for 25 min at 4°C. The supernatant was discarded and the pellet was collected. The pellet was resuspended in distilled water and dialyzed against 50 mMTris-HCl buffer pH 7.5 for 24 h at 4°C. The obtained dialysate was freeze-dried for further use. To prepare hydrolysates, a solution of the extracted protein in Tris-HCl buffer (50 mM, pH 7.5) was subjected to enzymatic hydrolysis using papain. The ratio of protein substrate to each enzyme was 50:1. Each enzyme was dissolved separately (0.1 mg/ml) in the same buffer. The protein extract was incubated at optimum temperature and pH range enzymes (optimum temperature 65°C and pH 7) for 1.5 h. The enzymatic hydrolysis was inactivated by heating in boiling water for 15 min, centrifuged at 7000 g for 10 min; the supernatant was then transferred to fresh tubes for subsequent studies. The prepared hydrolysates were passed through an ultra-membrane with a 5 kDa cut-off. The retentate was taken for encapsulation [Citation17].
Preparation of calcium alginate beads
About 50 ml of 2-2.5% w/v sodium alginate solution was prepared. The protein hydrolysate was loaded in a concentration ranging from 2-3% w/v. The solution of sodium alginate and peptides was mixed properly. The solution was introduced dropwise into 100 ml of calcium chloride solution (concentration ranging from 2-3% w/v) through glass syringe with a size-21 G needle. The aqueous solution of calcium chloride was stirred at 4000 rpm. The stirring was continued for 1h, and the calcium alginate beads were harvested by filtration, washed with distilled water, and air-dried overnight [Citation18].
Entrapment efficiency
The beads were separated from the dispersion by centrifugation at 15,000 rpm for 20 min. The supernatant obtained after centrifugation was suitably diluted and analysed for free protein by Bradford method at 595 nm [Citation19, Citation20]. The per cent entrapment efficiency was calculated as follows:
Fourier transform infra-red (FTIR) analysis
The IR analysis of peptide and peptide-loaded beads prepared were analysed with FTIR spectrophotometer. All the samples were crushed with potassium bromide to get pellets at 600kg cm−Citation2. Spectral scanning was done in the range of 400-4000 cm−Citation1 [Citation21].
Scanning electron microscope (SEM)
The surface morphology structure of the calcium alginate and papain hydrolysate calcium alginate beads samples was investigated by Analytical-SEM (type: JEOL, JSM-6360LA, Japan) with 10 kV voltage for secondary electron imaging. The hydrogel samples were first soaked in deionised water (after dissolution in medium pH of 7.5) for 24 h to bulge the internal channels and remove any impurities as well. The samples were then dehydrated by suddenly freezing using liquid nitrogen followed by lyophilisation conditions at 90°C under 0.5 mbar and coated with Au using an ion sputter coater in (model: 11430, USA, combined with vacuum base unit or SPi module control, model: 11425, USA) [Citation22].
Sample preparation for release of peptide entrapped in calcium alginate bead
10 mg of beads were placed in 10 mL of 50 mM Tris-HCl pH 7.5 for 24 h for 4°C. The solution was filtered. The filtrate was used for measuring the antioxidant activity, metal chelation activity, and reducing power [Citation18, Citation23].
Antioxidant activity
ABTS radical scavenging activity
The ABTS radical cation (ABTS+) was prepared with final concentrations of 7mM ABTS+and 2.45 mM potassium persulphate. The resultant mixture was left for 16 h in the dark at room temperature. The ABTS+ solution was diluted in 5mM phosphate buffered saline (PBS) pH 7.4, to an absorbance of 0.70 ± 0.02 at 734 nm. Two millilitres of diluted ABTS+ solution were mixed with 20 µl of the sample (peptide and entrapped peptide solution). The obtained mixture was shaken vigorously for 1 min, and the absorbance value was measured at 6 min intervals for a total of 10 min. An equivalent volume of distilled water was used as the blank. A standard curve was plotted by reacting 2ml of diluted ABTS+ solution with 20 µl of different concentrations of trolox (0-25 µM/L) in 5 mM PBS (pH 7.4). The percentage of the absorbance reduction at 734 nm was measured and plotted as a function of the concentration of trolox (as a standard reference) or antioxidant samples [Citation24]. To calculate TEAC (trolox equivalent antioxidant capacity) value, the gradient of the plot of the absorbance reduction (%) versus sample concentration was divided by the gradient of the trolox plot. All experiments were carried out in triplicates. The ABTS scavenging activity of the samples was expressed as TEAC (µmol TE/mg protein) value.
