ABSTRACT
Sweet potato (Ipomoea batatas) is a commonly cultivated root crop in tropical and subtropical regions, including Ghana. Different varieties of sweet potato have been bred, in order to expand its utilisation within the food and industrial sector. This study analysed flours made from 12 recently developed Ghanaian sweet potato varieties in terms of their amylose and amylopectin molecular fractions and amylopectin chain length distribution. Starch content of the sweet potato flours ranged from 49 to 77 g/100 g dry matter, with 11 of the varieties containing above 60 g/100 g dry matter. An orange-fleshed variety, Apomuden, had the lowest amount of starch (48.9 g/100 g dry matter), while the cream-fleshed variety Histarch had the highest (77.3 g/100 g dry matter). The flours from the 12 sweet potato varieties had intermediate amounts of amylose, within the range 10–30 g/100 g dry matter, and showed typical molecular distribution according to size-exclusion chromatography (SEC). The fine structures of amylopectin, as revealed by anion-exchange chromatography, contained features common for starches of C-type X-ray pattern, but some structural differences were also observed.
Introduction
Sweet potato is an important root crop and is widely cultivated in Asia, Africa, and other parts of the world. The crop has great potential for tackling malnutrition and contributing to enhancing food security in many developing countries, and also as a raw material for manufacturing industries.[Citation1] Although it has industrial applications, including acting as a source of flour and starch, it is usually consumed in its fresh form or processed into animal feed. Sweet potato yields about 27% flour, but this is low as per industrial terms. In the food industry, flour is used as a raw material in the production of noodles, confectionery, and bakery products.
Sweet potato flour consists of about 50–80% starch.[Citation2] Starch is a natural polymer made of glucose residues bound by glycosidic linkages. It is essentially made from two structurally different polymers, amylose and amylopectin, which are responsible for the properties of starch pastes, gels, and starchy food systems.[Citation3] Amylose is essentially linear, with a few branches, whereas amylopectin is highly branched and has greater molecular weight. Differences in the composition and structure of these two polymers have been reported to generally account for 10–25% of the variation in starches.[Citation4] Amylose acts as both a diluent and an inhibitor of swelling, and is responsible for retrogradation in starches.[Citation5] Amylopectin, accounting for about 75% of most native starches, is primarily responsible for the gelatinisation behaviour of starches. In previous studies, the chain length distribution and average chain length of amylopectin have been correlated with the functional properties of starches from different botanical sources.[Citation6,Citation7] Starches with higher amounts of amylopectin with long chains result in gels with higher viscosity and stability compared with their short-chain counterparts.[Citation8,Citation9]
In response to renewed efforts to broaden the food and industrial raw material base, a number of new varieties of sweet potato have been developed for different applications through conventional and modern breeding techniques. However, utilisation of these varieties for domestic and industrial applications is largely dependent on the properties of the starch, which are affected mainly by its amylose/amylopectin composition and by its molecular structure and distribution.[Citation10] The aim of this study was to produce flours from 12 new Ghanaian sweet potato varieties, determine their amylose and amylopectin molecular fractions and the chain length distribution of the amylopectin, and assess their suitability for food and industrial applications.
Materials and methods
Twelve sweet potato varieties newly developed by the CSIR-Crops Research Institute, Fumesua, Kumasi, were obtained from demonstration farms for the study. The characteristics of these sweet potato varieties are presented in .
Table 1. Characteristics of the 12 Ghanaian sweet potato varieties analysed in this study.
Preparation of sweet potato flour
The freshly harvested, mature sweet potato roots were washed in potable water, hand-peeled, and washed again. The roots were then manually cut into slices (2–4 mm thick). The cut slices were dipped in 0.01% sodium metabisulphite solution for 5 min to prevent browning and then spread thinly on trays and dried at 60ºC for 14 h in a mechanical dryer (Apex Dryer, CSIR-FRI, Accra, Ghana). The dried slices were milled into a fine powder and passed through a 400-µm sieve using a hammer mill. The fine flour obtained was packaged in high density polyethylene (HDPE) bags, sealed airtight with an impulse sealer (Oalink, QNS-3200HI), and stored at room temperature for later use.
