ABSTRACT
Daqu is an essential starter for the brewing of Shanxi Aged Vinegar, and high contents of total acid and total ester are two important indices for highly qualified vinegar. The present study is amied to investigate the aroma-enhancing role of a yeast strain screened from Daqu in fortified vinegar fermentation. A Daqu-originated yeast strain Y14 showed the highest ester-producing ability, up to 36.055 g/L, and was identified as Pichia manshurica by sequence analysis of the D1/D2 domain of the 26S rRNA gene in combination with morphological and physiological properties. When it was re-inoculated into the Daqu-based fermentation, Gas Chromatography Mass Spectrometry (GC/MC) analysis for alcohol fermentation showed a significant increase of total alcohol and total ester. The levels of ethanol, ethyl acetate, and isoamyl acetate increased from 31.912%, 9.839%, and 1.025% to 39.861%, 22.707%, and 1.291%, respectively. Moreover, an additional seven volatile aroma components appeared in the Y14 fortified fermentation (FF). Correspondingly in the resulting vinegar, the total acid and total ester markedly increased from 37.4 and 15.3 g/L to 38.0 and 21.5 g/L, respectively. Sensory assay revealed no abnormal odour but a distinct fruity, flowery fragrance and a lingering ester taste in the vinegar of the FF. With this approach, Pichia manshurica isolated from Daqu, when reintroduced into the Daqu-based fermentation, can significantly increase the acid and the ester production, improve the vinegar quality by endowing it flowery and fruity notes, and a long-lingering buttery and nut-like taste.
Introduction
Different regions of China possess their own local vinegar types.[Citation1] Shanxi Aged Vinegar, with a history of more than 3000 years, is a typical and representative product of Shanxi province in China. It is produced by spontaneous fermentation and undergoes a series of complex traditional techniques listed as a “National Non-Material Cultural Heritage” item. Its unique characteristics are also closely linked with local water, soil, climate, and the regional ecosystem because all these factors determine the quality of Daqu, which is a saccharifying and fermenting agent during the traditional Shanxi Aged Vinegar production and has a significant impact on the flavour of the product.[Citation2]
As a Chinese industrial fermentation starter[Citation3] and because of its importance for the yield and the quality of vinegar, Daqu is currently attracting more and more research interests. Many studies aim to reveal the microbiota structure and microbial dynamics during Daqu-making and Daqu-based spontaneous fermentations.[Citation4] As a critical constituent flora in Daqu, yeasts are classified into alcohol-producing types and aroma-producing types. Saccharomyces (S.), Pichia (P.), and Candida (C.) are known genera involved in traditional Chinese alcoholic and vinegar fermentation. Additionally S. cerevisiae is the most abundant alcohol-producing yeast species, accounting for 95% of all yeasts[Citation1] that dominate alcoholic fermentation.
Although extremely low in abundance, aroma-producing yeasts greatly decide the quality and the flavour of the alcoholic beverage and the vinegar, whereas there are still many unsolved problems derived from the lack of knowledge about them during the process of making Shanxi Aged Vinegar.
Over a period of more than 3000 years of selection and taming, Daqu microbes have evolved to be compatible with each other and have formed a stable ecology.[Citation5] The introduced yeast strains that are not of Daqu origin are ready to be inhibited or killed by the autochthonous yeast strains and other aboriginal inhabitants in Daqu, or even worse, the Daqu indigenous yeasts will be killed by the introduced microbes.[Citation6] Naturally, it is hard to increase the yield or improve the flavour of the vinegar, and their introduction even negatively affects the quality of the vinegar. Hence, a better alternative is to use Daqu-originated autochthonous yeasts to fortify the fermentation to optimize the microbiota in Daqu; thus, a desired increase of the production and a satisfied improvement of the quality can be obtained.[Citation7]
Organic acid is the main component of the vinegar, and esters formed by the reaction between organic acids and alcohols will enhance the flavour of the vinegar. Thus, for the vinegar, the higher the content of total acid and total ester, the better the quality. The aim of the study presented here was to isolate and screen an aroma-producing yeast from Daqu and reintroduce it into the Daqu-based vinegar fermentation and thus enhance Daqu’s ability to produce esters without negative disturbance of its saccharifying and fermenting ability. Such work would be economically significant in improving the quality and modifying the traditional brewing techniques, and finally open a new international market for local products.
