ABSTRACT
The economic and sustainable use of fish-processing by-products has received considerable attention. Large amounts of fish skin discarded by industry processes can be a source of collagen extraction. This study developed a method to extract collagen with high purity and yield from Southern catfish skins through improved pretreatment methods. Multiple degreasing methods effectively removed fat from fish skins and obtained a maximum degreasing rate (90.24%). The results of electrophoretic, hydroxyproline-content, and extraction-rate analyses revealed that the collagen extracted from defatted skin, which decoloured in 0.5% hydrogen peroxide solution (pH 10), exhibited higher purity and the highest yield (23.14% wet weight and 78.57% dry weight, respectively) as compared with other decolouration conditions. The histological appearance of pretreated fish skin indicated that the non-collagenous substances were removed effectively and the fibres loosened. Amino acid analysis, ultraviolet spectra, Fourier transform infrared (FTIR) spectroscopy, and thermal-stability analysis indicated that the collagen isolated from fish skin under optimal pretreated conditions was classified as type I. Our method for the optimal pretreatment of collagen extracted from fish skin following degreasing and decolouring procedures resulted in improved collagen purity and yield.
Introduction
Fish processing by-products, such as skin, bone, viscera, scales, and the head, constitute ~50–70% of the original raw materials and are discarded as waste.[Citation1] With the increased demand for processed fish products and the rapid development of fish-processing industries, large quantities of by-products are either discarded, potentially causing pollution and emitting offensive doors, or used as cheap feedstuff or fertilizer.[Citation2] However, there are potentials for increasing profits derived from fish-processing by-products. Collagen is the most abundant protein in vertebrates and a main component of connective tissue that is widely used in the food, biomedical, pharmaceutical, and cosmetic industries due to its excellent biocompatibility, biodegradability, and weak antigenicity based on its unique triple-helical structure.[Citation3,Citation4] Collagen is abundant in fish-processing by-products, which has received increasing attention as a collagen resource.[Citation5] Therefore, use of these by-products as alterative sources for collagen production can both increase economic return to fish-processing industries and avoid environmental problems.[Citation6]
Fish skin constitutes the primary waste associated with industry by-products and is considered as a raw material for collagen extraction. Previous studies reported that fish skins exhibited a high moisture content (60–80%), with the content of fat, protein, and ash at 0.5–15%, 50–95%, and 0–45% (dry weight), respectively.[Citation2,Citation7–Citation10] Collagen is the main component of skin protein; however, non-collagenous components, such as fat and ash, will affect the collagen purity and yield. Therefore, pretreatment to remove non-collagenous substances prior to collagen isolation is necessary.
To date, most studies have focused on collagen isolated from the skin of different species of fish, with some studies reporting the pretreatment of fish skin with 0.1 M sodium hydroxide (NaOH) and 10% butyl alcohol to remove non-collagenous proteins and fat, respectively, resulting in collagen yields ranging from 10 to 20% (wet weight).[Citation1,Citation7,Citation11–Citation14] Liu et al.[Citation15] investigated the effects of alkaline-pretreatment conditions on isolating acid-soluble collagen from grass carp (Ctenopharyngodon idella) skin and showed that pretreatment with 0.05 M and 0.1 M NaOH was capable of removing non-collagenous proteins without significant loss of collagen, while pretreatment with 0.2 M and 0.5 M NaOH caused significant loss and structural modification of collagen. Other studies reported the use of alkali or salt treatment, including NaOH,[Citation16,Citation17] sodium bicarbonate (NaHCO3),[Citation18] and sodium chloride (NaCl),[Citation5,Citation9,Citation19] on fish skin to remove non-collagenous proteins in the absence of a degreasing process. Kittiphattanabawon et al.[Citation20] soaked shark skin in 0.1 M NaOH at a solid to alkali solution ratio of 1:10 (w/v) to remove non-collagenous proteins, and then isolated the collagen using 0.5 M acetic acid (ASC) and pepsin (PSC). The yields of ASC and PSC were 9.38% and 8.86% (wet weight), respectively. Additionally, pigment is part of the skin ash and influences the purity and appearance of collagen. Nagai et al.[Citation21] isolated the collagen from cufflesh (Sepia lycidas) in the absence of a decolouring procedure, with the obtained collagen exhibiting a pinkish fibre. In order to obtain collagen with high purity, the decolouring process is necessary. Several studies focused on the fish skin treated with H2O2 to remove pigment following alkaline treatment and degreasing processes.[Citation6,Citation22–Citation24] Sadowska et al.[Citation25] soaked Baltic cod (Gadus morhua) skins in 10% NaCl and bleached the skins with a 1% H2O2 solution in 0.01 M NaOH to remove fish-skin pigments and obtained a colourless form of collagen with a yield of 21.5% (wet weight).
