ABSTRACT
Pumpkin oil cake protein isolate (POCPI) was hydrolysed using two food-grade enzymes alcalase and trypsin. Alcalase-hydrolysed POCPI (POCPH1) was selected as the optimum treatment based on the DPPH radical scavenging, total antioxidative, and ferrous ion chelating activities. Amino acid composition showed a direct relationship between the antioxidative activity of POCPH1 and the amount of hydrophobic amino acids that formed 33.49% of its total amino acids. Surface hydrophobicity decreased as a result of hydrolysis and potent thermal and pH stability was observed in POCPH1 (p ˂ 0.05). In terms of molecular weight distribution, size exclusion chromatogram indicated that the majority of peptides possessed molecular weight less than 6.5 kDa. Based on the results, POCPH1 could be employed as a natural antioxidative agent with strong pH and thermal stability.
Introduction
Currently, plant proteins are getting more attention as alternatives for animal proteins in different areas such as human nutrition, functional agents, and pharmaceutical products. However, some properties like low solubility have limited their use. There are different approaches like chemical and physical modifications and enzymatic hydrolysis to improve the functional properties as well as the bioactivity and nutritive value of plant proteins. In recent years, enzymatic hydrolysis has become the most common way to modify plant proteins.[Citation1]
Bioactive peptides produced during fermentation or enzymatic hydrolysis are known to have functional properties like emulsifying and foaming activities,[Citation2] as well as health-promoting properties such as antioxidative,[Citation3–Citation5] antimicrobial,[Citation6] and angiotensin converting enzyme (ACE) inhibitory activities.[Citation7–Citation11] Yang et al.,[Citation12] studying the effect of enzymatic hydrolysis of soy sauce lees by alcalase, reported that it is possible to release antioxidative peptides during hydrolysis, and the obtained hydrolysates possessed strong DPPH radical scavenging activity in a dose-dependent manner. The amino acid compositions of hydrolysates obtained from the enzymatic hydrolysis of Phaseolus lunatus (L.) and Phaseolus vulgaris (L.) seeds by alcalase and flavourzyme were studied by Torruco-Uco et al.[Citation9] It was reported that both Phaseolus lunatus (L.) and Phaseolus vulgaris (L.) hydrolysates had high amounts of hydrophobic amino acids like valine, proline, methionine, phenylalanine, leucine, and tryptophan. In a research carried out by Wang et al.[Citation5] rapeseed protein was hydrolysed using Alcalase 2.4 L. The results indicated that the peptide fraction with amino acid sequence of Trp-Ile-Tyr had the highest antioxidative activity. Alfalfa leaf protein hydrolysates prepared by alcalase, neutrase, protamex, and flavourzyme showed reducing power as well as DPPH and superoxide radical scavenging activities. Molecular weight determination showed that a high portion of peptides had molecular weight of less than 1000 Da and the antioxidative activities of these fractions were much more than those with higher molecular weights.[Citation13] Enzymatic hydrolysis of yellow stripe trevally (Selaroides leptolepis G.) meat by alcalase and flavourzyme exhibited an increase in protein solubility to above 85%; however, the functional properties decreased as a function of hydrolysis time.[Citation14] It was reported that the interfacial (emulsifying and foaming) properties of hydrolysates were dependent on the degree of hydrolysis (DH) and the proteases used.[Citation14]
Pumpkin (Cucurbita pepo L.) seeds are rich in proteins, unsaturated fatty acids, phytosterols, and essential minerals like Zn, K, Ca, Mg, Fe, Cu, and P. The oil content of pumpkin seeds is about 40–60%, and mostly consists of oleic, palmitic, and stearic acids. On the other hand, its protein content is about 45–46%, and this amount will reach 55–56% after defatting.[Citation15,Citation16] To date, pumpkin seeds have been mainly used for pumpkin oil production. After the oil extraction, a protein-rich by-product (pumpkin oil cake) remains, which is often used for animal feeding.[Citation1,Citation16] To our knowledge, surface properties, amino acid composition, and pH and thermal stability of hydrolysed pumpkin oil cake hydrolysates have not been the topic of evaluation in previous studies; therefore, in this study the enzymatic hydrolysis of pumpkin oil cake protein by two food-grade proteases alcalase and trypsin was attempted and the optimum treatment was selected based on the antioxidative properties. Thereafter, amino acid composition, surface hydrophobicity, molecular weight distribution, and thermal and pH stability of optimum treatment were investigated.