DPPH radical scavenging activity
The peptide and entrapped peptide solutions were dissolved in distilled water at 0.3 mg/ml. Two hundred microliters of the sample were then mixed with 600 µl of methanol and 200 µl of DPPH (0.15 mM in methanol). Shaken vigorously for 2 min, the mixture was kept for 30 min in the dark at room temperature. The absorbance of the mixture was measured at 517 nm using a UV–vis spectrophotometer. The control contained 800 µl of methanol and 200 µl of DPPH (0.15 mM) [Citation24]. DPPH radical scavenging ability was calculated using the following equation:
All experiments were carried out in triplicate.
Metal chelation activity
In the chelation test, 200 µl of peptide and entrapped peptide solution was mixed with 10 µl of FeCl2 (2 mM) and 600 µl of double distilled water. Subsequently, 20 µl of ferrozine solution (5 mM) was added to the mixture, followed by vigorous mixing for 2 min. The mixture was then kept for 10 min at room temperature. Afterward, the colour reduction, due to the chelation of FeCitation2+, was recorded by measuring the absorbance at 562 nm. The control sample contained 800 µl of double distilled water, 10 µl of FeCl2, and 20 µl of ferrozine solution (5 mM) [Citation25]. All experiments were carried out in triplicate. The chelating activity was calculated as a percentage using
All experiments were carried out in triplicate.
Reducing power assay
The peptide and entrapped peptide solution were dissolved in distilled water. Five hundred microliters of the sample (peptide or hydrolysate) and GSH solutions at different concentrations (0–2 mg/ml) were mixed with 500 µl of sodium phosphate buffer(0.2 M, pH 6.6) and 500 µl of potassium ferricyanide (1% (w/v)). After incubation at 50 ± 1°C for 20 min, 500 µl of 10% TCA was added. The resulting mixture was centrifuged at 4000g for 10 min. The supernatant (500 µl) was collected and mixed with 500 µl of distilled water and 100 µl of 0.1% (w/v) FeCl3 [Citation26]. The mixture was kept for 5min at room temperature, and the resulting absorbance was recorded at 700 nm. All determinations were performed in triplicate.
Statistical analysis
Statistical analysis was performed by using an MS Excel (2007) t-test. All data were expressed as mean from triplicate samples. Differences were considered statistically significant at p<0.05 level.
Results and discussion
Entrapment efficiency
The encapsulation efficiency depends on more availability of CaCitation2+ ions cross-link with guluronic units of alginate providing more amounts of protein entrapped, or similar phenomenon was also happened by increasing alginate concentration, and the entrapment efficiency was also increased [Citation27-Citation30]. showed that entrapment efficiency changes in concentrations of sodium alginate and calcium chloride had a significant effect on peptide entrapment. It can be seen that good entrapment efficiency was obtained from a 2.5% sodium alginate, 3% protein concentration, and 2% calcium chloride as compared to other formulations in this study. This optimised formulation was used for further study. The various factors such as the concentration of CaCl2 and concentration of sodium alginate were responsible for encapsulation. The concentration of sodium alginate was between 2 and 2.5% due to the viscosity property of sodium alginate. Zam et al. [31] reported the decrease in the encapsulation efficiency by an increase in the sodium alginate concentration due to increasing in viscosity [Citation31].