During alkaline treatment, skin fibres may swell unevenly after treatment with a reagent, such as NaOH, which can be detrimental to the subsequent degreasing, decolouring, and extraction processes. To avoid the problems of uneven swelling of skin and loss of collagen, NaOH can be replaced by a weakly alkaline salt to remove portions of skin fat and promote even swelling of the fibre. Additionally, for skins with high fat content, the fibres can be loosened effectively by a degreasing procedure to improve the purity and yield of collagen extraction. Zhao et al.[Citation26] investigated the influence of different pigskin-degreasing methods on collagen yield, finding that the collagen-extraction rate increased to 28.52% when the pigskin-degreasing rate was >90%. Furthermore, because the high pigment content can affect collagen purity and appearance, decolouring processes to remove skin pigments is necessary. According to previous work, H2O2 can oxidize the pigment to achieve decolouration, especially under alkaline conditions.[Citation25] Fu et al.[Citation27] studied the effects of H2O2-pretreatment concentration, time, and pH on de-hairing bovine hides and reported that the skin fibres were loosened effectively and the natural collagen extracted with high yields from bovine skins following pretreatment with 60 g L−Citation1 H2O2 at pH 10 for 4.5 h.
Southern catfish (Silurus meridionalis Chen) is widely distributed in the Changjiang (China) river system and is an important commercial freshwater fish without scales. During fish processing, large amounts of skin from this fish are generated as a by-product and can be used as a potential source for collagen extraction. Because catfish skin is rich in fat,[Citation28] the degreasing process is necessary. Additionally, the colour of catfish skin is deep, indicating that it contains additional pigment, making the decolouring process also essential. The objective of this study was to extract collagen from Southern catfish skins with higher purity and yield after degreasing and decolouring processes. The purity of collagen was investigated by electrophoretic analysis, and the optimal pretreatment conditions were determined based on the results of degreasing rate and extraction rate. The properties of the collagen extracted under optimal pretreatment conditions were partly investigated by amino acid analysis, ultraviolet spectra, Fourier transform infrared (FTIR) spectroscopy, and thermal stability. This study provided optimal pretreatment processes for the extraction of collagen from Southern catfish skins. The pretreatment methods were expected to further present guidelines for the collagen extracted from fish skins of other species and for the industrial production of fish-skin collagen.
Materials and methods
Pretreatment optimization
Degreasing process
All chemicals and reagents used were of analytical grade. Southern catfish (Silurus meridionalis Chen) skins were collected from a fish-processing factory in Hangzhou (Zhejiang, China). The residual flesh was scraped off the fish skin with a knife and then the sample was washed with distilled water three times. Cleaned skins were cut into small pieces (0.2 × 0.2 cm) manually, and referred to as NFS. Following NFS treatment with 10% sodium carbonate (Na2CO3), the skin was referred to as NFS1. Following NFS1 defatting by treatment with 15% (v/v) isopropyl alcohol, the skin was referred to as NFS2. Following NFS2 treatment with 6% (v/v) non-ionic degreasing agent, the skin was referred to as NFS3. All procedures were processed using a sample:solution ratio of 1:20 at 4°C for 24 h with gentle stirring, with the solution changed every 12 h. The fat contents of NFS, NFS1, NFS2, and NFS3 were measured according to Soxhlet extraction described by Bligh et al.,[Citation29] respectively. The dry weight of the skin was accurately weighed (W1), and skins were packed in filter paper and placed in the Soxhlet extractor for fat extraction using ether for 12 h. The fat content was constantly measured at 105°C to obtain the dry weight (W2) and calculated as follows:
All samples were measured in triplicate.
Decolouring process
According to , H2O2 solutions at different concentrations and at various pH values were initially prepared. To remove the skin pigment, multiple defatted skins were soaked in each H2O2 solution at a sample:solution ratio of 1:20 at 4°C for 24 h, and the solutions were changed every 12 h. The skins were then treated with 7.5% NaCl solution, which was dissolved using 0.05 M Tris–HCl buffer (pH 7.2) at a sample:solution ratio of 1:20 at 4°C for 24 h to remove non-collagenous proteins more effectively. The solutions were changed every 12 h.
Table 1. Design of decolouring experimental program.
Collagen extraction
All processes were performed at 4°C with gentle stirring. The pretreated skins were washed with distilled water, followed by soaking in 0.5 M acetic acid containing 1% (w/w) pepsin (1:10000; EC 3.4.23.1; Sigma–Aldrich, St. Louis, MO, USA) at a sample:solution ratio of 1:30 (w/v) for 48 h. The supernatants were then collected by refrigerated centrifugation at 9000 g for 10 min. The precipitates were salted out from the supernatants by adding NaCl to a final concentration of 0.7 M and dissolved in 0.5 M acetic acid. Successively, the obtained solutions were respectively dialyzed against 0.05 M acetic acid for 3 days, with a change of solution every 12 h. The dialysate was eventually lyophilized by a freeze dryer [Labconco FreeZone (6 L); Ft. Scott, KS, USA] at −50°C for 3 days and stored at 4°C until use.