Materials and methods
Materials
Pumpkin oil cake (Cucurbita pepo con. Pepo var. Styriaca, 48.6% ± 3.5 protein, 8.9% ± 0.6 fat, 6.2% ± 0.5 moisture, and 7.1% ± 0.1 ash) was obtained from soybean company (Gorgan, Iran). Alcalase 2.4 L (E.C. 3.4.21.62. from Bacillus licheniformis) and trypsin (E.C. 3.4.21.4. from porcine pancreases), DPPH (2, 2-diphenyl-1-picrylhydrazyl), anilino naphthalene sulfonic acid (ANS) and size exclusion chromatography molecular weight standards were provided by Sigma-Aldrich Co. (St. Louis, MO, USA). All other chemicals used were of analytical grade.
Preparations of pumpkin oil cake protein isolate
Defatted pumpkin oil cake was dispersed in distilled water (1:10 w/v). The pH was adjusted to 10 with 1 N NaOH, mixed for 1 h at room temperature and centrifuged at 5000 g for 20 min (Combi514R, South Korea). The supernatant was collected, pH was adjusted to 5 with 1 N HCl, and centrifugation was performed at the same condition. The supernatant was discarded and the precipitate was collected as pumpkin oil cake protein isolate (POCPI).[Citation1]
Enzymatic hydrolysis
POCPI was dispersed in tris-HCl at pH = 9 and pH = 8 for alcalase and trypsin enzymatic treatments, respectively (5% w/v). After that, alcalase or trypsin was added at 1%, 1.5%, and 2% and hydrolysis was carried out for 2, 3.5, and 5 h at 200 rpm in a shaker incubator (8480-VS, South Korea). Hydrolysis temperatures were 45, 50, and 55°C for alcalase and 35, 40, and 45°C for trypsin. At the end of hydrolysis, the enzyme was inactivated for 15 min at 85°C; dispersion was centrifuged at 4000 g for 30 min, and the supernatant was collected and freeze dried.[Citation17]
Degree of hydrolysis
Degree of hydrolysis (DH) was measured regarding the method suggested by Kaewka et al.[Citation18] A 10 ml of hydrolysed sample was mixed with 10 ml of 20% TCA (Trichloroacetic acid) and centrifuged at 10,000 g 25°C for 15 min. The soluble nitrogen in supernatant and total nitrogen were determined and DH was measured as follows:
DPPH radical scavenging activity
An aliquot of 1000 μl POCPH was mixed with 1000 μl of 0.1 mM DPPH solution prepared in 96% ethanol. The mixture was allowed to stand for 60 min in the dark and the absorbance was read at 517 nm. The blank was prepared in the same manner except that 1000 μl water was used instead of 1000 μl POCPH.[Citation19] DPPH radical scavenging activity was calculated as follows:
Total antioxidative activity
Total antioxidative activity was evaluated by the method described by.[Citation20] An aliquot of 100 microliter of POCPH was mixed with 1000 microliter reagent (0.6 M H2SO4, 28 mM sodium phosphate, 4 mM ammonium molybdate) and kept for 90 min in boiling water. After cooling, the absorbance of the samples was read at 695 nm. The control sample was prepared in the same manner except that 1000 μl water was used instead of POCPH. The higher the absorption, the higher the total antioxidative capacity.
Ferrous ion chelating activity
Protein hydrolysate (4.7 ml) was mixed with 0.1 ml 2 mM FeCl2 and 0.2 ml 5 mM ferrozine and allowed to stand at room temperature for 20 min. The absorbance was read at 562 nm. The blank sample was prepared in the same manner except that 4.7 ml distilled water was used instead of the sample.[Citation4] Metal ion chelating activity was determined by the following equation:
Amino acid composition
Amino acid composition of POCPH1 was determined using the RP-HPLC 150 × 4.6 mm C18 column (Teknokroma, Spain). The sample was digested with 6 M hydrochloric acid at 110°C for 24 h and injected into the column after derivatization with orthophthaldehyde. The mobile phase was acetate buffer with a flow rate of 1.3 mlmin−Citation1.[Citation12] Amino acid content of POCPH1 was expressed as mg amino acid per 100 g protein.
Size exclusion chromatography
Molecular weight distribution of POCPH1 was determined using size exclusion chromatography on a Synchropack-GPC100 column (250 × 4.6 mm, Varian, USA). The mobile phase was 50 mM phosphate buffer containing 50 mM NaCl (pH = 7.2) at the flow rate of 0.6 ml/min and UV detection at 214 nm.[Citation12] Bovine serum albumin (66 kDa), cytochrome C (12.5 kDa), and aprotinin (6.5 kDa) (Sigma Co., USA) were used as molecular weight standards.
Surface hydrophobicity
Surface hydrophobicity was determined according to Bamdad et al.,[Citation21] with slight modifications. Briefly, protein solution (1% w/v) was prepared in 0.1 M phosphate buffer (pH 7.4) and diluted in the same buffer to obtain a final concentration ranging from 0.005 to 0.025% (v/v). A quantity of 20 µl of ANS (8.3 mM in 0.1 M phosphate buffer, pH 7.4) was added to 4 ml of the sample and the fluorescence intensity was measured by a fluorescence spectrophotometer (Shimadzu, Japan) with excitation and emotion wavelengths of 390 and 470 nm, respectively. The initial slope of the fluorescence intensity versus protein concentration plot was reported as surface hydrophobicity.