Materials and methods
Isolation of yeast strains from Daqu
The materials used for yeast isolation were sampled towards the end of Daqu-based solid-phase alcoholic fermentation (on day 6) from Tongbao Vinegar Company in Taigu, Shanxi province, China. Samples were transported to the laboratory in 10 min and immediately suspended in sterile distilled water by a vigorous vortex. Serially diluted suspension was then plated on rose-bengal plates (5 g peptone, 10 g glucose, 1 g KH2 PO4, 0.5 g MgSO4·7H2O, 15 g agar, 100 mL 1/3000 rose-bengal solution, 1000 mL distilled water, and supplemented with 100 mg chloramphenicol). The plates were incubated at 28°C for 48 h. Representatives of each colony morphotype were purified by repeated streaking on rose-bengal agar plates.
Analysis of ester-producing ability
Around 3% (V/V) activated yeast isolate was inoculated into 80 mL ester-producing medium (8% glucose, 1% yeast extract, 2% peptone, natural pH) in a 150 mL conical flask. The liquid was incubated statically at 28°C for 7 days. Commercial aroma-producing yeast (ANGEL YEAST CO., LTD, China) labelled as Y21 as the control strain was used.
Microbiological analysis
Y14 was seeded into the liquid medium of malt extract (130 g malt extract powder, 0.1 g chloramphenicol, 1000 mL distilled water, pH 5.6) and was incubated at 25°C for 3 days, and the cell size was measured. Y14 was streaked onto the malt extract solid medium and incubated at 25°C for 1 week, and then the colony features were recorded. Cornmal agar Dalmau plate (AMRESCO, Shanghai, China) and spore-producing agar were used to check whether Y14 can form pseudohypha and ascospore. Ascospores can be dyed into green when stained by malachite green. Physiological and biochemical properties were determined as described by Yarrow.[Citation8] All assimilation tests were performed in duplicate on a 10-mL scale in test tubes and the results were recorded after 1 and 3 weeks.
The parameters for the tolerance test were set according to the practicality of Shanxi Aged Vinegar fermentation. All the tests were carried out in duplicates in Yeast Extract Peptone Dextrose (YPD) medium (pH 6.5) containing 1% yeast extract, 2% peptone, 2% glucose, and 2% agar, and all tubes were semi-anaerobically incubated. To test the pH tolerance, the pH was adjusted to 1.5, 2.0, 2.5, 3.0, and 4.0 with 1 M HCl. To test the ethanol tolerance, ethanol was supplemented to a final concentration (V/V) of 6%, 8%, 12%, 14%, 16%, and 18%. To test the heat tolerance, the medium (pH 5.5) was incubated at 30°C, 35°C, 40°C, 45°C, and 50°C. All 10-mL tubes were inoculated with 1% (V/V) of Y14 culture grown at 28°C overnight. Growth was determined by wet weight of cell pellet after centrifugation.
D1/D2 domain sequence analysis
Genomic DNA of Y14 was extracted and purified.[Citation9] To amplify the D1/D2 region of Y14 26s rDNA, the primers used for Polymerase Chain Reaction (PCR) were synthesized by SBS Genetech Co., Ltd (Peking). NL1: 5ʹ-GCA TAT CAA TAA GCG GAG GAA AAG-3ʹ, NL4:5ʹ-GGT CCG TGT TTC AAG ACG G-3ʹ. After 30 cycles of PCR (94°C 1 min, 52°C 1 min, and 72°C 50 s), the purified amplicons were sequenced by Invitrogen. BLAST searches were performed at the NCBI GenBank data library. Clustal X (1.83) was used to perform sequence alignments of the D1/D2 region (26s rDNA) of Y14 with homologous sequences available in GenBank. The distance tree was produced using BLAST pairwise alignments by the method of neighbour joining.