Determination of collagen purity and yield
The purity and yield of all collagen samples under different pretreatment conditions were measured by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE), hydroxyproline content, and extraction rate. SDS-PAGE was performed according to the method of Laemmli[Citation30] with a slight modification that involved the use of a 7.5% resolving gel and a 4% stacking gel. The collagen isolated from differently pretreated skins was mixed with Tris–HCl buffer containing 2% β-mercaptoethanol and 0.016% bromophenol blue to reach a final collagen concentration of 1 mg/mL, and the samples were boiled for 5 min at 100°C. Each boiled sample (15 μL) was injected into the stacking gel and electrophoresed under a 12-mA current. The gel was stained for 45 min in the presence of 0.25% Coomassie brilliant blue R-250 solution and distained with a 5% methanol/7.5% acetic acid solution until the bands were clear.
The hydroxyproline content of all collagen samples was determined using the method described by Bargman et al.[Citation31] with a slight modification. Briefly, samples were hydrolysed with 6 M HCl at 110°C for 24 h, followed by pH adjustment to 6.5 using 10 M and 1 M NaOH and filtering. The neutralized sample (0.3 mL) was transferred into a test tube, and isopropanol (0.6 mL) was added. The oxidant solution [0.3 mL of a mixture of 7% (w/v) chloramine T and acetate/citrate buffer (pH 6) at a ratio of 1:4 (v/v)] was added and mixed thoroughly. The chromophore was then developed with the addition of 4 mL Ehrlich’s reagent solution [a mixture of solution A (2 g p-dimethylamino-benzaldehyde in 3 mL of 60% (v/v) perchloric acid (w/v)) and isopropanol at a ratio of 3:13 (v/v)] and incubated for 25 min at 60°C in a water bath. Absorbance was measured at 558 nm. The dry and wet weights of all pretreated skins were accurately weighed and referred to as W3 and W4, respectively. The collagen extracted from differently pretreated skins was weighed and referred to as W5. The extraction rates associated with the dry and wet weights were calculated as follows:
All the samples were measured in triplicate. Optimal pretreatment conditions were determined based on the SDS-PAGE results, hydroxyproline content, and extraction rate.
Determination of fish skin before and after pretreatment (NFS and PFS)
Proximate analysis of NFS and PFS
Fish skins were pretreated according to the optimal conditions and referred to as PFS. The moisture, fat, ash, and protein contents of NFS and PFS were measured according to the methods described in the references. [Citation1,Citation2,Citation7,Citation8] All experiments were performed in triplicate.
Histological analysis of NFS and PFS
To determine the degrees of degreasing, decolouring, and fibre swelling of the pretreated fish skin, histological analysis was performed. NFS and PFS were immersed in 10% (w/v) formalin solution for 24 h and then cut into 15-μm-thick specimens in both the transverse and longitudinal directions using a freezing microtome (Jung Frigocut 2800 N; Leica, Inc., Heidelberg, Germany). The specimens were stained using hematoxylin and eosin (H&E) and then sealed in neutral resin for observation by a light microscope (BH-2; Olympus, Tokyo, Japan) at 100× magnification.[Citation32]
Determination of collagen properties
The lyophilized collagen extracted from PFS was referred to as FC, and the calf skin collagen powder purchased from Sigma–Aldrich (EC No. 232-697-4, Type I) was referred to as CC.
Amino acid analysis
The content of amino acid in the collagen was assessed using the method described by Zhang et al.[Citation22] Each collagen sample (5 mg) was dissolved in 3 mL of 6 M HCl, and hydrolysed in vacuum-sealed glass tubes at 110°C for 24 h. The hydrolysate was vaporized and the residue was dissolved in 25 mL citric acid buffer solution. Then, 0.05 mL sample was applied to an automated amino acid analyser (Hitachi’s L-8900, Tokyo, Japan).
Ultraviolet spectra measurement
The fish and calf collagen (FC and CC, respectively) were dissolved in 0.1 M acetic acid to obtain final concentrations of 0.5 mg/mL. The ultraviolet absorption spectra of FC and CC were measured using a spectrophotometer (PE Lambda 25; Perkin Elmer, Waltham, MA, USA) at wavelengths from 200 nm to 400 nm with a scanning rate of 240 nm/min.
FTIR measurement
FTIR spectra of FC and CC were obtained from discs containing 2-mg collagen samples in ~200 mg potassium bromide (KBr) under dry conditions. The spectra were recorded using a Nicolet iS10 spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) from 4000 cm−Citation1 to 500 cm−Citation1 and at a data-acquisition rate of 2 cm−Citation1 per point, with 32 scans conducted for each sample.
Thermal stability measurement
The thermal transition of collagen was determined by differential scanning calorimetry (Netzsch DSC 200PC; Bavaria, Germany). FC and CC were dissolved in 0.1 M acetic acid to a concentration of 5 mg mL−Citation1, and ~10 mg FC and CC solutions were weighed in aluminium pans, sealed, and then scanned over a temperature range of 25°C–50°C at a heating rate of 3°C min−Citation1 under nitrogen atmosphere. A sealed aluminium pan containing a solution consisting of 10 mg of 0.1 M acetic acid was used as the reference.