Table 1. Entrapment efficiency of different formulation of calcium alginate beads.
The difference between the encapsulation efficiency of A4 and B4 formulation was observed. The possible reason may be damage of microcapsule due to saturation of calcium binding sites in the glucoronic acid chain, preventing further calcium ion entrapment as reported by Ostberg et al. [Citation32]or to osmotic stress as reported by Takayuki et al. [Citation33].
Fourier transform infra-red (FTIR) analysis
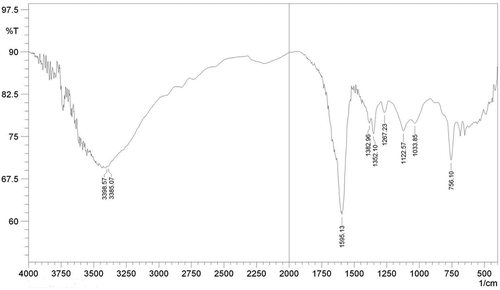
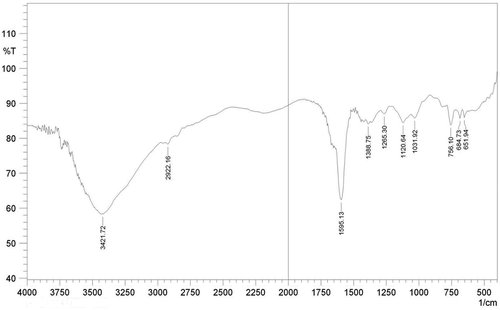
FT-IR spectra with the specific transmittance bands are presented in and . represents the FTIR spectra of whole protein entrapped in calcium alginate bead. The FTIR spectrum of whole protein showed characteristics peaks of O-H and N-H stretching at 3398 cm−Citation1 -3385 cm−Citation1, C=O, C-N, N-H (amide I) stretching at 1595 cm−Citation1, and COO− stretching at 1382 cm−Citation1 The bands located at 1200–700 cm−Citation1 corresponding to the carbohydrate region, respectively, to C-O and the C-O-H vibrations. represents the FTIR spectra of papain peptide entrapped in calcium alginate bead. The FTIR spectrum of papain entrapped bead showed characteristics peaks of O-H and N-H stretching at 3421 cm−Citation1, C=O, C-N, N-H (amide I) stretching at 1595 cm−Citation1, CH2 stretching at 2922.16 cm−Citation1, and COO− stretching at 1388.76 cm−Citation1 The bands located at 1200–600 cm−Citation1 corresponding to the carbohydrate region, respectively, to C-O and the C-O-H vibrations [Citation21].
Morphological investigation of papain hydrolysate calcium alginate beads
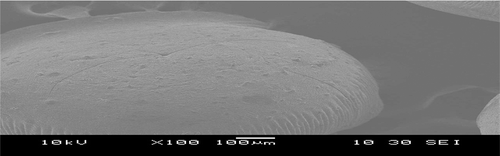
The scanning electron micrographs of calcium alginate beads and papain hydrolysate calcium alginate beads are presented in and , respectively. The morphological surface of alginate beads is very rough, rugged, and uniform in shape whereas cross-linked papain hydrolysate calcium alginate beads; the surface of grafted alginate beads tends to be smoother, spherical, and has almost turned into an entirety, which may be ascribed to the introduction of papain hydrolysate with alginate side chain.
Antioxidant activity of encapsulated peptides
The antioxidant activity of peptide and encapsulated peptide is presented in . The ABTS radical scavenging activity of protein (9.411 µ M of TE/g) was found to be high than encapsulated one (6.78 µ M of TE/g). The DPPH radical scavenging activity of peptide was 17.54%, whilst that of encapsulated was 13.016%. The peptides exhibit ion chelating capacity. The metal ion chelation of peptide and encapsulated peptide can be seen in . Both the peptide and encapsulated peptide exhibit the metal ion chelation capacity. The peptide showed 57.18% of metal chelation capacity which was slightly higher than encapsulated peptide was 42.43%.