Y14-enhanced alcoholic fermentation
Y14 in YPD (30°C) was subcultured for two passages and inoculated (7%, V/V) into the Daqu-based fermentation in triplicates. The recipe for the fermentation matrix was composed of 100 g dried corn flour, 300 mL sterilized water, 25 g Daqu powder, and 300 u (0.35 g) activated glucoamylase (Ruiyang biotech LTD, China). In this study, 80 g corn flour was used for each fermentation after being soaked (24 h, with 60% humidity) and steamed (1 ~ 1.5 h). The alcohol fermentation was carried out in a 500-mL Erlenmeyer flask capped with a cotton plug by static culture at 25°C. Flasks were stirred once a day for the first 2 days. Immediately after the second stirring, all the flasks were sealed with double layers of plastic film and left to ferment for another 6 days. Then ethanol contents were determined and sensory evaluations were organized. In the control fermentation, Daqu was the only starter with no fortification of Y14.
Y14-enhanced acetic acid fermentation
Both the alcoholic fermentations enhanced by Y14 and the control ones were transferred to plastic buckets and made into a solid form by directly supplementing the rice husk and wheat bran at a ratio of 1:1.2:1.6 (1 stands for dried corn flour used in alcoholic fermentation, 80 g in this study). Acetic acid bacteria were inoculated in the form of 6% (V/V) fermenting matrix, which was collected on the second day of acetic acid fermentation from Tongbao vinegar company in Taigu, China. The buckets were covered with straw mats and were incubated at 25°C. The matrix was turned over daily from day 3 to day 9. NaCl (4 g) was added to terminate the fermentation. Then the matrix was soaked in 350 mL water for 24 h. The collected vinegar was subject to quality evaluation according to the methods described in Chinese national standards GB 18187-2000 dedicated for vinegar. To determine the content of total acid, phenolphthalein was used as the indicator to judge the end point of the titration with 0.05 mol/L NaOH, and the content of the acid was calculated in light of the volume consumed. Then the above-mentioned solution underwent saponification by 0.05 mol/L NaOH, and then was titrated by 0.05 mol/L H2SO4 to pH 8.7, and the content of total ester was calculated according to the volume of H2SO4 consumed. The content of non-volatile acid was also determined by the method of NaOH titration after the vinegar sample was distilled for 2 min, and the reducing sugar was analysed by Fehling’s reagent. For the content of amino nitrogen, 20 mL 12.5% vinegar was titrated with 0.05 mol/L NaOH to pH 8.2 and titrated again with NaOH to pH 9.2 after 10 mL methanol being added. The content was calculated according to the consumed volume of NaOH.
Aroma compounds analysis for ethanol fermentation
Three replicates of alcoholic fermentation liquid were collected and mixed thoroughly. Freeze centrifugation was performed for 15 min at 2400 × g, 4°C (Spin Dryer Standard VC-96R, TAITEC Co. Ltd., Tokyo, Japan). Around 10 mL of the supernatant with 3 g NaCl dissolved in it was transferred into a head-space bottle; 2 mL sample was injected by a head-space autosampler (ThermoFisher, Scientific, USA) and vibrated at 70°C for 30 min. GC/MS analysis was performed on a TRACE-ISQ (ThermoFisher) equipped with a TR-5MS column (0.25 mm × 30 m, 0.25 μm, ThermoFisher). System control and data acquisition were conducted using the Thermo X-calibur 2.2 sp 1.48 software. The sample (2 μL) was injected in the split-less mode, with an injection temperature of 250°C. The carrier gas (He) flow was 1 mL/min. The column temperature was held at 35°C for 2 min, then increased by 5°C/min to 230°C, and maintained for 5 min. The transfer line and ion source temperatures were 280°C and 250°C, respectively. Ions were generated by electron ionization (EI) at 70 eV. Mass spectra were recorded at 5 scan/s over the mass range m/z 30 ~ 350. Peak detection and calculation of the peak area were conducted using the X calibur. Retention indices of the eluted compounds were calculated based on the standard alkane mixture (C6–C25). Peaks were annotated by comparing the unique mass spectra with the NIST and Wiley reference. The peak area was normalized against the 3-octanol internal standard.
