ABSTRACT
Residue change of six pesticides (triadimefon, lambda-cyhalothrin, beta-cypermethrin, esfenvalerate, pyraclostrobin, and difenoconazole) in Chinese liquor produced from field incurred sorghum was studied. The Chinese liquor was produced through soaking, steaming, fermentation, and distillation process. Residue magnitudes of the six pesticides were determined by gas chromatography coupled with tandem mass spectrometry (GC-MS/MS). The results showed soaking process did not significantly reduce the pesticide residues, while the steaming process involving continuous heating could obviously reduce the pesticide residues by 54–72%. Moreover, the fermentation process could remove pesticide residues by 37–65% due to biological degradation. Furthermore, the distillation process could completely remove the residue of lambda-cyhalothrin, beta-cypermethrin, esfenvalerate, pyraclostrobin, and difenoconazole, and remarkably reduce the residue of triadimefon with the processing factor between 0.002 and 0.004. This study demonstrated that the whole process could obviously reduce the residue of contaminated pesticides in Chinese liquor, especially the distillation process.
Introduction
Chinese liquor takes an important role in Chinese culture and people’s daily life in China.[Citation1] It is typically produced from cereals, mainly sorghum.[Citation2] However, as the pests and diseases often occur in the growth of sorghum, it is unavoidable to use plenty of pesticides in order to ensure the quality and yield.[Citation3] Although these pesticides control of pests and diseases, they also induce potential risk to human health.[Citation4] Therefore, monitoring the residue magnitudes of pesticides in sorghum and its processed products is important for human health.
Food safety issue involving pesticide residues is becoming increasingly important.[Citation5] Most raw agricultural commodities (RAC) are consumed after variety of processing.[Citation6] These processing procedures usually decrease the residue magnitudes of pesticides in foods, such as blanching, washing, juicing, baking, fermentation, etc.[Citation7] However, in some cases, the processing procedures may increase the concentration of residue magnitudes in processed products, for example crude rapeseed oil production[Citation8] or fruit drying[Citation9] etc. Moreover, some processing procedures may produce more toxic metabolites of pesticides.[Citation10,Citation11] As far as we know, the research concerning liquors was mainly about the single process, such as fermentation[Citation12,Citation13] and distillation.[Citation14,Citation15] However, no paper has reported the change of pesticide residues during Chinese liquor production. Therefore, good knowledge of the residue change of pesticides in Chinese liquor production is necessary to properly assess the human exposure from such pesticides.
The processing factor (PF) is evaluated by the ratio of residue magnitudes in processed commodities and the RAC. The estimation of PF is used to conduct refined dietary exposure assessments of related pesticides in processed commodities. Moreover, the PF is also used to establish maximum residue limits (MRLs) for processed commodities when the processing procedure induces the increase of residue magnitudes.[Citation16] To our knowledge, there is little report about the study of PFs relating to pesticide residues during the production of Chinese liquor. Therefore, it is of importance to clarify the PFs of pesticides during Chinese liquor production.
This work was aimed to study the residue change of six pesticides in Chinese liquor produced from field incurred sorghum, and throw light on the PFs during each process of Chinese liquor production. The six pesticides (triadimefon, lambda-cyhalothrin, beta-cypermethrin, esfenvalerate, pyraclostrobin, and difenoconazole) were selected on the basis of the frequency of detection and registration in sorghum.