Thermal and pH stability
Thermal and pH stabilities were determined according to Nalinanon et al.[Citation4] For thermal stability measurement, 5 ml of POCPH1 was kept in boiling water (100°C) for 0, 15, 30, 60, 90, 120, and 180 min (pH = 7). The treated samples were immediately transferred into cold water and the residual antioxidative activities were determined using total antioxidative activity assay as described before. The untreated POCPH1 (25°C) was used as control.
For pH stability measurement, POCPH1 was adjusted to different pHs from 1 to 11 and left at room temperature for 30 min. After that the pH was adjusted to 7 and the residual antioxidative activities were determined using total antioxidative activity assay.
Statistical analysis
Data were statistically analysed by completely randomized design with the general linear models (GLM) procedure using SPSS software (ver. 16, SPSS Inc., Chicago). Significant differences were evaluated using Duncan’s multiple range tests at the 5% level and optimization was performed using the Design Expert (trial version 6.0.2, Stat- Ease Inc., Minneapolis, USA) statistical package.
Results and discussion
DPPH radical scavenging activity
The antioxidative activities of hydrolysates prepared by alcalase and trypsin are presented in and , respectively. Based on the results, samples hydrolysed with 2% alcalase at 50°C for 3.5 h (POCPH1, DH= 28.0 ± 0.7, 90% DPPH radical scavenging activity, RCitation2 = 0.96, and adjusted RCitation2 = 0.92) and 1% trypsin at 35°C for 5 h (POCPH2, DH = 23.0 ± 0.5, 78% DPPH radical scavenging activity, RCitation2 = 0.92, and adjusted RCitation2 = 0.98) were suggested by response surface methodology (RSM)Citation1 as two best treatments in terms of DPPH radical scavenging activity.
Table 1. DPPH radical scavenging activities of POCPHs prepared by alcalase.
Table 2. DPPH radical scavenging activities of POCPHs prepared by trypsin.
The results obtained from and show that there was not necessarily a direct correlation between increase in enzyme concentration, hydrolysis time, and temperature (increase in DH) and enhanced antioxidative properties. In other words, enzyme specificity and the size, type, and composition of free amino acids and small peptides produced through hydrolysis are among the main reasons behind the antioxidative properties. In this case, the enzyme activity probably produced peptides that were electron or hydrogen donors, which could react with free radicals to make more stable products and terminate the radical chain reaction.[Citation22] Regarding the results reported by Sun et al.,[Citation23] disruption of proteins followed by hydrolysis leads to unfolding and subsequently exposing the amino acid residues with antioxidative properties. Wiriyaphan et al.[Citation24] reported that high DH may suppress the peptide’s ability to act as a physical barrier against oxidative agents. Decrease in the antioxidative activities of alcalase-produced hydrolysates from yellow stripe trevally (Selaroides leptolepis G.), peanut protein, and alfalfa leaf protein with increase in DH were reported by Klompong et al.,[Citation14] Jamdar et al.,[Citation25] and Xie et al.,[Citation13] respectively. In a research conducted by Kamau and Lu[Citation22] no relationship was established between DH and antioxidative activity. The highest DH (29.53%) and the lowest antioxidative activity (62.77%) were assigned to whey protein hydrolysed by alcalase. In contrast, hydrolysed whey protein made by flavourzyme (DH=9.06%) showed an antioxidative power of 72.60%. They believed that excessive increase in DH may cause the release of free amino acids that have lower antioxidative activity compared to peptides. Opposite results were reported by Vaštag et al.,[Citation26] from the hydrolysis of pumpkin oil cake protein with alcalase and flavourzyme. They claimed that in both alcalase and flavourzyme hydrolysates, the radical scavenging activity increased as the DH increased. The results obtained in this study were in agreement with the results reported by many researchers .[Citation13,Citation14,Citation22,Citation24,Citation25]
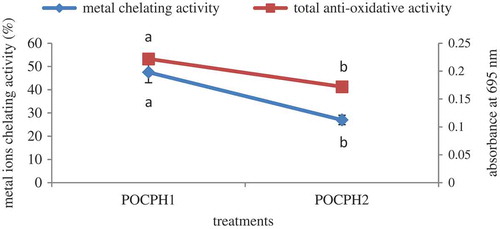
Total antioxidative and ferrous ion chelating activities
Optimum treatment was selected based on the comparison between the total antioxidative and ferrous ion chelating activities of POCPH1 and POCPH2. As shown in the total antioxidative and metal ion chelating activities of POCPH1 (prepared by alcalase) were higher than POCPH2 (prepared with trypsin) and therefore POCPH1 was selected as the optimum treatment.