Results and discussion
Pretreatment optimization
The optimal degreasing method was determined according to the fat content of fish skin treated with different defatting processes, and the optimal decolouring method was determined according to the results of SDS-PAGE analysis, hydroxyproline content, and extraction rate.
Optimization of the degreasing process
The fat content and degreasing rate of NFS, NFS1, NFS2, and NFS3 are shown in . The fat content of Southern catfish skin (dry weight) was 3.68%, which was comparatively higher than those obtained for squid (Doryteuthis singhalensis) (2.774%),[Citation2] bigeye snapper (Priacanthus tayenus) (2.728%),[Citation7] brown-backed toadfish (Lagocephalus gloveri) (1.3%),[Citation8] and brown-banded bamboo shark (Chiloscyllium punctatum) (0.50%).[Citation20] To obtain collagen with high purity, the degreasing procedure was necessary for the efficient extraction of collagen. Obtaining an effective degreasing method for Southern catfish skins can also offer a potential reference for other types of fish skin with a low fat content. Na2CO3 solution was used to degrease the skin and remove non-collagenous proteins during pretreatment; however, the fat content (1.13%) and degreasing rate (69.37%) of NFS1 indicated that individual treatment with 10% Na2CO3 solution was insufficient. The fat content of NFS2 (0.70%) was 0.43% lower than that of NFS1, indicating that the addition of isopropyl alcohol might further remove the lipids from the skin. NFS3 exhibited the lowest lipid (0.36%) content and maximum degreasing rate (90.24%) following sequential treatment of NFS2 with a non-ionic degreasing agent. Our results indicated that skin fat was effectively removed by the combination of multiple degreasing methods.
Table 2. The fat content and skim rate of NFS, NFS1, NFS2, and NFS3.
Optimization of decolouring process
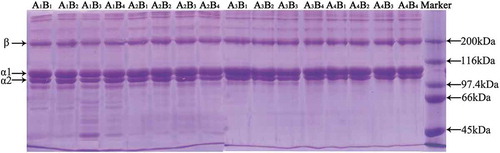
shows SDS-PAGE patterns of collagen from Southern catfish skins pretreated under different conditions. All collagen samples were comprised of at least the different α-chains (α1 and α2) and 1 β-chain. The intensity of α1 was ~2-fold higher than that of α2, which was similar to the pattern observed for type I collagen from other fish species[Citation33, Citation34] and suggested that the collagen extracted from Southern catfish skins was likely type I collagen. With respect to the different decoloring conditions, different intensities associated with low-molecular-weight components (<97.4 kDa) derived from the non-collagenous proteins were observed in the banding patterns, indicating that the amounts of removed non-collagenous proteins differed based on the various pretreatment conditions. The intensities of the low-molecular-weight bands were invisible at pH values of between 10 and 11 (A3B1 and A4B4), indicating that the extracted collagen exhibited higher purity.
The extraction rate and hydroxyproline content of collagen isolated following different pretreatment methods are shown in . The maximum extraction rate was 23.14% (wet weight) and 78.57% (dry weight) under the process A3B1, which was comparatively higher than the rates observed for cod skin (20%, wet weight),[Citation25] Spanish mackerel (Scomberomorous niphonius) skin (17.17%, wet weight),[Citation1] brown-backed toadfish skin (54.3%, dry weight),[Citation8] chub mackerel (Scomber japonicus) skin (49.8%, dry weight), and bullhead shark (Heterodontus japonicus) skin (50.1%, dry weight).[Citation35] Additionally, the hydroxyproline content under process A3B1 was 391.26 μg mL−Citation1, indicating that the extracted collagen exhibited higher purity.
Table 3. The yield and Hyp content of collagen extracted from the different pretreatment technics.
According to the results associated with the degreasing rate, SDS-PAGE, extraction rate, and hydroxyproline content, multiple degreasing methods effectively removed skin fat to allow acquisition of higher collagen purity and yield following decolouring treatment involving 0.5% H2O2 at pH 10. The fish skin might exhibit a loosened matrix via swelling mechanisms after multiple defatting treatments. Afterwards, the decolouring agent and pepsin would have easier access to regions between skin fibres. As a consequence, we obtained collagen exhibiting higher yield and purity.