Quality assessment and sensory analysis of vinegar
The vinegar was heated to 45°C and subjected to a sensory analysis by a trained tasting panel. The descriptors including sour, ester-taste, fruity, flowery, and aftertaste intensity were expressed as numerical values ranging from 0 to 100 and were presented as averages. General comments and characteristics were also given by the panel.
Statistical analysis
The results were expressed as the mean value plus or minus the standard deviation. Data were subjected to one-way analysis of variance (ANOVA) at 5% and 1% levels of significance.
Results
Ester-producing ability analysis of the isolated yeast strains
Typical colonies of Saccharomyces cerevisiae accounted for the majority during alcoholic fermentation. Other 19 representative yeast strains were obtained on the selective plates and marked Y1–Y19. The results showed that 11 isolated strains demonstrated higher capability to produce esters than the control strain Y21, and Y14 displayed the highest performance ().
Table 1. Eleven isolated yeast strains with good ester-producing performance.
Morphological and physiological properties of Y14
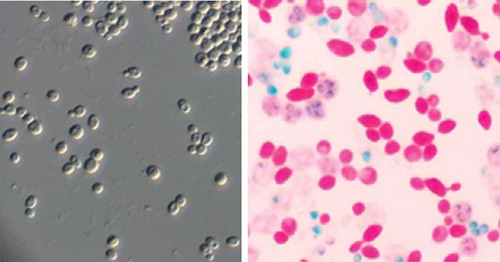
When cultured in a malt extract medium, cells of Y14 were spherical or ovoid, (4.6–6.5) × (3.8–6.5) μm in size, and occurred singly, in pairs, in chains, or in groups (, left). The colony appeared pale grey without lustre, with a smooth surface and a smooth margin. No pseudohyphae were observed and 2 ~ 4 ascospores became visible in the asci after dying (, right). It is noticeable that unlike other yeast strains isolated from Daqu, the ascospores of Y14 were hard to stain for unknown reason. After dying, some were not dyed into green, but blue or colourless. The results in suggested that the ability of the tested strain to ferment glucose was quite weak. It can assimilate glycerol, D-mannitol, succinic acid, and lactic acid and can utilize nitrate, nitrite, and many organic nitrogenous compounds. Results of the litmus milk test confirmed that Y14 cannot utilize lactose.
Table 2. Physiological and biochemical traits of Y14, later identified as Pichia manshurica.
Vinegar fermentation-associated physiological characterization
Tolerance assay showed that Y14 still grew well at 40°C and the growth was apparently inhibited above 40°C. It also proliferated vigorously at pH 2–4, but decreased sharply at pH 1.5. Y14 was capable of growing in the presence of ethanol up to 10%, while it was unable to grow at 12%. It grew even better at 40% glucose concentration than at 10%, 20%, and 30%, and nearly no growth occurred at 50% and 60%. Hence, the critical parameters associated with vinegar fermentation for Y14 were 40°C, pH 1.5, 10% ethanol, and 40% glucose.
Molecular identification
Results of the microscopic and physiological analyses above directed Y14 to the genus of Pichia. For further identification, the D1/D2 region of 26s rDNA of Y14 was amplified and sequenced. It was 514 nt in length (GenBank accession: KP027538.1). According to the currently most frequently detected intra-species sequence variability of 0–3 nucleotide differences in the D1/D2 LSU rRNA region, Y14 is assigned to Pichia manshurica (the type strain of Pichia galeiformis), because their sequences of D1/D2 26s rRNA gene showed 100% similarity. shows the resulting distance tree of Y14 produced by BLAST pairwise alignments. The strain was named Pichia manshurica sxdqY-14 and deposited at China General Microbiological Culture Collection Center (CGMCC) numbered as CGMCC 2.5274.
Ethanol content and aroma components of alcoholic fermentation
The alcohol degree of Y14 fortified fermentation (FF) was markedly higher than that of Daqu-alone fermentation (p < 0.01), which were 9.59% and 9.42%, respectively. In the HS-GC-MS maps of all tested fermentations, ethanol constituted a larger percentage of area at around 2.50 min both in Y14 FF and in the controlled fermentation (CF). Hence, fractional ion maps from 6 min to 40 min were used for analysis.