Table 2. Bioactive properties of peptide and encapsulated peptide.
showed the reducing power of peptide and encapsulated peptide. The reducing power assay was carried out for encapsulated and peptide. The absorbance of the peptide (0.957) was slightly higher than that absorbance of encapsulated peptide was 0.723 at 700nm. The difference between the antioxidant activity of peptides and encapsulated peptides was observed due to drying of encapsulated peptides. Because drying antioxidant activity decreases, decrease in the antioxidant activity was observed by Sonawane and Arya [Citation34].
Storage stability of antioxidant activity of encapsulated peptides
The storage study of peptide and encapsulated peptide for antioxidant activity, metal ion chelation activity, and reducing power assay was carried out for 30 days at the interval of five days. The sample was kept at 4ºC during the study. The effect of storage on antioxidant activity of peptide and encapsulated peptide by using ABTS and DPPH radical scavenging assay is presented in . In ABTS radical scavenging assay, at 0 days, the antioxidant activity of peptide was 9.411 µ M of TE/g and encapsulated peptide was 6.78 µ M of TE/g. The values were considered as 100% to denote the stability. The encapsulated peptide showed same activity as 0 days till 10 days and then slightly decreased, while peptide showed same activity as 0 days till five days and then gradually decreased. At 30th day, the encapsulated peptide showed 6.0 µ M of TE/g (88.49%) and peptide showed 1.28 µ M of TE/g (13.60%). In DPPH radical scavenging assay, at 0 days the % RSA of peptide and encapsulated peptide was 17.54% and 13.016%, respectively. The %RSA of 0 days was considered as 100%. The DPPH activity of peptide decreased gradually than encapsulated peptide. At 30th day, %RSA of encapsulated peptide was 12% (92.19) and that of the peptide was 1.754% (10%).
Table 3. Antioxidant activity of peptide and encapsulated peptide in storage study.
The metal ion chelation activity of peptide and encapsulated peptide is shown in . The metal chelation at 0 days for peptide and encapsulated peptide was 57.18% and 42.43%, respectively. The % chelation at 0 days was considered as 100%. At 30th day, encapsulated peptide showed 39.53% (93.16%) and peptide showed 6.97% (12.19%).
Table 4. Metal ion chelation and reducing power of peptide and encapsulated peptide in storage study
Reducing the power of peptide and encapsulated peptide can be seen in . The absorbance of the peptide at 0 days was 0.957 and that of encapsulated peptide was 0.723 at 700 nm. The absorbance at 0 days was considered as 100%. There was a gradual decrease in absorbance in peptide as compared to encapsulated peptide. At 30th day, encapsulated peptide showed 0.681 (94.17%) and peptide showed 0.137 (14.28%) ().
Conclusion
The peptide was effectively entrapped in 2.5% sodium alginate and 2% calcium chloride. Cross-linked papain hydrolysate in calcium alginate beads was seen through FTIR and SEM. High antioxidant activity, metal ion chelation activity, and reducing power were observed in the case of the peptide as compared to that of encapsulated peptide on 0 days. However, retention of activity was observed during 30 days of storage of encapsulated peptide. Thus, encapsulation of peptide contributes to the maintenance of antioxidant activity, metal ion chelation, and reducing power.
Acknowledgments
The authors thank Department of Biotechnology (DBT), Government of India for their financial support for carrying out this work.
References
- Yoon, J.I.; Al-Reza, S.M.; Kang, S.C. Hair Growth Promoting Effect of Zizyphus Jujuba Essential Oil. Food and Chemical Toxicology 2010, 48, 1350–1354.
- Benammar, C.; Hichami, A.; Yessoufou, A.; Simonin, A.M. Belarbi, M.; Allali, H.; Khan, N.A. Zizyphus lotus L. (Desf.) Modulates Antioxidant Activity and Human T-Cell Proliferation. BMC Complementary and Alternative Medicine 2010, 10(54), 1–9.