Starch determination of the flour
Starch content was determined according to Åman et al.[Citation11] with slight modifications concerning the glucose oxidase reaction and the final reagent volume. Each flour (40 mg) sample was washed three times with 80% ethanol. Then 25 mL acetate buffer (0.1 M, pH 5.0) and 50 µL termamyl (α-amylase, EC 3.2.1.1; 3000 U/mL; Megazyme, Wicklow, Ireland) were added to the sample and it was placed for 30 min in a boiling water bath and agitated three times during incubation. After cooling to 40°C, 100 µL amyloglucosidase (EC 3.2.1.3, 260U/ml; Megazyme, Wicklow, Ireland) was added and the sample was placed in a 60°C shaking water bath overnight. After centrifuging (10 min at 900 g), 40 µL of supernatant was diluted with distilled water (1:25) and 3 mL GOPOD reagent (glucose oxidase plus peroxidase and 4-aminoantipyrine; Megazyme, Wicklow, Ireland) was added. The sample was then placed in a 50°C water bath for 20 min and absorbance was read at 510 nm using a Shimadzu UV spectrophotometer. Glucose concentration in the flour samples was determined from a standard curve with solutions ranging from 0.025 to 0.100 mg/mL. Starch content was calculated as follows:
Amylose determination of the flour
Amylose content was determined according to Chrastil.[Citation12] A 50 mg portion of flour was combined with 6 mL UDMSO (0.6 M urea in 90% dimethyl sulphoxide) and washed three times with ethanol. After decanting the solvent, 100 µL UDMSO was added to the pellet and it was placed in a boiling water bath for 15 min for complete dissolution. Trichloroacetic acid (5 ml, 0.5%) and 50 µL iodine solution (1.27 g I2 and 3.00 g KI per litre) were added and mixed immediately. After 30 min at room temperature, absorbance was read at 620 nm (Shimadzu 1800, Tokyo, Japan) with distilled water as the reference. The amylose content of the flour was calculated using a standard curve as described by Menzel et al.[Citation13] Amylopectin was calculated by difference using the following equation[Citation14]:
Elution profiles of amylopectin and amylose in the flour
Molecular characterisation of starch fractions was performed according to Menzel et al.[Citation15] In brief, a 25 mg flour sample was weighed into a centrifuge tube with 250 µL water, the tube was capped, and the contents were stirred using a magnetic stirrer. After 10 min, 250 µL 2 M NaOH was added and the mixture was stirred for a further 20 min. Thereafter, 750 µL water was added at 20-min intervals on six successive occasions while stirring. A portion of the flour suspension (3.0 mL) was centrifuged (Kubota 8100, Tokyo, Japan.) at 2500 g for 10 min. The supernatant was filtered (0.45µm nylon filter) and 1 mL of the clear filtrate was fractioned by size-exclusion chromatography (SEC) on a Sepharose CL-2B (GE Healthcare, Uppsala, Sweden) (80 × 1.6 cm), with 0.01 M NaOH as eluent and a flow rate of 0.4 mL/min. Fractionated portions (1 mL) were collected on a fraction collector (Gilson FC 204, Middleton, USA) for further analysis of each odd-numbered fraction with phenol-sulphuric acid[Citation16] and each even-numbered fraction with iodine staining methods.[Citation17]
Amylopectin chain length distribution of the flour
Chain length distribution of amylopectin in the sweet potato flour was analysed by high-performance anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD). In brief, 3 mg flour was dissolved in 50 µL 90% DMSO and stirred at elevated temperature (70°C). To this suspension, 450 µL warm water was added after 4–6 h and it was allowed to cool to room temperature before adding 50 µL acetate buffer (pH 5.0, 0.1M), 1 µL isoamylase, and 2 µL pullulanase (Megazyme, Bray, Ireland). The solution was left overnight for de-branching while stirring. The enzyme action was stopped by heating the solution in boiling water for 5 min, the sample was allowed to cool to room temperature, and 300 µL of distilled water was added. The solution was filtered through a 0.45 µm nylon syringe filter (Titan 3, Thermo Scientific, USA) directly into glass vials. HPAEC-PAD analysis was then performed using a Dionex DX 500 device (Sunnyvale, CA, USA) equipped with a PAD system (ED40), as described by Koch et al.[Citation18]
Statistical analysis
All determinations were performed in duplicate. The data obtained were analysed by ANOVA using SPSS 16.0. (SPSS Inc., USA), and the Duncan Multiple Range Test (DMRT) was performed to separate varieties with significantly different (p < 0.05) means. Results are reported as mean ± standard error (SE).