It can be seen from that the basic major volatile components were roughly the same in the Y14 FF and the CF. It can be calculated from that the aroma components of CF included 60.744% terpinoid alcohols, 12.196% esters, 0.624% aldehyde, and 0.168% others. However, in FF, the content of total alcohols and esters increased significantly to 63.482% and 26.128%, respectively. Although the content of isoamyl alcohol lowered, ethanol increased significantly. The fortification of Y14 decreased the level of isoamyl alcohol (pungent taste), which in turn caused a direct increase of the corresponding ester of isoamyl acetate, which exhibits a strong fresh fruity smell and taste.
Table 3. Peak area and aroma feature of volatile compounds from ethanol fermentation.
Figure 3. Gas chromatography-mass spectrometry ion map (6–40 min) of Y14-fortified alcoholic fermentation (upper) and Daqu-alone alcoholic fermentation (bottom).
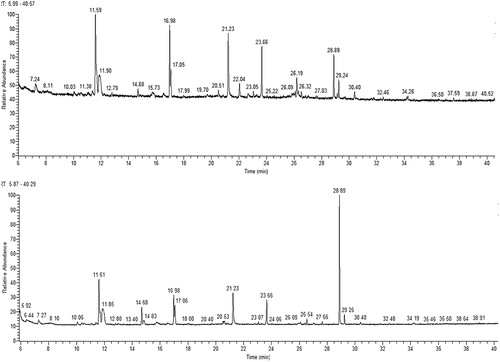
It was also promising that in FF ethyl acetate increased by 135%, which was the most abundant and contributing fragrant component in both Daqu-based liquors and vinegars. Although the levels of some few esters decreased, many others elevated. Aldehyde accounted for less than 1% and also contributed to many kinds of fruity flavours. Seven components, including pentadecanal, 1, 2-propanediol, tetradecanal, 3-octanone, 2-ethoxyethanol, isovaleric acid ethyl ester, and benzoic acid methyl ester, appeared in FF but were not detected in CF (). Among them, benzoic acid methyl ester had a powerful wintergreen oil taste and a strong flowery and cherry-like smell.
Quality assessment and sensory analysis of the Y14-fortified vinegar
It is shown in that in the resulting Y14-fortified vinegar both the total acid and total ester contents were significantly increased (p < 0.01), while the levels of non-volatile acid and amino nitrogen obviously decreased. Additionally, the content of reducing sugar in the vinegar was not affected by the additional inoculation of Y14.
Table 4. The contents (g/L) of five types of basic compounds in the vinegar of the present study.
As shown in , all vinegar tested was sour with different intensities. Compared with Daqu-alone vinegar, Y14-fortified vinegar gained high scores of sour, fruitiness, and floweriness. The ester taste and aftertaste of Y14-fortified vinegar were better than the control ones. Nevertheless, Y14-fortified vinegar tasted less astringent and much better than Daqu-alone vinegar.
Table 5. Sensory evaluation of Y14-fortified Daqu vinegar versus Daqu-alone vinegar.
Discussion
The new ability to ferment or assimilate can be developed during the long history of Daqu making and vinegar brewing in Shanxi. If phenotypic identifications were solely applied, P. manshurica would be identified as P. membranifaciens and many other isolates would be misidentified.[Citation10,Citation11] In this study, the sequence of the D1/D2 domain of the 26S rRNA gene was used in combination with phenotypic traits for the identification of Y14 since it has been widely accepted as a standard procedure for yeast identification.[Citation12,Citation13] Although Y14 was identified as P. Manshurica (P. galeiformis), it showed some differences in phenotypic characteristics from the reported P. manshurica NRRL Y-27978T[Citation14] and the same species isolated from cocoa fermentation.[Citation11] These differences could be interpreted as intra-species variation.