Analysis of NFS and PFS
Proximate compositions of NFS and PFS
To illustrate the changes in the composition of fish skin before and after pretreatment, the proximate compositions of NFS and PFS are given in . NFS exhibited high moisture content (65.63%), which was similar to the results obtained for brown-banded bamboo shark skin (61.96%),[Citation20] balloon fish (Diodon holocanthus) skin (62.23%),[Citation36] bigeye snapper skin (64.08%),[Citation7] and Nile perch (Lates niloticus) skin (68.4%).[Citation9] The crude protein, ash, and fat contents of Southern catfish skin (dry weight) were 89.67%, 0.56%, and 3.68%, respectively. The higher protein content (dry weight), which was similar to those of bigeye snapper skin (89.09%)[Citation7] and brown-backed toadfish skin (90.30%),[Citation8] suggested that Southern catfish skins could be used as a potential source for collagen extraction. Due to the absence of scales on Southern catfish skin, the ash content (dry weight) was lower than that of brown-backed toadfish skin (8.4%),[Citation8] balloon fish skin (15.87%),[Citation36] and bigeye snapper skin (8.99%).[Citation7] Additionally, the fat content (3.68%) of NFS was higher than that of other fish species,[Citation2,Citation5] indicating that the degreasing process was essential. The moisture content of PFS (76.1%) was 10.47% higher than that measure for NFS due to loosened collagen fibres resulting from alkaline treatment. Following pretreatment, the fat content of PFS (dry weight) was 0.36%, which decreased along with an increase in the protein content of 3.38%, indicating that the collagen isolated from Southern catfish skins would be of higher quality following treatment using a combination of degreasing methods. The proximate composition of Southern catfish skin showed that FPS was more suitable as compared to NFS for collagen extraction.
Table 4. Proximate analyses of NFS and PFS.
Histological analysis of NFS and PFS
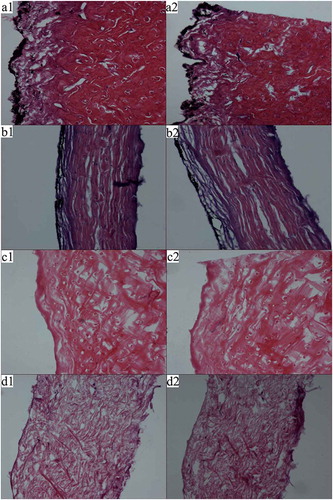
Tissue images of NFS and PFS at 100× magnification are displayed in . According to histology in the presence of H&E staining, the NFS epidermis appeared black following staining, and the collagen fibres were dyed red accompanied with blue dots representing nuclei. NFS collagen fibres appeared closely knit in the form of bundles, with vertical and horizontal distributions. Additionally, collagen fibres near the epidermis were looser than those in the dermis. Following pretreatment, the black region of the epidermis disappeared, illustrating effective removal of the pigment by H2O2. Compared with NFS, the PFS collagen fibres exhibiting a loose distribution were attributed to swelling initiated by incubation in an alkaline solution and the removal of non-collagenous protein and fat. These results indicated that following pretreatment, fish-skin fibres swelled and non-collagenous substances were removed, resulting in collagen isolation to a higher yield and purity.
Characteristics of collagen
Amino acid compositions
shows the amino acid composition of the collagen from Southern catfish skin as compared with that of calf skin. The collagen from Southern catfish skin contained glycine (347 residues/1000 residues) as the major amino acid and was also rich in alanine (115 residues/1000 residues) and proline (120 residues/1000 residues). The collagen had low methionine (8 residues/1000 residues), tyrosine (1 residue/1000 residues), and histidine (6 residues/1000 residues) contents, similar to other forms of collagen.[Citation37] The number of imino acid residues, proline, and hydroxyproline, in collagen from Southern catfish skin, was 197 residues/1000 residues, which was higher than those reported for bighead carp collagen (181 residues/1000 residues)[Citation38] and grass carp (Ctenopharyngodon idella) collagen (186 residues/1000 residues)[Citation6] skin, but lower than those reported for calf skin collagen (215 residues/1000 residues). The higher the imino acid content, the more stable the helices,[Citation39] with the stability of collagen helix structures affected by proline hydroxylation. The collagen of Southern catfish skin had a hydroxyproline content of 39.1%, which was higher than that of collagen from bighead and grass carp skin (36.46% and 34.9%, respectively),[Citation38] but lower than that of collagen from calf skin (43.7%). These findings suggested that the difference in the imino acid content of fish-skin collagen might be associated with differences in habitat. Our results indicated that the collagen isolated from Southern catfish skin was type I collagen based on the result of electrophoretic analysis.
Table 5. Amino acid composition of collagens (residues/1000 residues).
Ultraviolet spectra measurement
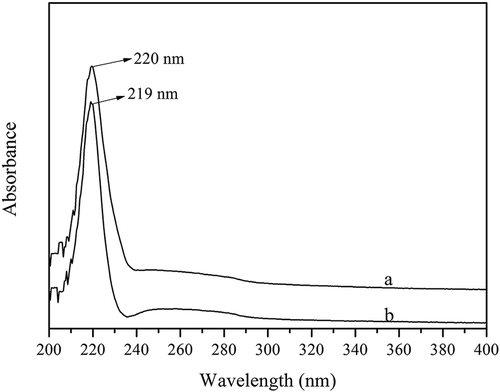
The ultraviolet spectra of FC and CC at wavelengths between 200 nm and 400 nm are presented in . The maximum absorption wavelength of most proteins appeared at 356 nm, with some proteins exhibiting strong ultraviolet absorption at 280 nm due to the content of tyrosine and tryptophan. However, there was no observable absorption for FC and CC at this wavelength due to the low concentration of tyrosine (one and three residues per 1000 residues for FC and CC, respectively). As presented in , the maximum absorption wavelengths of FC and CC occurred at 220 nm and 219 nm, respectively, which was similar to that of collagen extracted from squid skin (222 nm). Furthermore, this result provided additional proof of the isolation of highly pure collagen from Southern catfish skin.