The results indicated that both POCPH1 and POCPH2 had antioxidative activity; therefore these hydrolysates may decrease the rate of oxidation. However, the antioxidative activity of POCPH1 was higher than that of POCPH2, which can be associated with the fact that hydrolysis using different enzymes may cause peptides with different lengths and structures and subsequently different antioxidative potentials. Alcalase is an endoenzyme with a broad specificity, which can cleave the peptide bonds from the interior of the peptide chain, and will cause the release of short- or medium-chain oligo/poly peptides containing hydrophobic amino acids such as Phe, Tyr, Trp, Leu, Ile, Val, and Met.[Citation26,Citation27] Considering the results, it can be said that the amount of antioxidative amino acids especially hydrophobic species in POCPH1 was probably higher than POCPH2 as also reported by Vaštag et al.[Citation26] Furthermore, since radical scavenging activity is also related to hydrogen donor capacity of the hydroxyl groups of aromatic amino acid residues,[Citation27] the amount of such amino acids in POCPH1 was likely higher than POCPH2. Moreover, amino acid sequence and molecular weight of peptides in protein hydrolysates, which are influenced by the source of protein and conditions of hydrolysis, may be effective in the antioxidative activity of final hydrolysates.[Citation26] In general, it can be concluded that the antioxidative activity of protein hydrolysates may be their mechanism of free radical scavenging and it will be beneficial to show the protective effect against oxidative agents.
Amino acid composition
Amino acid composition has a potential role in the antioxidative properties of protein hydrolysates. This parameter was investigated to find the relationship between the antioxidative activity of POCPH1 and its amino acid profile. It is observable from that Glu, Asp, and Arg are the main amino acids in both POCPI and POCPH1. Similar results were reported by Amza et al.[Citation3] about the amino acid profile of gingerbread plum seed flour and its protein isolates, and by Zhu et al.[Citation28] on the amino acid composition of wheat germ protein isolate and related hydrolysates. On the other hand, Thr and Tyr were found to be present in small amounts and His was the most limiting amino acid in POCPI and POCPH1.
Table 3. Amino acid composition of POCPH1 (mg100 g−Citation1).
The amino acid profile of hydrolysates is greatly affiliated to the protease used. Alcalase has a broad specificity on aromatic (Phe, Trp, and Tyr), acidic (Glu), sulphur-containing (Met), aliphatic (Leu and Ala), hydroxyl (Ser), and basic (Lys) amino acids.[Citation24] It has been proven that Asp and Glu as well as Pro, Arg, His, Met, Leu, Ile, Ala, Tyr, and Val[Citation29] have potential to show antioxidative properties. Comparing the amino acid profiles of POCPH1 and POCPI, it is possible to correlate the antioxidative activity of POCPH1 to the amount of Leu, Ile, Ala, and Val, followed by Asp, Glu, and Arg. Moreover, for hydrolysed proteins, an increase in hydrophobicity will enhance their solubility in lipid and subsequently increase the antioxidative activity.[Citation30] In case of POCPH1, the amount of hydrophobic amino acids was 26016 mg/100g, which was much higher than the hydrophobic amino acid content of POCPI (25561 mg/100g).
Based on the FAO-suggested pattern for adults,[Citation31] it is possible to consider both POCPH1 and POCPI as balanced sources of essential amino acids, especially His, Thr, Met, Val, Leu, and Ile. Therefore POCPI and its hydrolysate (POCPH1) meet the most nutritional requirements for body performance and may be used as protein supplements for poor diets in terms of essential amino acids. Torruco-Uco et al.,[Citation9] studying the hydrolysis of Phaseolus lunatus (L.) and Phaseolus vulgaris (L.) protein concentrates with alcalase and flavourzyme, found that both Phaseolus lunatus (L.) and Phaseolus vulgaris (L.) protein hydrolysates had a balanced amino acid composition as well as high amounts of hydrophobic amino acids like Val, Ile, Pro, Met, Phe, Leu, and Trp. It was found by Xie et al.[Citation13] that the amino acid composition of alfalfa leaf protein hydrolysates was close to that recommended by FAO for adult humans. They claimed that antioxidative activity depends on the amounts of hydrophobic amino acids and the amino acid sequence of peptides. The presence of high quantities of hydrophobic as well as essential amino acids in different protein hydrolysates was also reported by other researchers.[Citation12,Citation32,Citation33]
Size exclusion chromatography
Molecular weight of peptides changes during hydrolysis and plays a key role in the bioactivity and properties of final hydrolysates.[Citation12] Two peaks were obtained from the chromatogram of POCPH1 (): the main peak was attributed to the peptides with molecular weight less than 6.5 kDa, which was much larger than the smaller one related to peptides with the molecular weight of higher than 66 kDa. It can be concluded from the graph that the majority of peptides had a molecular weight less than 6.5 kDa.