- Ambasta, S.P. Useful Plants of India. Publications and Information Directorate; CSIR: New Delhi, India, 1986, 703 pp.
- Glombitza, K.W.; Mahran, G.H.; Mirhom, Y.W.; Michel, K.G.; Motawi, T.K. Hypoglycemic and Antihyperglycemic Effects of Zizyphusspina-Christi in Rats. Planta Medica 1994, 60, 244–247.
- Anand, K.K., B.; Singh, D.; Chand, B.K.; Chandan, V. Gupta, Effect of Zizyphussativa Leaves on Blood Glucose Levels in Normal and Alloxan-Diabetic Rats. Journal of Ethnopharmacology 1989, 27, 121–125.
- Han, B.H.; Park, M.H.; Han, Y.N. Cyclic Peptide and Peptide Alkaloids from Seeds of Ziziphus Vulgaris. Phytochemistry 1990, 29, 3315–3319.
- Tschesche, R.; Kaubmann, E.U. In The Alkaloids; Manske R.; Ed.; HF Academic Press: New York, vol XV, 1975; 165–205.
- Borgi,W.; Bouraoui, A.; Chouchane, N. Antiulcerogenic Activity of Zizyphus Lotus (L.) Extracts. Journal of Ethnopharmacology 2007, 112, 228–231.
- Borgi W.; Recio, M.C.; Rı´os, J.-L.; Chouchane, N. Anti-Inflammatory and Analgesic Activities of Flavonoid and Saponin Fractions from Zizyphus Lotus (L.) Lam. South African Journal of Botany 2008, 74, 320–324.
- Ahmad B.; Khan, I.; Bashir, S.; Azam, S.; Hussain, F. Screening of Zizyphusjujuba for Antibacterial, Phytotoxic and Haemagglutination Activities. Afrcan Journal of Biotechnology 2011, 10, 2514–2519.
- Mohan A.; Subin R. C. K.; Sophia He Q.; Bazinetc L.; Udenigwe CC. Encapsulation of Food Protein Hydrolysates and Peptides: A Review. RSC Advance 2015, 5, 79270–79278
- Vasile, F. E.; Romero A. M.; Judis, M. A.; Mazzobre, M. F. Prosopis alba Exudate Gum as Excipient for Improving Fish Oil Stability in Alginate–Chitosan Beads. Food Chemistry 2016, 190, 1093–1101.
- Milani, J.; Maleki, G. Hydrocolloids in Food Industry. Food Industrial Processesemethods and Equipment; InTech: Croatia, 2012; 17e38 pp.
- Khoury, D. E.; Goff, H. D.; Berengut, S.; Kubant, R.; Anderson, G. H. Effect of Sodium Alginate Addition to Chocolate Milk on Glycemia, Insulin, Appetite and Food Intake in Healthy Adult Men. European Journal of Clinical Nutrition 2014, 68, 613–618
- Toker, O. S.; Dogan, M.; Canıyılmaz, E.; Ersöz, N. B.; Kaya, Y. The Effects of Different Gums and their Interactions on the Rheological Properties of a Dairy Dessert: A Mixture Design Approach. Food and Bioprocess Technology 2013, 6(4), 896–908.
- Lim F.; Sun A. M. Microencapsulated Islets as Bioartificial Endocrine Pancreas. Science 1980, 210(4472), 908–910.
- Kanbargi, K. D.; Sonawane, S. K.; Arya, S .S. Functional and Antioxidant Activity of Ziziphus Jujube Seed Protein Hydrolysates. Journal of food measurement and characterization 2016, 10(2), 226–235
- Mandal, S.; Kumar, S.; Krishnamoorthy, B.; Basu, S. K. Development and Evaluation of Calcium Alginate Beads Prepared By Sequential and Simultaneous Methods. Brazilian Journal of Pharmaceutical Sciences 2010, 46(4), 785–793.