FTIR spectroscopy
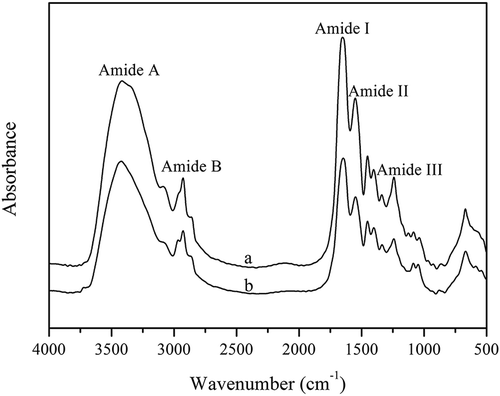
shows the FTIR spectra of FC and CC, which were similar to those of collagen from other fish species.[1,2] Amide A-band positions in FC and CC were observed at 3420 cm-1 and 3428 cm-1, respectively, and were ascribed to the N–H-stretching vibration involved in hydrogen bonding according to Doyle et al.[40] The amide B-band positions of FC and CC were observed at 2926 cm-1 and 2928 cm-1, respectively, representing the asymmetrical stretching of CH2 groups. Amide I bands from FC and CC and mainly associated with C=O-stretching vibrations along the polypeptide backbone were observed at 1655 cm-1 and 1653 cm-1,[41] respectively. Amide II bands from FC observed at 1550 cm-1 were lower than those from CC (1560 cm-1), indicating that the N–H bonds in FC were involved in additional interactions with adjacent a-chains relative to those in CC. Amide III bands were represented by coupled peaks between C–N-stretching vibrations and N–H deformation from amide linkages, as well as absorption originating from wagging vibrations from CH2 groups in the glycine backbone and proline sidechains.[42] The amide III bands from FC and CC were observed at 1240 cm-1 and 1239 cm-1, respectively. The absorption ratios between the amide III bands and the 1450 cm-1 bands from FC and CC were 0.903 and 0.917, respectively, suggesting that the triple-helical structure of collagen from each sample maintained its integrity.[43]
Thermal stability
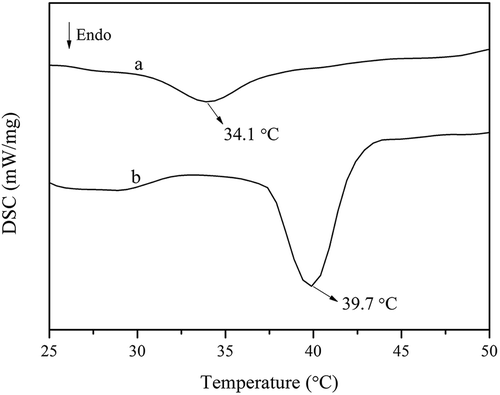
Differential scanning calorimetry thermograms for FC and CC are shown in . The maximum endothermic temperature (Tmax) for FC and CC was 34.1°C and 39.7°C, respectively, both of which were higher than those of collagen from other fish skins, such as Baltic cod skin (15°C),[Citation25] deep-sea redfish (Sebastes mentella) (16.1°C),[Citation5] bigeye snapper (31°C),[Citation7] and brown-striped red snapper (Lutjanus vitta) (30.5°C).[Citation12] The difference in Tmax among the collagen from various species might be related to the imino acid content, the degree of hydroxylation of proline residues, body temperature, and environmental temperature.[Citation33] The comparatively high Tmax values broadened the region of collagen found in Southern catfish skin.
Conclusion
The economic and sustainable use of fish skins from industrial processing has gained increasing attention. In this paper, the optimal pretreatment conditions for isolating the collagen from Southern catfish skins to acquire collagen were studied. The results demonstrated that fat and pigment were removed, and skin fibres were effectively loosened following the pretreatment processes. The yield and purity of the extracted collagen were obviously improved. Additionally, the properties of the collagen extracted from fish skins pretreated under these optimal conditions were compared with those from calf skin collagen, revealing that the Tmax of fish collagen was close to that of calf skin collagen. These results indicated that Southern catfish skin collagen can be used as an alternative source of mammalian collagen in industrial applications. Furthermore, these pretreatment methods can be utilized for the industrial processing of collagen.
Funding
This research was financially supported by the National Natural Science Foundation of China (No. 21276167 and No. 21476147).