Figure 2. Molecular weight distribution of POCPH1 by size exclusion chromatography on SynChropak gpc-100 column (1: bovine serum albumin, 2: cytochrome C; 3: aprotinin).
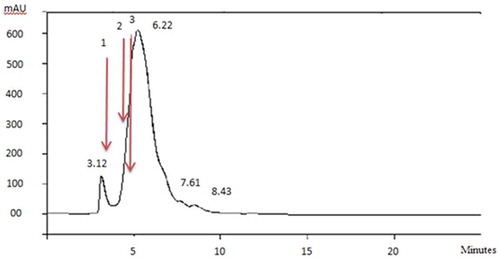
Molecular weight is an important factor that correlates with the progress in hydrolysis and subsequently influences the bioactivity of final hydrolysates.[Citation12] Hydrolysing the muscle food by-products with alcalase[Citation34] reported that the molecular weight of hydrolysates was lower than 6.5 kDa, which is in agreement with the present results. Considering this fact that low molecular weight peptides show higher antioxidative properties, it is possible to establish a logical correlation between the molecular weight of POCPH1 and its antioxidative activity.
Surface hydrophobicity
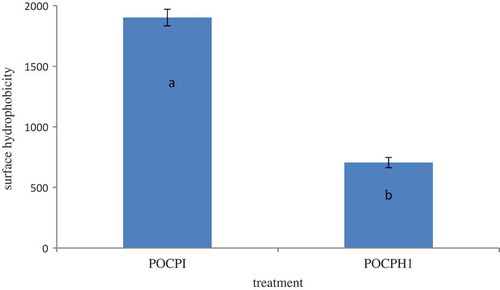
Surface hydrophobicity of POCPH1 in comparison with POCPI is shown in . POCPI showed higher surface hydrophobicity compared to POCPH1. In other words, hydrolysis caused a significant decrease in surface hydrophobicity (p ˂ 0.05). Surface hydrophobicity indicates the number of hydrophobic groups present at the surface of the protein and in contact with the aqueous environment.[Citation35] Depending on the nature, molecular weight, and the size of peptides produced through the hydrolysis, an increase or decrease in surface hydrophobicity may occur.[Citation36] As instance, tryptic hydrolysis of casein led to a reduction in surface hydrophobicity, while hydrolysed hemp protein made by trypsin and flavourzyme possessed higher surface hydrophobicity compared to intact protein.[Citation37] The lower surface hydrophobicity of POCPH1 compared to POCPI demonstrated that smaller peptides have had fewer hydrophobic binding sites for ANS, or these binding sites have changed during the hydrolysis.[Citation38] Considering the amino acid composition of POCPI and the changes of its surface hydrophobicity after hydrolysis, POCPI has probably a special tertiary structure with a hydrophilic core covered by a hydrophobic surface. Moreover, the partial entrapment of hydrophobic residues in cross-linked molecules as a result of peptide–peptide interactions may have a reducing effect on surface hydrophobicity. Similarly, increase in charged amino acids (aspartic and glutamic acid) followed by alcalase-induced deamidation caused a significant decrease in surface hydrophobicity.[Citation35] Enzyme specificity also plays an important role in surface hydrophobicity. Since alcalase is an endoenzyme, its hydrolysis occurs from the interior to exterior of protein molecules and thus less hydrophobic groups are exposed. Moreover, alcalase can further digest hydrophobic groups that released during the initial step of POCPI hydrolysis.[Citation37]
Thermal and pH stability
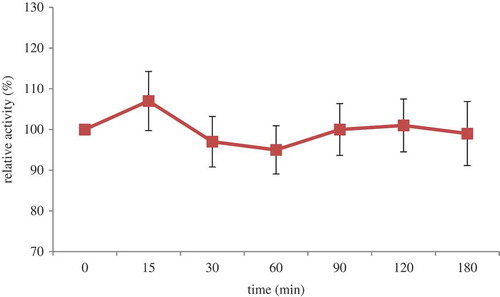
Thermal stability of POCPH1 is shown in . There was no significant difference between the antioxidative activities of samples left at 100°C for 0–180 min (p ˃ 0.05). This indicated the thermal stability of POCPH1. The slight decrease in the antioxidative activity of some samples may be related to either the degradation or aggregation of peptides due to heat treatment. In general, proteins are heat sensitive, which can lead to their aggregation. However, it has been reported that low molecular weight peptides are heat-stable.[Citation4] As suggested by Muhamyankaka et al.,[Citation38] hydrolysed proteins have thermal stability because in most cases the secondary structure of proteins was destroyed, followed by hydrolysis. Moreover, the presence of Pro, Hyp, and Ileu as well as amino acids with similarly charged side chains inhibits the formation of the secondary structure and subsequently enhances the heat stability. Thermal stability can be a result of the formation of hydrophobic bonds in the interior of the protein molecule as well.[Citation39] Considering the results, it is possible to use POCPH1 as a natural antioxidative agent in cooked food systems, without significant loss of its antioxidative activity. The obtained results confirmed the results reported by Nalinanon et al.,[Citation4] about the heat stability of peptic hydrolysates from ornate threadfin bream (Nemipterus hexodon Q & G.) muscle.