S. cerevisiae was the most sensitive yeast species towards low pH values,[Citation11] while Y14 could still proliferate at pH 2.0 or in the presence of 10% ethanol or at 40°C. Y14 was isolated at the late stage of alcoholic fermentation, so the tolerance results indicated that the Daqu autochthonous Y14 could survive throughout the multiple-stage vinegar fermentation both in lab-scale investigation and in manufacturing practices. During the subsequent acetic acid fermentation, it could produce more esters and further utilize the substrates by producing pectinase.[Citation15] Nevertheless, it could be killed later by the added table salt and pasteurization. This was evidenced by no detection of any yeast in the vinegar. The results were in agreement with the report by Saez JS et al.[Citation16] where small quantities of artificially inoculated P. manshurica are viable and culturable in red wine with 12.6% (v/v) ethanol.
Although in low abundance, P. manshurica was a naturally occurring yeast in the fermenting substrate of Maotai-flavoured liquor,[Citation17] Sichuan sauerkrau, and kombucha.[Citation18] It was the main yeast species present through the table olive fermentation processes.[Citation19,Citation20] It was also the second prevailing yeast species found through all successful cocoa bean fermentation necessary for chocolate making.[Citation21] It was one of the 12 constituent yeast species in koumiss fermentation and could be isolated from all koumiss samples from Inner Mongolia, Qing Hai, and Xin Jiang.[Citation22] As a less-frequently encountered species, P. manshurica was identified for the first time from Daqu during Shanxi Aged Vinegar fermentation in this study.
This study was carried out under strict control to ensure all the isolated yeast strains were from Daqu. Reinforcement of Daqu with Y14 originating from Daqu markedly increased the ethanol production and later increased the acetic acid content in the vinegar. As an aroma-producing yeast, its fermentation ability was quite weak; such an increase of ethanol content was possibly due to an increased abundance of Y14 at an early period of alcoholic fermentation, which favoured the amplification or the activity of ethanol-producing yeasts in Daqu. It was also partially due to the ability of P. manshurica to produce extracellular pectinase, which would hydrolyse the plant material and produce additional fermentable carbohydrates for S. cerevisiae and other microbes.
The fortification of Y14 also greatly boosted the production of esters, especially ethyl acetate, which is the most quality-decisive fragrant component in traditional fermented liquor and vinegar. Other increased esters, such as isoamyl acetate, exhibited strong fresh fruity or flowery flavour. Most of the seven aroma components presenting only in FF fermentation were also of flowery and/or fruity flavouring. Moreover, a significant increase of phenethyl alcohol (phenylethanol, PEA) was also detected, which has a distinct rose-like flavour and is involved in the aroma and taste of many fermented foods. Research showed that although the content of PEA in brandy was extremely low, it greatly and positively affected the aroma and the quality of brandy. It was also reported that an increase of PEA also improved the organoleptic features of saké[Citation23,Citation24] and bread.[Citation25] The results of sensorial analysis further confirmed that both the distilled alcohol and the final vinegar fermented on a lab-scale with Y14-fortified Daqu as starter were characterized by an excellent quality, an integrated organoleptic attribute, rich aroma, prominently pleasant fresh fruity and floral notes, and a long-lingering buttery and nut-like taste. No other negative impacts were discovered in the vinegar.
Conclusion
Re-inoculation of the indigenous yeast strain comprehensively identified as P. manshurica to the Daqu-based fermentation not only increased ethanol and acetic acid production but also increased the total ester content in the vinegar, endowing the vinegar with a pleasant fruity and flowery flavour and a better aftertaste. The results of this study would increase the quality of Shanxi Aged Vinegar, which, with an annual production of 600,000 ton, accounts for nearly 20% of the vinegar production in China.
Acknowledgments
The authors greatly acknowledge Katherine Xenna Goh for her correction of the English writing of this paper.
Funding
This research work was supported by the Science and Technology Key Program (Guide) of Shanxi Province, China (No. 2015-TN-10) and National High Technology Research and Development Program of China (863 program) (2011AA1009040102).
Additional information
Funding
References
- Wu, J.J.; Ma, Y.K.; Zhang, F.F.; Chen, F.S. Biodiversity of Yeasts, Lactic acid Bacteria and Acetic Acid Bacteria in the Fermentation of “Shanxi Aged Vinegar”, a Traditional Chinese Vinegar. Food Microbiology 2012, 30, 289–297. doi: 10.1016/j.fm.2011.08.010.