Additional information
Funding
References
- Li, Z.R.; Wang, B.; Chi, C.F.; Zhang, Q.H.; Gong, Y.D.; Tang, J.J.; Luo, H.Y.; Ding, G. Isolation and Characterization of Acid Soluble Collagens and Pepsin Soluble Collagens from the Skin and Bone of Spanish Mackerel (Scomberomorous niphonius). Food Hydrocolloids 2013, 31, 103–113.
- Veeruraj, A.; Arumugam, M.; Ajithkumar, T.; Balasubramanian, T. Isolation and Characterization of Collagen from the Outer Skin of Squid (Doryteuthis singhalensis). Food Hydrocolloids 2015, 43, 708–716.
- Woo, J.W.; Yu, S.J.; Cho, S.M.; Lee, Y.B.; Kim, S.B. Extraction Optimization and Properties of Collagen from Yellowfin Tuna (Thunnus albacares) Dorsal Skin. Food Hydrocolloids 2008, 22, 879–887.
- Liu, W.; Li, G.; Miao, Y.; Wu, X. Preparation and Characterization of Pepsin-Solubilized Type I Collagen from the Scales of Snakehead (Ophiocephalus argus). Journal of Food Biochemistry 2009, 33, 20–37.
- Wang, L.; An, X.; Xin, Z.; Zhao, L.; Hu, Q. Isolation and Characterization of Collagen from the Skin of Deep-Sea Redfish (Sebastes mentella). Journal of Food Science 2007, 72, E450–E455.
- Zhang, Y.; Liu, W.; Li, G.; Shi. B.; Miao. Y.; Wu. X. Isolation and Partial Characterization of Pepsin-Soluble Collagen from the Skin of Grass Carp (Ctenopharyngodon idella). Food Chemistry 2007, 103, 906–912.
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Nagai, T.; Tanaka, M. Characterisation of Acid-Soluble Collagen from Skin and Bone of Bigeye Snapper (Priacanthus tayenus). Food Chemistry 2005, 89, 363–372.
- Senaratne, L.S.; Park, P.J.; Kim, S.K. Isolation and Characterization of Collagen from Brown Backed Toadfish (Lagocephalus gloveri) Skin. Bioresource Technology 2006, 97, 191–197.
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Characterisation of Acid Soluble Collagen from Skins of Young and Adult Nile Perch (Lates niloticus). Food Chemistry 2004, 85, 81–89.
- Hsieh, C.H.; Shiau, C.Y.; Su, Y.C.; Liu, Y.H.; Huang, Y.R.; Isolation and Characterization of Collagens from the Skin of Giant Grouper (Epinephelus lanceolatus). Journal of Aquatic Food Product Technology 2014, 25, 93–104.
- Singh, P.; Benjakul, S.; Maqsood, S.; Kishimura, H. Isolation and Characterisation of Collagen Extracted from the Skin of Striped Catfish (Pangasianodon hypophthalmus). Food Chemistry 2011, 124, 97–105.
- Jongjareonrak, A.; Benjakul, S.; Visessanguan, W.; Nagai, T.; Tanaka, M. Isolation and Characterisation of Acid and Pepsin-Solubilised Collagens from the Skin of Brownstripe Red Snapper (Lutjanus vitta). Food Chemistry 2005, 93, 475–484.
- Nalinanon, S.; Benjakul, S.; Visessanguan, W.; Kishimura, H. Use of Pepsin for Collagen Extraction from the Skin of Bigeye Snapper (Priacanthus tayenus). Food Chemistry 2007, 104, 593–601.
- Nagai, T.; Araki, Y.; Suzuki, N. Collagen of the Skin of Ocellate Puffer Fish (Takifugu rubripes). Food Chemistry 2002, 78, 173–177.
- Liu, D.; Wei, G.; Li. T.; Hu, J.; Lu, N.; Regenstein. J.M.; Zhou, P. Effects of Alkaline Pretreatments and acid Extraction Conditions on the Acid-Soluble Collagen from Grass Carp (Ctenopharyngodon idella) Skin. Food Chemistry 2015, 172, 836–843.
- Nagai, T.; Suzuki, N. Preparation and Partial Characterization of Collagen from Paper Nautilus (Argonauta argo, Linnaeus) Outer Skinn. Food Chemistry 2002, 76, 149–153.
- Hwang, J.H.; Mizuta, S.; Yokoyama, Y.; Yoshinaka, R. Purification and Characterization of Molecular Species of Collagen in the Skin of Skate (Raja kenojei). Food Chemistry 2007, 100, 921–925.
- Chen, J.; Li, L.; Yi, R.; Xu, N.; Gao, R.; Hong, B. Extraction and Characterization of Acid-Soluble Collagen from Scales and Skin of Tilapia (Oreochromis niloticus). LWT - Food Science and Technology 2016, 66, 453–459.
- Ciarlo, A.S.; Paredi, M.E.; Fraga, A.N. Isolation of Soluble Collagen from Hake Skin (Merluccius hubbsi). Journal of Aquatic Food Product Technology 1997, 6, 65–77.