Results and discussion
Starch content of sweet potato flour
The starch content of the flours ranged from 48.9 to 77.0 g/100g dry matter, with 11 varieties containing >60 g/100 g dry matter (). An orange-fleshed variety, Apomuden, had the lowest amount (48.9 g/100 g dry matter) of starch of all 12 sweet potato varieties, while the variety Histarch had the highest (77.3 g/100 g dry matter). Statistical analysis showed some varieties as having significantly higher (p < 0.05) starch than others. Similarities in starch content among some other varieties were also observed, particularly for Otoo and Santom pona. These were comparable to the eight other varieties, i.e. excluding Histarch and Apomuden. The starch content of sweet potato reported by previous authors varies widely, from 50 to 80 g/100 g dry matter.[Citation2,Citation19]
Table 2. Starch (g/100 g dry wt.), amylose (g/100 g dry wt.), and amylopectin (g/100 g dry wt.) contents of flour made from the 12 Ghanaian sweet potato varieties analysed in this study.
The ratio of the two major polysaccharide components in starch, i.e. amylose and amylopectin, is responsible for the textural and thermodynamic properties of a particular starch.[Citation20,Citation21] Amylose content of the 12 flours analysed here was the highest for Ligri (20.2%) and the lowest for Apomuden (10.1%), with a median value of 17.5%. In fact, 10 varieties had an amylose content lower than 20%. Notably, none of the 12 varieties could be classified as a high-amylose (>30%) or low-amylose (<10%) sweet potato, as also observed by Noda et al.[Citation4] The range of variation among the various flours compared well with the previous findings of 14–24% amylose by Noda et al.[Citation4], 18.6% by Badu et al.[Citation22], and 17.8% by Yadav et al.[Citation23] However, it was lower than the amylose content of 22.8% reported by McPherson and Jane[Citation24] or 20.5–25.5% reported by Garcia and Walter[Citation25] for starch from sweet potato. Fractions of starch components have been established to vary among different varieties of the same crop and therefore disparities were expected among the 12 Ghanaian sweet potato varieties analysed here. The influence of growing conditions on the amylose:amylopectin ratio has been studied by several groups for different starch crops.[Citation26–Citation28] Cottrell et al.[Citation27] reported an increase in the amylose content with increasing growing temperature in potato, whereas Haase and Plate[Citation28] only found little variation in the amylose content in potato due to growing conditions. The 12 different sweet potato varieties screened in the present study were all grown in the same year and under reasonably stable climate/weather conditions, so it is reasonable to assume that the growing conditions had little influence, if any, on the samples.
Starches with high amylose content have good film-forming properties[Citation29] and their low gelatinisation temperature can partly be explained by their lower crystallinity.[Citation30] The differences in starch composition observed among the 12 varieties screened here suggest that they may exhibit different pasting and gel texture properties.[Citation2] This in turn will confer different sensory properties on their finished products. For instance, Ligri, which has a higher amylose content, is likely to have a higher paste stability and may be better suited for the manufacture of noodles than, e.g., Apomuden.
Elution profiles of amylopectin and amylose in the flour
Elution profiles showing the distribution of amylopectin and amylose in the flours are presented in –D. The peaks showed good separation of large and small molecular weight starch components. In SEC, the components are separated based on their molecular size, with the larger fractions eluting first, i.e. amylopectin elutes first before amylose. The chromatograms indicated two peaks, the relative proportions of which indicated the concentration of amylose and amylopectin in flour from each sweet potato variety,[Citation31] together with a third and less suggestive peak. The initial high peak corresponded to amylopectin and occurred at an elution volume ranging between 48 and 70 mL. The composition of this peak was confirmed by its wavelength at maximum absorbance (λmax), which varied from 550 to 560 nm, as is typical for amylopectin, resulting in a dark-brown-coloured complex with iodine. The second peak, which was wider and had no peak maximum, corresponded to amylose and smaller and partially degraded amylopectin molecules.[Citation15,Citation31,Citation32] Elution of these smaller polymers occurred over a much wider volume that ranged between approximately 72 and 140 mL.