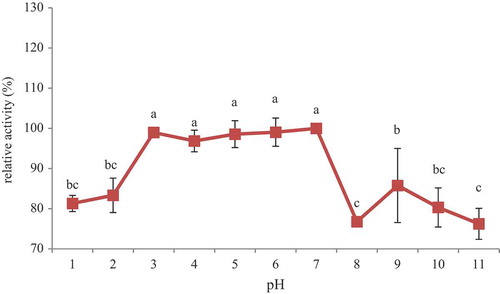
The pH stability of POCPH1 is shown in . A significant decrease in antioxidative activity was observed at high acidic (1 and 2) and basic (8, 9, 10, and 11) pHs. However, there was no significant decrease in the antioxidative activity of samples with pH ranging from 3 to 7 (p ˃0.05).
Proteins are sensitive to changes in pH; thus their activity may decrease or even become disabled at extremely high or low pH values. The stability of proteins at a particular pH may be attributed to the net charge of protein at that pH. Therefore the decrease in the antioxidative activity of POCPH1 at high acidic or basic pH values may be attributed to the accumulation of homonymous charges and the effect of this aggregation on the peptide structure. However, it should be noted that even at extremely high (11) or low (1 and 2) pH values, 70–80% of antioxidative activity of POCPH1 was retained. Based on the results, POCPH1 can be considered an antioxidative agent in food systems, especially with neutral and mild acidic pH values. Investigating the pH stability of peptides produced through the enzymatic hydrolysis of ornate threadfin bream (Nemipterus hexodon Q & G.) muscle by pepsin, Nalinanon et al.[Citation4] reported that the antioxidative activity of peptides remained constant over the pH range of 1–10, but this feature decreased at pH 11. They believed that antioxidative activity may be partly decreased at high pH values.
Conclusion
It can be concluded from the results that enzymatic hydrolysis may be beneficial to improve antioxidative activity. In contrast, hydrolysis led to decreased surface hydrophobicity and molecular weight. Considering the fact that antioxidative activity is more evident in low molecular weight peptides, it is possible to establish a logical relation between the reduction in molecular weight and increase in the antioxidative activity of the final hydrolysate. Alcalase hydrolysate possessed high amounts of hydrophobic amino acid residues and was in appropriate condition in terms of essential amino acids, particularly histidine, threonine, methionine, valine, leucine, and isoleucine, according to the FAO pattern for adults. Considering the results, enzymatic hydrolysis may be used to valorize the industrial by-products such as pumpkin oil cake protein and produce cost-effective and natural antioxidative agents with appropriate thermal and pH stabilities.
References
- Živanović, I.; Vaštag, Z.; Popović, S.; Popović, L.; Peričin, D. Hydrolysis of Hull-less Pumpkin Oil Cake Protein Isolate by Pepsin. International Journal of Biological and Life Sciences 2011, 1, 30–34.
- Guan, X.; Yao, H.; Chen, Z.; Shan, N.; Zhang, M. Some Functional Properties of Oat Bran Protein Concentrate Modified by Trypsin. Food Chemistry 2007, 101, 163–170.
- Amza, T.; Balla, A.; Tounkara, F.; Man, L.; Zhou, H.M. Effect of Hydrolysis Time on Nutritional, Functional and Antioxidant Properties of Protein Hydrolysates Prepared from Gingerbread Plum (Neocarya macrophylla) Seeds. International Food Research Journal 2013, 20, 2081–2090.
- Nalinanon, S.; Benjakul, S.; Kishimura, H.; Shahidi, F. Functionalities and Antioxidant Properties of Protein Hydrolysates from the Muscle of Ornate Threadfin Bream Treated with Pepsin from Skipjack Tuna. Food Chemistry 2011, 124, 1354–1362.
- Wang, L.; Yang, J.; Wang, Y,; Zhang, J.; Gao, Y.; Yuan, J.; Su, A.; Ju, X. Study on Antioxidant Activity and Amino Acid Analysis of Rapeseed Protein Hydrolysates. International Journal of Food Properties 2016, 19, 1899–1911.
- Tan, Y.N.; Matthews, K.; Di, R.; Ayob, M. Comparative Antibacterial Mode of Action of Purified Alcalase-and Tryptic-hydrolyzed Palm Kernel Cake Proteins on the Food-Borne Pathogen (Bacillus cereus). Food Control 2013, 31, 53–58.