Materials and methods
Standards, reagents, and materials
The pesticide standards triadimefon (purity ≥99.0%), lambda-cyhalothrin (purity ≥98.0%), beta-cypermethrin (purity ≥95.0%), esfenvalerate (purity ≥97.0%), pyraclostrobin (purity ≥97.0%), and difenoconazole (purity ≥95.0%) were obtained from the National Institute of Metrology (Beijing, China). Commercial 25% triadimefon wettable powder (WP) and 250 g/L pyraclostrobin EC were obtained from Jiangsu State Farm Biochemistry Co., Ltd. (Jiangsu, China). Chromatography grade acetonitrile was obtained from Fisher Scientific (New Jersey, USA). Analytical grade anhydrous magnesium sulphate (MgSO4) and sodium chloride (NaCl) were purchased from Sinopharm Chemical Reagent Co. Ltd (Beijing, China). Multi-walled carbon nanotubes (MWCNTs, 5–10 nm), octadecylsilane (C18, 40 μm), and primary secondary amine (PSA, 40 μm) were obtained from Tianjin Bonna-Agela (Tianjin, China). Ultra-pure water was acquired from a Milli-Q system (Bedford, MA, USA). Standard stock solutions of 100 mg L−Citation1 for mixture of the six pesticides were prepared in acetonitrile and diluted daily.
Field trials
The field trials were conducted in the experimental farm of China agricultural university. The fields were divided into 50 mCitation2 blocks for the control. Three independent field test sites were taken for the treatment. All four sides of the plots were protected by blank sorghum. According to the OECD guideline for the testing of magnitude of the pesticide residues in processed commodities, all the pesticide formulations were mixed and sprayed on sorghum at fivefold of the recommended dosage.[Citation16] Twenty kilograms sorghum was sampled in each block after 3 days. All the sorghum samples were placed in polyethylene bags and transported to the laboratory for Chinese liquor production.
Sample preparation and processing
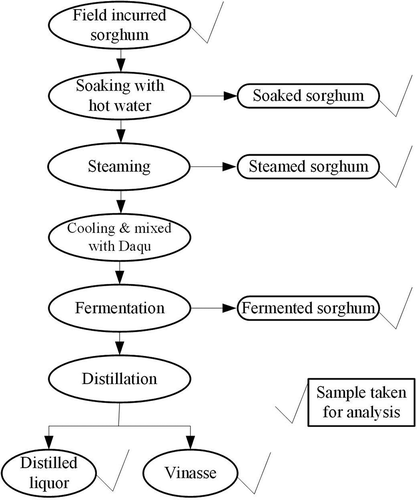
Generally speaking, the production of Chinese liquor mainly includes soaking, steaming, fermentation, and distillation processes, as presented in . So as to increase the yield of Chinese liquor, the sorghum is usually subjected to secondary fermentation and distillation processes. The blank rice hull and Daqu were obtained from a local market, and the rice hull was steamed about 30 min prior to use. In current study, the samples after each processing step were collected to study the residue change of the pesticides during Chinese liquor production. The detailed production procedures are as follows: first, 5 kg of sorghum was crushed for each independent test. Then the crushed sorghum was soaked with boiled water for 24 h and the sorghum heap was turned over every 8 h. After soaking, the sorghum was steamed for 1.5 h. Subsequently, the sorghum was mixed with Daqu (9–10%) when it was cooled down. Then the mixture was transferred to an airtight glass jar (10 L) and fermented at 26–30°C for 28 days. The secondary fermentation was done for 21 days. Finally, the fermented sorghum was distilled to produce liquor when it was mixed with rice hull (20–25%) equably. And the secondary distillation was conducted to increase the yield of Chinese liquor.
GC-MS/MS analytical conditions
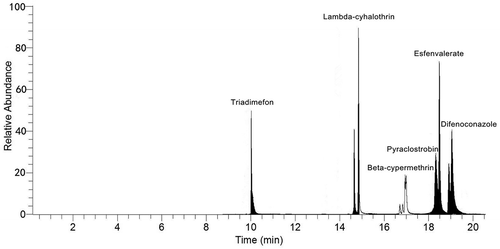
The analysis of the target compounds was conducted with the Thermo Scientific TSQ 8000 EVO triple quadrupole mass spectrometer coupled with a Trace 1300 gas chromatograph and a TriPlus AI 1310 autosampler (Thermo Fisher Scientific, San Jose, CA, USA). Chromatographic separation was achieved with a Thermo Fisher Scientific TR-Pesticide column (30 m × 0.25 mm i.d., 0.25 μm film thickness) with the following temperature programs: 80°C hold for 1 min, ramp to 180°C at 25°C min−Citation1, then increase to 280°C at 10°C min−Citation1, and hold for 9 min. The total run time was 24 min. The inlet, ion source, and transfer line temperatures were set at 250°C, 250°C, and 290°C, respectively. Triple quadrupole MS was applied in the selected reaction monitoring (SRM) mode with emission current of 25 μA and electron energy of 70 eV. A volume of 1 μL extraction was injected in splitless mode with a splitless time of 1.0 min and a split flow of 50 mL min−Citation1. Argon gas was chosen as collision gas with the pressure of 1.5 mTorr, and helium gas was used as carrier gas with a constant flow of 1.2 mL min−Citation1. The product ion and collision energy (CE) were optimized for each pesticide which are summarized in . The data were acquired and processed using the Xcalibur software. The typical chromatogram of standard can be seen in .