Figure 1. Elution profiles of flours on Sepharose CL-2B showing amylopectin and amylose distribution A) Patron, Histarch, and Ogyefo, B) Bohye, Dadanyuie, and Faara, C) Ligri, Okumkom, and Sauti, D) Otoo, Patron, and Apumuden.
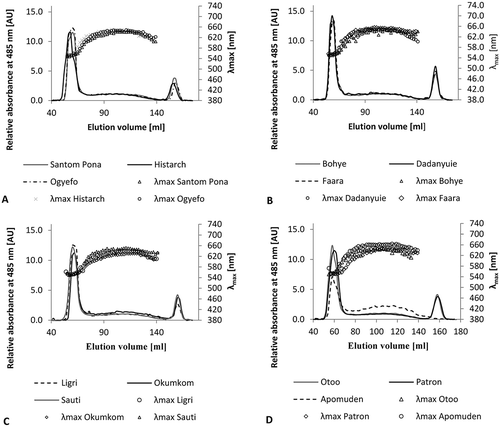
The elution profiles reveal nuanced differences in the distribution of amylose and amylopectin in the flour samples ( and ). For example, although there was a striking resemblance between the chromatograms, the amylopectin peak observed in Apomuden was distinctly shorter than the peak obtained for the other varieties. Moreover, the varieties Histarch, Apomuden, and Otoo showed an increase in λmax at slightly lower elution volume compared with all other varieties. Higher λmax indicates the presence of longer starch chains. These results imply that Histarch, Apomuden, and Otoo contain amylopectin with longer unit chains, or that the elution of low molecular weight amylose interferes with the elution of amylopectin. However, analysis of the amylopectin fine structure showed no significant increase in long amylopectin chains.
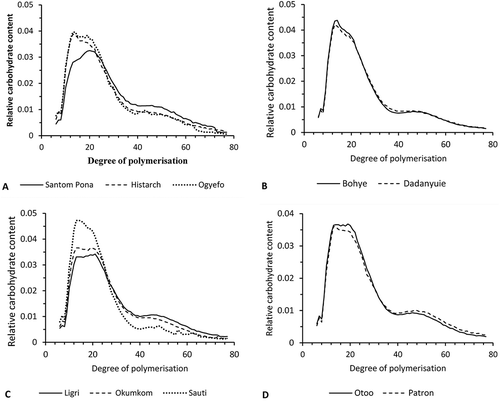
Figure 2. Chain length distribution of the glucan chains of amylopectin measured by HPAEC-PAD A) Santom pona, Histarch, and Ogyefo, B) Bohye and Dadanyuie, C) Ligri, Okumkom, and Sauti, D) Otoo and Apomuden.
The third peak, which occurred after 148 mL, possibly resulted from other components/sugars that were much smaller than amylose and therefore eluted later. Interestingly, the third peak was absent for the variety Apomuden, indicating that there was probably no sugar and/or oligosaccharides in that flour. The concentration of amylose and amylopectin and the presence of other components may account for these observed variations.