- Fritz, M.; Vecchi, B.; Rinaldi, G.; Cristina Anon, M. Amaranth Seed Protein Hydrolysates Have In Vivo and In Vitro Antihypertensive Activity. Food Chemistry 2011, 126, 878–884.
- Li, G.H.; Qu, M.R.; Wan, J.Z.; You, J.M. Antihypertensive Effect of Rice Protein Hydrolysate with in Vitro Angiotensin I-converting Enzyme Inhibitory Activity in Spontaneously Hypertensive Rats. Asia Pacific Journal of Clinical Nutrition 2007, 16, 275–280.
- Torruco-Uco, J.; Chel-Guerrero, L.; Martı´nez-Ayala, A.; Da´vila-Ortı´z, G.; Betancur-Ancona, D. Angiotensin-I Converting Enzyme Inhibitory and Antioxidant Activities of Protein Hydrolysates from Phaseolus Lunatus and Phaseolus Vulgaris Seeds. LWT-Food Science and Technology 2009, 42, 1597–1604.
- Li, F.; Liu, W.; Yamaki, K.; Liu, Y.; Fang, Y.; Li, Z.; Chen, M.; Wang, C. Angiotensin I-Converting Enzyme Inhibitory Effect of Chinese Soypaste along Fermentation and Ripening: Contribution of Early Soybean Protein Borne Peptides and Late Maillard Reaction Products. International Journal of Food Properties 2016, 19, 2805–2816.
- Wang, H.; Meng, F.; Yin, L.; Cheng, Y.; Lu, A.; Wang, J. Changes of Composition and Angiotensin I-Converting Enzyme (ACE)-inhibitory Activity during Douchi Fermentation. International Journal of Food Properties 2016, 19, 2408–2416.
- Yang, B.; Yang, H.; Li, J.; Li, Z.; Jiang, Y. Amino Acid Composition, Molecular Weight Distribution and Antioxidant Activity of Protein Hydrolysates of Soy Sauce Lees. Food Chemistry 2011, 124, 551–555.
- Xie, Z.; Huang, J.; Xu, X.; Jin, Z. Antioxidant Activity of Peptides Isolated from Alfalfa Leaf Protein Hydrolysate. Food Chemistry 2008, 111, 370–376.
- Klompong, V.; Benjakul, S.; Kantachote, D.; Shahidi, F. Antioxidative Activity and Functional Properties of Protein Hydrolysate of Yellow Stripe Trevally (Selaroides leptolepis) as Influenced by the Degree of Hydrolysis and Enzyme Type. Food Chemistry 2007, 102, 1317–1327.
- Mohamed, R.A.; Ramadan, R.S.; Ahmed, L.A. Effect of Substituting Pumpkin Seed Protein Isolate for Casein on Serum Liver Enzymes, Lipid Profile and Antioxidant Enzymes in CCl4-intoxicated Rats. Advances in Biological Research 2009, 3, 9–15.
- Popović, L.; Peričin, D.; Vaštag, Z.; Popović, S.; Krimer, V.; Torbica, A. Antioxidative and Functional Properties of Pumpkin Oil Cake Globulin Hydrolysates. Journal of the American Oil Chemists’ Society 2013, 90, 1157–1165.
- Villanueva, A.; Vioque, J.; Sánchez-Vioque, R.; Clemente, A.; Pedroche, J.; Bautista, J.; Millán, F. Peptide Characteristics of Sunflower Protein Hydrolysates. Journal of the American Oil Chemists’ Society 1999, 76, 1455–1460.
- Kaewka, K.; Therakulkait, C.; Cadwallader, K. Effect of Preparation Conditions on Composition and Sensory Aroma Characteristics of Acid Hydrolyzed Rice Bran Protein Concentrate. Journal of Cereal Science 2009, 50, 56–60.
- Bougatef, A.; Hajji, M.; Balti, R.; Lassoued, I.; Triki-Ellouz, Y.; Nasri, M. Antioxidant and Free Radical-scavenging Activities of Smooth Hound (Mustelus mustelus) Muscle Protein Hydrolysates Obtained by Gastrointestinal Proteases. Food Chemistry 2009, 114, 1198–1205.
- Prieto, P.; Pineda, M. Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Annal Biochemistry 1999, 269, 337–341.
- Bamdad, F.; Wu, J.; Chen, L. Effects of Enzymatic Hydrolysis on Molecular Structure and Antioxidant Activity of Barley Hordein. Journal of Cereal Science 2011, 54, 20–28.
- Kamau, S.M.; Lu, R.R. The Effect of Enzymes and Hydrolysis Conditions on Degree of Hydrolysis and DPPH Radical Scavenging Activity of Whey Protein Hydrolysates. Current Research in Dairy Sciences 2011, 3, 25–35.