Table 1. Parameters of the six pesticides determined by GC-MS/MS.
Figure 2. Typical chromatogram of standard (0.05 mg kg–Citation1).
Extraction and purification procedure
Sorghum, fermented sorghum, and rice hull: a total of 5.0 g homogenized sorghum and fermented sorghum (rice hull was 2.0 g) were weighed into a 50 mL centrifuge tube. Then 5.0 mL of ultra-pure water was added to swell the sample. Subsequently, 5.0 mL of acetonitrile was added and the mixture was shaken for 2 min on a VX-Ⅲ Multi-Tube Vortexer (Beijing Targin Technology, China). Following, 3.0 g of NaCl was added and the mixture was shaken for 1 min again. And then the mixture was centrifuged for 5 min at 3800 rpm. An aliquot of 1 mL acetonitrile portion was transferred to a new centrifuge tube containing 5 mg MWCNTs, 15 mg PSA, 15 mg C18 sorbents plus 150 mg anhydrous MgSO4. Then the mixture was shaken for 1 min and centrifuged for 1 min at 10,000 rpm. Finally, the supernatant was filtered through 0.22 μm Nylon syringe filters (Agela Technologies, China) into an autosampler vial for GC-MS/MS analysis.
Sorghum, fermented sorghum, and rice hull
An amount of 5.0 g Chinese liquor sample was weighed into a 50 mL spin steaming bottle. Before extraction, the ethanol was removed under vacuum distillation at 41°C for 5 min. After the addition of 5.0 mL acetonitrile, the mixture was shaken for 2 min on a VX-Ⅲ Multi-Tube Vortexer. Then 3.0 g of NaCl was added and the mixture was shaken for 1 min and centrifuged for 5 min at 3800 rpm. Following, an aliquot of 1 mL acetonitrile was transferred to a new centrifuge tube containing 5 mg MWCNTs plus 150 mg anhydrous MgSO4. Then the tube was shaken for 1 min and centrifuged for 1 min at 10,000 rpm. After that, the supernatant was filtered through 0.22 μm Nylon syringe filters into an autosampler vial for GC-MS/MS analysis.
Method performances
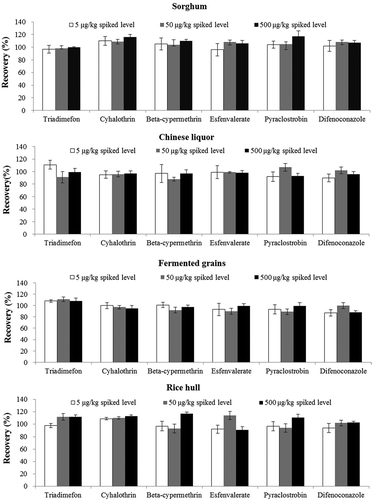
The method was validated through linearity, matrix effect, limit of quantification (LOQ), and limit of detection (LOD), trueness, and precision. Linearity was studied with matrix-matched calibration curves. The matrix effect was estimated by comparing the calibration curves slopes of matrix and solvent. The LOQs and LODs were calculated for each pesticide by the lowest concentration producing a signal-to-noise (S/N) ratio of 10 and 3, respectively. The trueness and precision were investigated through recovery assays with five replicates spiked at three levels (5, 50, and 500 µg kg−Citation1).