Chain length distribution of amylopectin in the flour
The chain length distribution of de-branched amylopectin was studied essentially according to Koch et al.[Citation18], who also developed a calibration for their method of analysis. However, in a modification of that method, in the present study enzymatic de-branching was performed on whole flour instead of isolated amylopectin. The samples from the varieties Faara and Apomuden could not be de-branched to a satisfactory degree for reliable and reproducible analysis by HPAEC-PAD, and therefore had to be omitted. It is plausible that the more complex matrix in whole sweet potato flour compared with isolated starch hindered efficient and complete enzymatic de-branching of the amylopectin. –D shows the chain length distribution of the different sweet potato samples with the same subdivision as the elution profiles in –D. The chain length profiles of the 10 samples analysed differed mainly regarding the degree of polymerisation (DP) 13–21 of chains. All samples also had a relatively high amount of chains longer than DP 60, which is common for tuber starches.[Citation18] For all samples, the fingerprint A-chains, Afp,[Citation33] showed a peak at DP 7. This has also been found by Zhu et al.,[Citation34] who investigated 11 Chinese sweet potato genotypes. This pattern for Afp chains is common for starches with a C-type X-ray pattern, such as pea[Citation18] and also sweet potato.[Citation19] Similar observations have been made by Mar et al.[Citation35] regarding the chain length distribution of amylopectin in starch in Myanmar rice cultivars. Furthermore, similar patterns of amylose and amylopectin have been observed by Torruco-Uco et al.[Citation36] in characterisation of these compounds isolated from diverse botanical sources. In the present study, the amylopectin chain distribution observed for Histarch (), Bohye and Dadanyuie (), and Sauti (Fig. 2C) was comparable to that reported for amylopectin from the Chinese sweet potato cultivar Eshu-6 by Zhu et al.[Citation34] In those four Ghanaian varieties, there was a distinct peak at DP 13–14, a pronounced shoulder at DP 19–20, and a broad peak with a maximum at DP 46–49. In fact, all 10 Ghanaian sweet potato flour samples showed peaks or shoulders at these DP intervals, but for Patron the internal relationship between the major peak and shoulder was reversed, giving a shoulder at DP 13 and a broad peak with a maximum at DP 20 (). For the varieties Ligri, Okumkom (Fig. 2C), and Otoo (Fig. 2D), a broad peak with two shoulders at around DP 13 and DP 20 was observed. Hence, there were considerable differences in the fine structure of starch from the 10 different sweet potato samples. Similarly, other groups have reported significant variations in the physicochemical properties of various sweet potato flours.[Citation34,Citation37]
Conclusion
Flours from the 12 Ghanaian sweet potato varieties analysed here showed varying amounts of starch and the polymer distribution (amylose and amylopectin content) of these starches also differed. Flour from the variety Apomuden contained less than 50 g starch/100 g dry matter, while the remaining flours contained more than 60 g/100 g dry matter, with Histarch containing the highest amount (77.0 g/100 g dry matter). The starches from the varieties Ligri and Ogyefo contained about 20% amylose, whereas Apomuden, which is an orange-fleshed sweet potato variety, had 10% amylose. Overall, the 12 varieties could be classified as intermediate-amylose (10–30%) starches. The molecular distribution of amylose and amylopectin in the flours generally followed a similar pattern as observed in their chromatograms. However, there were slight variations in the height and width of the elution peaks because of the differences observed in the starch polymers. Moreover, there were pronounced differences in the fine structure of amylopectin between the 10 samples analysed for this parameter.
Declaration of interest
The authors hereby state that there is no conflict of interest regarding this publication.
Funding
The authors are grateful to the West Africa Agricultural Productivity Programme (WAAPP2A)-Ghana for sponsorship and to the Swedish University of Agricultural Sciences, Uppsala, Sweden, for financial support.
Additional information
Funding
References
- Jangchud, K.; Phimolsiripol, Y.; Haruthaithanasan, V. Physicochemical Properties of Sweet Potato Flour and Starch as Affected by Blanching and Processing. Starck/Starke 2003, 55, 258–264.
- Zhu, F.; Yang, X.; Cai, Y.Z.; Bertoft, E.; Corke, H. Physicochemical Properties of Sweet Potato Starch. Starch/Starke 2011, 63, 249–259.
- Lindeboom, N.; Chang, P.R.; Tyler, R.T. Analytical, Biochemical and Physico-Chemical Aspects of Starch Granule Size, with Emphasis on Small Granule Starches: A Review. Starch/Starke 2004, 56, 89–99.
- Noda, T.; Takahata, Y.; Sato, T.; Suda, I.; Morishita, T.; Ishiguro, K.; Yamakawa, O. Relationships Between Chain Length Distribution of Amylopectin and Gelatinization Properties within the Same Botanical Origin for Sweet Potato and Buckwheat. Carbohydrate Polymers 1998, 37, 153–158.