- Zheng, X.; Tabrizi, M.R.; Robert Nout, E.J.; Han, B.Z.; Han, B.Z. Daqu-a Traditional Chinese Liquor Fermentation Starter. Journal of the Institute of Brewing 2011, 117, 82–90. doi: 10.1002/j.2050-0416.2011.tb00447.x.
- Zheng, X.W.; Zheng, M.J.; Robert Nout, E.J.; Smid, M.; Zwietering, T.; Han, J.S.; Han, B.Z. Microbiota Dynamics Related to Environmental Conditions during the Fermentative Production of Fen-Daqu, a Chinese Industrial Fermentation Starter. International Journal of Food Microbiology 2014, 182, 57–62. doi: 10.1016/j.ijfoodmicro.2014.05.008.
- Li, X.R.; Ma, E.B.; Liang, Z.Y.; Meng, H.; Du, X.W.; Quan, Z.X. Bacterial and Fungal Diversity in the Starter Production Process of Fen Liquor, a Traditional Chinese Liquor. Journal of Microbiology 2013, 51, 430–438. doi: 10.1007/s12275-013-2640-9.
- Li, P.; Li, S.; Cheng, L.; Luo, L. Analyzing the Relation between the Microbial Diversity of Daqu and the Turbidity Spoilage of Traditional Chinese Vinegar. Applied Microbiology and Biotechnology 2014, 13, 60–73.
- Nie, Z.; Zheng, Y.; Du, H.; Xie, S.; Wang, M. Dynamics and Diversity of Microbial Community Succession in Traditional Fermentation of Shanxi Aged Vinegar.[J]. Food Microbiology 2015, 47, 62–68. doi: 10.1016/j.fm.2014.11.006.
- Li, P.; Lin, W.; Liu, X.; Wang, X.; Gan, X.; Luo, L.; Lin, W.T. Effect of Bioaugmented Inoculation on Microbiota Dynamics during Solid-State Fermentation of Daqu Starter using Autochthonous of Bacillus, Pediococcus, Wickerhamomyces and Saccharomycopsis. Food Microbiology 2017, 61, 83–92. doi: 10.1016/j.fm.2016.09.004.
- Yarrow, D. Methods for the Isolation, Maintenance and Identification of Yeasts. The Yeasts, a Taxonomic Study 1998, 14, 77–100.
- Bhadra, B.; Rao, S.R.; Kumar, N.N.; Chaturvedi, P.; Sarkar, P.K.; Shivaji, S. Pichia cecembensissp. nov. Isolated from a Papaya Fruit (Carica papaya L., Caricaceae). Fems Yeast Research 2007, 7, 579–584. doi: 10.1111/fyr.2007.7.issue-4.
- Mikata, K.; Ueda-Nishimura, K. Reclassification of Pichia Membranifaciens Sensu Kurtzman. Antonie van Leeuwenhoek 2000, 77, 159–171. doi: 10.1023/A:1002425226917.
- Daniel, H.M.; Vrancken, G.; Takrama, J.F.; Camu, N.; De Vos, P.; De Vuyst, L. Yeast diversity of Ghanaian Cocoa Bean Heap Fermentations. Fems Yeast Research 2009, 9, 774–783. doi: 10.1111/j.1567-1364.2009.00520.x.
- Fell, J.W.; Boekhout, T.; Fonseca, A.; Scorzetti, G.; Statzell-Tallman, A. Biodiversity and Systematics of Basidiomycetous Yeasts as Determined by Large-subunit rDNA D1/D2 Domain Sequence Analysis. International Journal of Systematic and Evolutionary Microbiology 2000, 50, 1351–1371. doi: 10.1099/00207713-50-3-1351.
- Scorzetti, G.; Fell, J.W.; Fonseca, A.; Statzell-Tallman, A. Systematics of Basidiomycetous Yeasts: A Comparison of Large-subunit D1/D2 and Internal Transcribed Spacer rDNA Regions. Fems Yeast Research 2002, 2, 495–517. doi: 10.1111/fyr.2002.2.issue-4.