Statistical analysis
All assays were carried out at least three times. Data were statistically estimated by one-way ANOVA with SPSS base 17.0 software. The least significant difference (LSD) test was used to determine the differences among means when significant differences were found.
Results and discussion
Method validation
Calibration curves were constructed with matrix-matched standard calibration at five different concentrations in the range of 5–500 µg L−Citation1 for the six pesticides. Good linearity was found for the six pesticides with coefficients of determination (RCitation2) larger than 0.9990. Matrix effects often occurred owing to the co-elution of matrix components which play a significant role in the quality of the quantitative data.[Citation17] To study the matrix effects, the slopes of the matrix-matched calibration curves were compared with those of the solvent-based calibration curves. As shown in , the slope ratios for the six pesticides in sorghum and fermented sorghum matrices ranged from 1.2 to 2.7, which exceeded 10% of the slope ratio of 1.0 indicating that significant enhancement was found for sorghum and fermented sorghum matrices. In the case of the Chinese liquor matrix, the slope ratios for the six pesticides ranged from 0.9 to 1.1, which were within 10% of the slope ratio of 1.0 showing no matrix effect. As for the rice hull matrix, five of the pesticides showed significant enhancement except beta-cypermethrin presenting significant suppression with the slope ratios below 10% of the slope ratio of 1.0. Thus, matrix-matched calibration was commonly used to compensate for the matrix effects.[Citation18]
The LOQs and LODs were determined by the lowest spiked concentration of the six pesticides producing a signal-to-noise (S/N) ratio of 10 and 3, respectively. As presented in , the LOQs for the six pesticides in the four matrices were between 0.6 and 3.8 μg kg−Citation1, and the LODs ranged from 0.2 to 1.1 μg kg−Citation1. The trueness and precision of the method were investigated by recovery experiments at three spiked levels (5, 50, and 500 μg kg−Citation1) in quintuplicate for the four matrices. The trueness and precision were estimated by recovery (%) and relative standard deviation (RSD, %), respectively. As shown in , the mean recovery for the six pesticides was in the range of 70%–120% with RSDs below 20%, which were within the range expected for pesticide residue analysis.[Citation19]
Residue magnitudes of the six pesticides during Chinese liquor production
The residue magnitudes of the six pesticides in samples after various processes are summarized in . The six pesticides presented different change during the production of Chinese liquor. First, the sorghum was soaked with boiled water so as to accelerate the gelatinization of starch. The water was added about 50–60% of the weight of the sorghum. As summarized in , the soaking process reduced the residue of triadimefon, lambda-cyhalothrin, beta-cypermethrin, esfenvalerate, pyraclostrobin, and difenoconazole by 38%, 36%, 29%, 29%, 33%, and 30%, respectively. Considering the weight of water added to the sorghum, it was not hard to find that the reduction of the six pesticides residues was mainly owing to the dilution of water.
Table 3. Residue magnitudes of the six pesticides (mg kg−Citation1) in samples after various processes (n = 3).