- Sun, Q.; Shen, H.; Leu, Y. Antioxidant Activity of Hydrolysates and Peptide Fractions Derived from Porcine Hemoglobin. Journal of Food Science and Technology 2011, 23, 6646–6652.
- Wiriyaphan, C.; Chitsomboon, B.; Yongsawadigul, J. Antioxidant Activity of Protein Hydrolysates Derived from Threadfin Bream Surimi Byproducts. Food Chemistry 2012, 132, 104–111.
- Jamdar, S.; Rajalakshmi, V.; Pednekar, M.D.; Juan, F.; Yardi, V.; Sharma, A. Influence of Degree of Hydrolysis on Functional Properties, Antioxidant Activity and ACE Inhibitory Activity of Peanut Protein Hydrolysate. Food Chemistry 2010, 121, 178–184.
- Vaštag, Ž.; Popović, L.; Popović, S.; Krimer, V.; Peričin, D. Production of Enzymatic Hydrolysates with Antioxidant and Angiotensin-I Converting Enzyme Inhibitory Activity from Pumpkin Oil Cake Protein Isolate. Food Chemistry 2011, 124, 1316–1321.
- Cumby, N.; Zhong, Y.; Naczk, M.; Shahidi, F. Antioxidant Activity and Water-holding Capacity of Canola Protein Hydrolysates. Food Chemistry 2008, 109, 144–148.
- Zhu, K.; Zhou, H.; Qian, H. Antioxidant and Free Radical-scavenging Activities of Wheat Germ Protein Hydrolysates (WGPH) Prepared with Alcalase. Process Biochemistry 2006, 41, 1296–1302.
- Zhu, L.; Chen, J.; Tang, X.; Xiong, Y. Reducing, Radical Scavenging, and Chelation Properties of In Vitro Digests of Alcalase-treated Zein Hydrolysate. Journal of Agricultural and Food Chemistry 2008, 56, 2714–2721.
- Li, Y.; Jiang, B,; Zhang, T.; Mu, W.; Liu, J. Antioxidant and Free Radical-scavenging Activities of Chickpea Protein Hydrolysate (CPH). Food Chemistry 2008, 106, 444–450.
- FAO/WHO. 1973. Energy and Protein Requirements: Report of a Joint FAO/WHO Ad Hoc Expert Committee. Rome and Geneva: FAO Nutrition Meetings Report Series No. 52, WHO Technical Report Series No. 522.
- Hmidet, N.; Balti, R.; Nasri, R.; Sila, A.; Bougatef, A.; Nasri, M. Improvement of Functional Properties and Antioxidant Activities of Cuttlefish (Sepia officinalis) Muscle Proteins Hydrolyzed by (Bacillus mojavensis) A21 Proteases. Food Research International 2011, 44, 2703–2711.
- Klompong, V.; Benjakul, S.; Yachai, M.; Visessanguan, W.; Shahidi, F.; Hayes, K.D. Amino Acid Composition and Antioxidative Peptides from Protein Hydrolysates of Yellow Stripe Trevally (Selaroides leptolepis). Journal of Food Science 2009, 74, 126–133.
- Karamac, M.; Flaczyk, E.; Wanasundara, P.D.P.K.J.; Amarowicz, R. Angiotensin I-Converting Enzyme (ACE) Inhibitory Activity of Hydrolysates Obtained from Muscle Food Industry By-products: A Short Report. Polish Journal of Food and Nutrition Sciences 2005, 14, 133–138.
- Agyare, K.K.; Addo, K.; Xiong, Y.L. Emulsifying and Foaming Properties of Transglutaminase-Treated Wheat Gluten Hydrolysate as Influenced by pH, Temperature and Salt. Food Hydrocolloids 2009, 23, 72–81.
- Cheng, X.; Tang, X.; Wang, Q.; Mao, X.Y. Antibacterial Effect and Hydrophobicity of Yak κ-casein Hydrolysate and Its Fractions. International Dairy Journal 2013, 31, 111–116.
- Tang, CH.; Wang, X.S.; Yang, X.Q. Enzymatic Hydrolysis of Hemp (Cannabis sativa) Protein Isolate by Various Proteases and Antioxidant Properties of the Resulting Hydrolysates. Food Chemistry 2009, 114, 1484–1490.
- Muhamyankaka, V.; Shoemaker, C.F.; Nalwoga, M.; Zhang, X.M. Physicochemical Properties of Hydrolysates from Enzymatic Hydrolysis of Pumpkin (Cucurbita moschata) Protein Meal. International Food Research Journal 2013, 20, 2227–2240.
- Klomklao, S.; Benjakul, S.; Visessanguan, W.; Kishimura, H.; Simpson, B. Purification and Characterisation of Trypsins from the Spleen of Skipjack Tuna (Katsuwonus pelamis). Food Chemistry 2007, 100, 1580–1589.