- Tester, R.F.; Morrison, W.R. Swelling and Gelatinization of Cereal Starches I. Effect of Amylopectin, Amylose and Lipids. Cereal Chemistry 1990, 67, 551–557.
- Stevenson, D.G., Jane, Jay-lin, Inglett, G.E. Structures and Physicochemical Properties of Starch from Immature Seeds of Soybean Varieties (Glycine max (L.) Merr.) Exhibiting Normal, Low-Linolenic or Low-Saturated Fatty Acid oil Profiles at Maturity. Carbohydrate Polymers 2007, 70, 149–159.
- Tattiyakul, J.; Pradipasena, P., Asavasaksakul, S. Taro Colocasia esculenta (L.) Schott Amylopectin Structure and its Effect on Starch Functional Properties. Starch/Starke 2007, 59, 342–347. doi:10.1002/star.200700620.
- Jane, Y.; Chen, Y.Y.; Lee, L.F.; McPherson, A.E.; Wong, K.S.; Radosavljevic, M.; Kasemsuwan, T. Effects of Amylopectin Branch Chain Length and Amylose Content on the Gelatinization and Pasting Properties of Starch. Cereal Chemistry 1999, 76, 629–637.
- Jane, J.Y.; Chen, J.F. Effect of Amylose Molecular Size and Amylopectin Branch Chain Length on Paste Properties of Starch. Cereal Chemistry 1992, 69, 60–65.
- Katayama, K.; Komae, K.; Kohyama, K.; Kato, T.; Tamiya, S.; Komaki, K. New Sweet Potato Line Having Low Gelatinization Temperature and Altered Starch Structure. Starch/Starke 2002, 54, 51–57.
- Aman, P., Westerlund, E. and Theander, O. Determination of starch using thermostable amylase. In Methods in Carbohydrate Chemistry, BeMiller; Manners, D.J.; Sturgeon R.J. Eds. Vol X. Wiley, New York, 1994, 111–115.
- Chrastil, J. Improved Colorimetric Determination of Amylose in Starches or Flours. Carbohydrate Research 1987, 159, 154–158.
- Menzel, C.; Andersson, M.; Andersson, R.; Vázquez-Gutiérrez, J.L.; Daniel, G.; Langton, M.; Gällstedt, M.; Koch, K. Improved Material Properties of Solution-Cast Starch Films: Effect of Varying Amylopectin Structure and Amylose Content of Starch from Genetically Modified Potatoes. Carbohydrate Polymers 2015, 130, 388–397.
- Torruco-Uco, J.G.; Chel-Guerrero, L.A.; Betancur-Ancona, D. Isolation and Molecular Characterization of Makal (Xanthosoma yucatanensis) Starch. Starch/Starke 2006, 58, 300–307.
- Menzel, C.; Olsson, E.; Plivelic, T.S.; Andersson, R.; Johansson, A.; Kuktaite, R.; Jarnstrom, L.; Koch, K. Molecular Structure of Citric Acid Cross-Linked Starch Films. Carbohydrate Polymers 2013, 96, 270–276.
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Analytical Chemistry 1956, 28(3), 350–356.
- Morrison, W.R.; Laignelet, B. An Improved Colorimetric Procedure for Determining Apparent and Total Amylose in Cereal and Other Starches. Journal of Cereal Science 1983, 1(1), 9–20.
- Koch, K.; Andersson, R.; Åman, P. Quantitative Analysis of Amylopectin Unit Chains by Means of High-Performance Anion-Exchange Chromatography with Pulsed Amperometric Detection. Journal of Chromatograph 1998, A 800, 199–206.
- Aprianita, A.; Purwandari, U.; Watson, B.; Vasiljevic, T. Physico-Chemical Properties of Flours and Starches from Selected Commercial Tubers Available in Australia. International Food Research Journal 2009, 16, 507–520.
- Noda, T.; Kimura, T.; Otani, M.; Ideta, O.; Shimada, T.; Saito, A.; Suda, I. Physicochemical Properties of Amylose-Free Starch from Transgenic Sweet Potato. Carbohydrate Polymers 2002, 49, 253–260.