Then, the sorghum was steamed for 1.5 h to facilitate the gelatinization and expansion of starch granules. As shown in , the steaming process further decreased the residue of triadimefon, lambda-cyhalothrin, beta-cypermethrin, esfenvalerate, pyraclostrobin, and difenoconazole by 55%, 67%, 72%, 69%, 67%, and 63%, respectively. The steaming process reduced the six pesticide residues more than the soaking process. It demonstrated that the constant heating was more conducive to the reduction of pesticide residues.[Citation20,Citation21]
Fermentation is a conventional process of the production of Chinese liquor.[Citation22,Citation23] A large number of literatures reported that fermentation could reduce the pesticide residues.[Citation12,Citation13,Citation24,Citation25] As presented in , the residues of triadimefon, lambda-cyhalothrin, beta-cypermethrin, esfenvalerate, pyraclostrobins and difenoconazole were lowered by 47%, 59%, 45%, 42%, 37%, and 55% during primary fermentation, and further reduced by 46%, 46%, 38%, 58%, 65%, and 49% in secondary fermentation, separately. The result was in accordance with the study by Dordevic and Durovic-Pejcev, where it was found that yeast fermentation was especially effective for reduction of pirimiphos methyl causing dissipation for maximum 48.8%, and activity of Lactobacillus plantarum was especially effective for reduction of chlorpyrifos methyl causing dissipation for maximum 56.7%.[Citation26] Regueiro et al. also reported that the reduction of pesticide levels during fermentation could mainly be due to biological degradation.[Citation27] Therefore, the reductions of the six pesticide residues in this study might be owing to biological degradation.
After fermentation, the distillation process was carried out to produce distilled liquor. Some researchers found that it could obviously reduce the pesticide residues in distilled spirits.[Citation14,Citation28,Citation29] As shown in , the residues of lambda-cyhalothrin, beta-cypermethrin, esfenvalerate, pyraclostrobin, and difenoconazole were completely eliminated after distillation, which demonstrated that these pesticides could not transfer to the distilled liquor. However, 0.59% of the initial residues of triadimefon were detected in the primary distilled liquor indicating that a small amount of triadimefon could migrate into the distilled liquor. Generally, the commercial distilled liquor was produced through blending the primary distilled liquor and the secondary distilled liquor. Therefore, in view of the whole process of the production of Chinese liquor, the transfer ratio for triadimefon was 0.15% which was very low. These results demonstrated that the distillation process was effective for decreasing the pesticide residues in distilled liquor.[Citation28]
Processing factors
A wide range of RAC are processed before they are consumed. Different food processing technologies result in various changes of pesticide residues in foods. The Joint FAO/WHO Meeting on Pesticide Residues (JMPR) evaluates food processing data on residue behaviour where significant residues occur in plant or plant products which are processed into food.[Citation30] The processing factors are estimated by JMPR and OECD through the ratio of residue levels in processed commodities and the raw agriculture commodities. The mean value of the two PFs from two independent field test sites is calculated to provide the processing factor in a processing study. Nevertheless, the mean value is inappropriate if the processing factors from two independent trials are contradictory, because it would not on behalf of either process. In this situation, the highest processing factor should be selected as the conservative value when there is no reason to choose which one.[Citation16] PF < 1 (reduction factor) indicates a reduction of the residue in the processed commodity, while PF > 1 (concentration factor) indicates a concentration effect of the processing procedure.
As shown in , the PFs for the six pesticides were generally less than 1, showing that all processes could reduce the pesticide residues. The processing factors for all pesticides in soaking process were between 0.95 and 0.99, while the processing factors in steaming process ranged from 0.32 to 0.51, which indicated that the constant heating was more conducive to the reduction of the pesticide residues. Moreover, the fermentation process decreased the pesticide residues with the PFs between 0.39 and 0.79. The distillation process remarkably reduced the residue of triadimefon with the PF obviously lower than other processes. And the procedure completely eliminated the residue of lambda-cyhalothrin, beta-cypermethrin, esfenvalerate, pyraclostrobin, and difenoconazole. It demonstrated that the distillation process was the most effective for lowering pesticide residues in Chinese liquor.
Table 4. PFs for different processing type (n = 3).
Conclusion
Residue change of the six pesticides in Chinese liquor produced from field incurred sorghum was studied. The residue magnitudes of the six pesticides gradually reduced after each process. The steaming process involving continuous heating could obviously reduce the pesticide residues by 55–72% with the PFs ranging from 0.32 to 0.51. Moreover, the fermentation also decreased the pesticides residues by 37–65% with the PFs between 0.39 and 0.79. Especially, the distillation procedure completely eliminated the residue of lambda-cyhalothrin, beta-cypermethrin, esfenvalerate, pyraclostrobin, and difenoconazole, and reduced the residue of triadimefon remarkably with the PF of 0.004, indicating that the distillation process was the most effective for reducing pesticide residues in Chinese liquor production.