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Analytical Biochemistry 1976, 72, 248–254.
- Guhagarkar S. A.; Malshe V. C.; Devarajan P. V. Nanoparticles of Polyethylene Sebacate: A New Biodegradable Polymer. AAPS PharmSciTech 2009, 10(3), 935–942.
- Stoica, R.; Pop, S. F.; Ion R. M. Evaluation of Natural Polyphenols Entrapped In Calcium Alginate Beads Prepared by the Ionotropic Gelation Method. Journal of Optoelectronics and Advanced Materials 2013, 15, 893–898
- Mohy Eldin, M.S.; Kamoun E.A.; Sofan M.A.; Elbayomi S.M. L-Arginine Grafted Alginate Hydrogel Beads: A Novel pH-Sensitive System for Specific Protein Delivery. Arabian Journal of Chemistry 2015, 8(3), 355–365.
- Deladino, L.; Anbinder, P. S.; Navarro, A. S.; Martino, M. N. Encapsulation of Natural Antioxidants Extracted from ILEX PARAGUARIENSIS. Carbohydrate Polymers 2008, 71, 126–134.
- Memarpoor-Yazdi, M.; Asoodeh, A.; Chamani, J. A Novel Antioxidant and Antimicrobial Peptide from Hen Egg White Lysozyme Hydrolysates. Journal of Functional Foods 2012, 4, 278–286.
- Decker, E. A.; Welch, B. Role of Ferritin as a Lipid Oxidation Catalyst in Muscle Food. Journal of Agricultural and Food Chemistry 1990, 38, 674–677.
- Ahmad, F.; Kadivar, M.; Shahed, M. Antioxidant Activity of Kelussia Odoratissima Mozaff in Model and Food Systems. Food Chemistry 2007, 105, 57–64.
- Gulati N, Nagaich U, Sharma VK, Khosa RL. Effect of Polymer and Cross Linking Agent on In Vitro Release of Quercetin from Microbeads. Asian Journal of Pharmacy and Life Science 2011, 1(4): 1–5.
- Manjanna KM, Kumar TM, Pramod B, Shivakumar. Calcium Alginate Cross-Linked Polymeric Microbeads for Oral Sustained Drug Delivery in Arthritis. Drug Discoveries and Therapeutics 2010, 4(2): 109–122.
- Joshi S, Patel P, Lin S, Madan PL. Development of Cross-Linked Alginate Spheres by Ionotropic Gelation Tecnique for Controlled Release of Naproxen Orally. Asian Journal of Pharmaceutical Sciences 2012, 7(2): 134–142.
- Singh I, Kumar P. Formulation and Optimization of Tramadol Loaded Alginate Beads Using Response Surface Methodology. Pakistan Journal of Pharmaceutical Sciences 2012, 25(4): 741–749.
- Zam W, Bashour G, Abdelwahed W, Khayata W. Alginate-Pomegranate Peels’ Polyphenols Beads: Effects of Formulation Parameters on Loading Efficiency. Brazilian Journal of Pharmaceutical Sciences 2014, 50(4), 741–748
- Ostberg T; Lund ME; Graffner C. Calcium Alginate Matrices for Oral Multiple Unit Administration: Release Characteristics in Different Media. International Journal of Pharmaceutics 1994, 112, 241–248.
- Takayuki, T; Masahiro Y; Yasuo H; Kouichiro S; Shiro K. Preparation of Lactic Acid Bacteria-Enclosing Alginate Beads in Emulsion System: Effect of Preparation Parameters on Bead Characteristics. Polymer Bulletin 2009, 63, 599–607.
- Sonawane, S. K.; Arya S. S., Effect of Drying and Storage on Bioactive Components of Jambhul and Wood Apple. Journal of Food Science and Technology 2015, 52, 2833–2841.