- Bhaskar, B.; Zareena, B.; Sisinthy, S. Pichia garciniaesp. nov., Isolated from a Rotten Mangosteen Fruit (Garcinia mangostana L., Clusiaceae). International. Journal of Systematic and Evolutionary Microbiology 2008, 58, 2665–2669. doi: 10.1099/ijs.0.65770-0.
- Golomb, B.L.; Morales, V.; Jung, A.; Yau, B.; Boundy-mills, K.L.; Marco, M.L. Effects of Pectinolytic Yeast on the Microbial Composition and Spoilage of Olive Fermentations. Food Microbiology 2013, 33, 97–106. doi: 10.1016/j.fm.2012.09.004.
- Saez, J.S.; Lopes, C.A.; Kirs, V.E.; Sangorrín, M. Production of Volatile Phenols by Pichia manshurica and Pichia membranifaciens Isolated from Spoiled Wines and Cellar Environment in Patagonia. Food Microbiology 2011, 28, 503–509. doi: 10.1016/j.fm.2010.10.019.
- Sun, J.Q.; Liu, W.W.; Zang, W.; Shen, G.J.; Ping, W.X. Composition and Distribution of Yeasts at Different Fermentation Stages for Maotai-flavor Chinese Liquor Production. Acta Microbiologica Sinica 2012, 52, 1290–1296.
- Qiao, H.P.; Sha, D.N.; Jin, T.Y.; Hang, X.M. Isolation and Identification of Predominant Microbes from Kombucha and Phylogenic Analysis. Journal of Food Science and Biotechnology 2011, 30, 609–612.
- Abriouel, H.; Benomar, N.; Lucas, R.; Gálvez, A. Culture-independent Study of the Diversity of Microbial Populations in Brines during Fermentation of Naturally-fermented Aloreña Green Table Olives. International Journal of Food Microbiology 2011, 144, 487–496. doi: 10.1016/j.ijfoodmicro.2010.11.006.
- Durán Quintana, M.C.; Noé Arroyo, F.; García García, P.; Garrido Fernández, A. Evolución del crecimiento en salmuera, a bajas Temperaturas y Diferentes Acidulantes, de levaduras aisladas de aceitunas de mesa. Grasas y Aceites 2005, 56, 9–15.
- Papalexandratou, Z.; Falony, G.; Romanens, E.; Jimenez, J.C.; Amores, F.; Daniel, H.M.; De Vuyst, L. Species Diversity, Community Dynamics, and Metabolite Kinetics of the Microbiota Associated with Traditional Ecuadorian Spontaneous Cocoa Bean Fermentations. Applied and Environmental Microbiology 2011, 77, 7698–7714. doi: 10.1128/AEM.05523-11.
- Mu, Z.S.; Yang, X.J.; Li, Y.H. Detection and Identification of Wild Yeast in Koumiss. Food Microbiology 2012, 31, 301–308. doi: 10.1016/j.fm.2012.04.004.
- Fukuda, K.; Watanabe, M.; Asano, K.; Ouchi, K.; Takasawa, S. Isolation and Genetic Study of p-fluoro-DL-phenylalanine-resistant Mutants Overproducing β-phenethylalcohol in Saccharomyces cerevisiae. Current Genetics 1991a, 20, 449–452. doi: 10.1007/BF00334770.
- Fukuda, K.; Watanabe, M.; Asano, K.; Ouchi, K.; Takasawa, S. A Mutated ARO4 Gene for Feedback-Resistant DAHP Synthase which Causes both o-fluoro-DL-phenylalanine Resistance and β-phenethyl-alcohol Overproduction in Saccharomyces cerevisiae. Current Genetics 1991b, 20, 453–456. doi: 10.1007/BF00334771.
- Dueñas-Sánchez, R.; Pérez, A.G.; Codón, A.C.; Benítez, T.; Rincón, A.M. Overproduction of 2-phenylethanol by Industrial Yeasts to Improve Organoleptic Properties of Bakers’ Products. International Journal of Food Microbiology 2014, 180, 7–12. doi: 10.1016/j.ijfoodmicro.2014.03.029.