Funding
The authors are grateful for the support from National Instrumentation Program of China [grant number 2013YQ510391] from the Chinese Ministry of Science and Technology and partial support from Program of Beijing Municipal Science and Technology Commission [grant number Z151100001215015]. It was also partially supported by the program for Guangxi Special Invited Scientist (2013) in Agric-Environment and Agroproducts Safety. All authors declare that they have no conflict of interests.
Additional information
Funding
References
- Zheng, X.W.; Yan, Z.; Nout, M.R.; et al. Characterization of the Microbial Community in Different Types of Daqu Samples as Revealed by 16S Rrna and 26S Rrna Gene Clone Libraries. World Journal of Microbiology and Biotechnology 2015, 31(1), 199–208.
- Zheng, X.W.; Zheng, Y.; Han, B.Z.; et al. Complex Microbiota of a Chinese “Fen” Liquor Fermentation Starter (Fen-Daqu), Revealed by Culture-Dependent and Culture-Independent Methods. Food Microbiology 2012, 31(2), 293–300.
- He, Z.; Wang, L.; Peng, Y.; et al. Multiresidue Analysis of over 200 Pesticides in Cereals Using a Quechers and Gas Chromatography–Tandem Mass Spectrometry-Based Method. Food Chemistry 2015, 169, 372–380.
- Fan, S.; Zhao, P.; Yu, C.; et al. Simultaneous Determination of 36 Pesticide Residues in Spinach and Cauliflower by LC-MS/MS Using Multi-Walled Carbon Nanotubes-Based Dispersive Solid-Phase Clean-Up. Food Additives & Contaminants: Part A 2014, 31(1), 73–82.
- Ling, Y.; Wang, H.; Yong, W.; et al. The Effects of Washing and Cooking on Chlorpyrifos and Its Toxic Metabolites in Vegetables. Food Control 2011, 22(1), 54–58.
- Keikotlhaile, B.M.; Spanoghe, P.; Steurbaut, W. Effects of Food Processing on Pesticide Residues in Fruits and Vegetables: A Meta-Analysis Approach. Food and Chemical Toxicology 2010, 48(1), 1–6.
- Kaushik, G.; Satya, S.; Naik, S.N. Food Processing A Tool to Pesticide Residue Dissipation – A Review. Food Research International 2009, 42(1), 26–40.
- Jiang, Y.; Shibamoto, T.; Li, Y.; et al. Effect of Household and Commercial Processing on Acetamiprid, Azoxystrobin and Methidathion Residues during Crude Rapeseed Oil Production. Food Additives & Contaminants: Part A 2013, 30(7), 1279–1286.
- Peng, W.; Zhao, L.; Liu, F.; et al. Effect of Paste Processing on Residue Levels of Imidacloprid, Pyraclostrobin, Azoxystrobin and Fipronil in Winter Jujube. Food Additives and Contaminants Part A-Chemistry Analysis Control Exposure & Risk Assessment 2014, 31(9), 1562–1567.
- Han, Y.; Li, W.; Dong, F.; et al. The Behavior of Chlorpyrifos and Its Metabolite 3,5,6-Trichloro-2-Pyridinol in Tomatoes during Home Canning. Food Control 2013, 31(2), 560–565.
- Kontou, S.; Tsipi, D.; Tzia, C. Stability of the Dithiocarbamate Pesticide Maneb in Tomato Homogenates during Cold Storage and Thermal Processing. Food Additives and Contaminants 2004, 21(11), 1083–1089.
- Dordevic, T.M.; Durovic-Pejcev, R.D. Dissipation of Chlorpyrifos-Methyl by Saccharomyces cerevisiae during Wheat Fermentation. Lwt-Food Science and Technology 2015, 61(2), 516–523.