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Kishimura, H.; Shahidi, F. Isolation and Characterisation of Collagen from the Skin of Brownbanded Bamboo Shark (Chiloscyllium punctatum). Food Chemistry 2010, 119, 1519–1526.
- Nagai, T.; Yamashita, E.; Taniguchi, K.; Kanamori, N.; Suzuki, N. Isolation and Characterisation of Collagen from the Outer Skin Waste Material of Cuttlefish (Sepia lycidas). Food Chemistry 2001, 72, 425–429.
- Zhang, M.; Liu, W.; Li, G. Isolation and Characterisation of Collagens from the skin of Largefin Longbarbel Catfish (Mystus macropterus). Food Chemistry 2009, 115, 826–831.
- Montero, P.; Alvarez, C.; Marti, M.A.; Borderias, A.J. Plaice Skin Collagen Extraction and Functional Properties. Journal of Food Science 1995, 60, 1–3.
- Kołodziejska, I.; Sikorski, Z.E.; Niecikowska, C. Parameters Affecting the Isolation of Collagen from Squid (Illex argentinus) Skins. Food Chemistry 1999, 66, 153–157.
- Sadowska, M.; Kołodziejska, I.; Niecikowska, C. Isolation of Collagen from the Skins of Baltic Cod (Gadus morhua). Food Chemistry 2003, 81, 257–262.
- Shuai, Z.; Xu, G.; Li, G. Influence of Different Pigskin Degreasing Methods on Collagen Yield. China Leather 2007, 36, 33–36.
- Qing, F.; Guoying, L.; Xu, Y. Pretreatment Method of Bovine Hides for Natural Collagen Extraction at High Percentage. China Leather 2006, 35, 28–35.
- Peng, L.; Huishang, Z.; Ran, A.; Jianwen, C.; Chen, L. The Degreasing of Catfish Skin with Alkaline Lipase. Food science and technology 2015, 40, 147–150.
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Canadian Journal of Biochemistry and Physiology 1959, 37, 911–917.
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of Head of Bacteriophage T4. Nature 1970, 277, 680–685.
- Bergman, I.; R, L. Two Improved and Simplified Methods for the Spectrophotometric Determination of Hydroxyproline. Analytical Chemistry 1963, 35, 1961–1965.
- Koide, M.; Osaki, K.; Konishi, J.; Yoshizato, K. A New Type of Biomaterial for Artificial Skin: Dehydrothermally Cross-Linked Composites of Fibrillar and Denatured Collagens. Journal of Biomedical Materials Research 1993, 27, 79–87.
- Nagai, T.; Suzuki, N.; Nagashima, T. Collagen from Common Minke Whale (Balaenoptera acutorostrata) Unesu. Food Chemistry 2008, 111, 296–301.
- Liu, H.; Li, D.; Guo, S. Studies on Collagen from the Skin of Channel Catfish (Ictalurus punctaus). Food Chemistry 2007, 101, 621–625.
- Nagai, T.; Suzuki, N. Isolation of Collagen from fish Waste Material-Skin, Bone and Fins. Food Chemistry 2000, 68, 277–281.
- Huang, Y.R.; Shiau, C.Y.; Chen. H.H.; Huang, B.C. Isolation and Characterization of Acid and Pepsin-Solubilized Collagens from the Skin of Balloon Fish (Diodon holocanthus). Food Hydrocolloids 2011, 25, 1507–1513.
- Madeleine, M.; Guille, G.; Besseau, L.; Chopin, C.; Durand, P.; Herbage, D. Structural Aspects of Fish Skin Collagen Which forms Ordered Arrays Via Liquid Crystalline States. Biomaterials 2000, 21, 899–906.
- Liu, D.; Zhou, P.; Li, T.; Regenstein, J.M.; Comparison of Acid-Soluble Collagens from the Skins and Scales of Four Carp Species. Food Hydrocolloids 2014, 41, 290–297.
- Ikoma, T.; Kobayashi, H.; Tanaka, J.; Walsh, D.; Mann, S. Physical Properties of Type I Collagen Extracted from Fish Scales of Pagrus Major and Oreochromis Niloticas. International Journal of Biological Macromolecules 2003, 32, 199–204.
- Doyle, B.B.; Bendit, E.G.; Blout, E.R. Infrared Spectroscopy of Collagen and Collagen-Like Polypeptides. Biopolymers 1975, 14, 937–957.
- Pati, F.; Adhikari, B.; Dhara, S. Isolation and Characterization of Fish Scale Collagen of Higher Thermal Stability. Bioresource technology 2010, 101, 3737–3742.
- Jackson, M.; Choo, L.P.; Watson, P.H.; Halliday, W.C.; Mantsch, H.H. Beware of connective tissue proteins assignment and implications of collagen absorptions in infrared spectra of human tissues. Biochimica et Biophysica Acta 1995, 1270, 1–6.
- Plepis, A.M.D.G.; Goissis, G.; Das-Gupta, D.K. Dielectric and Pyroelectric Characterization of Anionic and Native Collagen. Polymer Engineering and Science 1996, 36, 2932–2938.