- Matveev, Y.I.; van Soest, J.J.G.; Nieman, C.; Wasserman, L.A.; Protserov, V.A.; Ezernitskaja, M.; Yuryev, V.P. The Relationship Between Thermodynamic and Structural Properties of Low and High Amylose Maize Starches. Carbohydrate Polymers 2001, 44, 151–160.
- Badu, S.A.; Parimalavalli, R.; Jagannadham, K.; Rao, S.J.; Gaur, S.R. Fat Mimicking Properties of Citric Acid Treated Sweet Potato Starch. International Journal of Food Properties 2016, 19(1), 139–153.
- Yadav, A.R.; Guha, M.; Tharanathan, R.N.; Ramteke, R.S. Changes in Characteristics of Sweet Potato Flour Prepared by Different Drying Techniques. LWT 2006, 39, 20–26.
- McPherson, A.E.; Jane, J. Comparison of Waxy Potato with Other Root and Tuber Starches. Carbohydrate Polymers 1999, 40, 57–70.
- Garcia, A.M.; Walter, W.M. Physicochemical Characterization of Starch from Peruvian Sweet Potato Selections. Starch/Starke 1998, 50(8), S331–337.
- Ahmed, N.; Tetlow, I.J.; Nawaz, S., Iqbal, A.; Mubin, M.; Rehman, M.S.N.; Butt, A.; Lightfoot, D.A.; Maekawa, M. Effect of High Temperature on Grain Filling Period, Yield, Amylose Content and Activity of Starch Biosynthesis Enzymes in Endosperm of Basmati Rice.Journal of Science Food and Agriculture 2015, 95, 2237–2243.
- Cottrell, J.E.; Duffus, C.M.; Paterson, L.; Mackay, G.R. Properties of Potato Starch: Effects of Genotype and Growing Conditions. Phytochemistry 1995, 40, 1057–1064.
- Haase, N.U.; Plate, J. Properties of Potato Starch in Relation to Varieties and Environmental Factors. Starch: Starke 1996, 48, 167–171.
- Lourdin, V.; Colonna, S. Influence of Amylose on Starch Films and Foams. Carbohydrate Polymers 1995, 27, 261–270.
- Sasaki, T.; Yasui, T.; Matsuki, J. Effect of Amylose Content on Gelatinization, Retrogradation, and Pasting Properties of Starches from Waxy and Non-Waxy Wheat and their F1 Seeds. Cereal Chemistry 2000, 77(1), 58–63.
- You, S.G.; Izydorczyk, M.S. Molecular Characterization of Barley Starches with Variable Amylose Content. Carbohydrate Polymers 2002, 49, 33–42.
- Altskar, A.; Andersson, R.; Boldizar, A.; Koch, K. Some Effects of Processing on the Molecular Structure and Morphology of Thermoplastic Starch. Carbohydrate Polymers 2008, 71, 591–597.
- Bertoft, E. On the Nature of Categories of Chains in Amylopectin and their Connection to the Superhelix Model. Carbohydrate Polymers 2004, 57, 211–224.
- Zhu, F.; Corke, H.; Bertoft, E. Amylopectin Internal Molecular Structure in Relation to Physical Properties of Sweet Potato Starch. Carbohydrate Polymers 2011, 84, 907–918.
- Mar, N.; Umemoto, T.; Siti Nor Akmar Abdulah, S.N.A.; Maziah, M. Chain Length Distribution of Amylopectin and Physicochemical Properties of Starch in Myanmar Rice Cultivars. International Journal of Food Properties 2015, 18 (8), 1719–1730.
- Torruco-Uco, J.G.; Chávez-Murillo, C. E.; Hernández-Centeno, F.; Salgado-Delgado, R.; Tirado-Gallegos, J.M; Zamudio-Flores, K.B. Use of High-Performance Size-Exclusion Chromatography for Characterization of Amylose Isolated from Diverse Botanical Sources. International Journal of Food Properties 2016, 19(6), 1362–1369.
- Tian, S.J.; Rickard, J.E.; Blanshard, J.M.V. Physicochemical Properties of Sweet Potato Starch. Journal of Science Food and Agriculture 1991, 57, 459–49.