- Dordevic, T.M.; Siler-Marinkovic, S.S.; Durovic-Pejcev, R.D.; et al. Dissipation of Pirimiphos-Methyl during Wheat Fermentation by Lactobacillus Plantarum. Letters in Applied Microbiology 2013, 57(5), 412–419.
- Nagatomi, Y.; Inoue, T.; Uyama, A.; et al. The Fate of Mycotoxins during the Distillation Process of Barley Shochu, a Distilled Alcoholic Beverage. Bioscience Biotechnology and Biochemistry 2012, 76(1), 202–204.
- Inoue, T.; Nagatomi, Y.; Suga, K.; et al. Fate of Pesticides during Beer Brewing. Journal of Agricultural and Food Chemistry 2011, 59(8), 3857–3868.
- OECD guideline for the testing of chemicals. Magnitude of the pesticide residues in processed commodities. 2008, NO.508.
- Zhao, P.; Wang, L.; Luo, J.; et al. Determination of Pesticide Residues in Complex Matrices Using Multi‐Walled Carbon Nanotubes as Reversed‐Dispersive Solid‐Phase Extraction Sorbent. Journal of Separation Science 2012, 35(1), 153–158.
- SANTE/11945/2015. Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticides Residues Analysis in Food and Feed. European Commission Directorate-General For Health And Food Safety. 2015.
- OECD. Guidance Document on Pesticide Residue Analytical Methods. ENV/JM/MONO 2007, 17, 18–28.
- Kim, S.W.; Abd El-Aty, A.M.; Rahman, M.M.; et al. The Effect of Household Processing on the Decline Pattern of Dimethomorph in Pepper Fruits and Leaves. Food Control 2015, 50, 118–124.
- Kwon, H.Y.; Lee, H.; Kim, J.; et al. Reduction of Pesticide Residues in Field-Sprayed Leafy Vegetables by Washing and Boiling. Journal of Food Hygiene and Safety 2009, 24(2), 182–187.
- Meng, X.; Wu, Q.; Wang, L.; et al. Improving Flavor Metabolism of Saccharomyces cerevisiae by Mixed Culture with Bacillus Licheniformis for Chinese Maotai-Flavor Liquor Making. Journal of Industrial Microbiology & Biotechnology 2015, 42(12), 1601–1608.
- Li, X.R.; Ma, E.B.; Yan, L.Z.; et al. Bacterial and Fungal Diversity in the Starter Production Process of Fen Liquor, a Traditional Chinese Liquor. Journal of Microbiology 2013, 51(4), 430–438.
- Abou-Arab, A. Degradation of Organochlorine Pesticides by Meat Starter in Liquid Media and Fermented Sausage. Food and Chemical Toxicology 2002, 40(1), 33–41.
- Ruediger, G.A.; Pardon, K.H.; Sas, A.N.; et al. Fate of Pesticides during the Winemaking Process in Relation to Malolactic Fermentation. Journal of Agricultural and Food Chemistry 2005, 53(8), 3023–3026.
- Dordevic, T.M.; Durovic-Pejcev, R.D. The Potency of Saccharomyces cerevisiae and Lactobacillus plantarum to Dissipate Organophosphorus Pesticides in Wheat during Fermentation. Journal of Food Science and Technology-Mysore 2016, 53(12), 4205–4215.
- Regueiro, J.; Lopez-Fernandez, O.; Rial-Otero, R.; et al. A Review on the Fermentation of Foods and the Residues of Pesticides-Biotransformation of Pesticides and Effects on Fermentation and Food Quality. Critical Reviews in Food Science and Nutrition 2015, 55(6), 839–863.
- Inoue, T.; Nagatomi, Y.; Kinami, T.; et al. Fate of Pesticides in a Distilled Spirit of Barley Shochu during the Distillation Process. Bioscience Biotechnology and Biochemistry 2010, 74(12), 2518–2522.
- Cabras, P.; Angioni, A.; Garau, V.L.; et al. Pesticides in the Distilled Spirits of Wine and Its Byproducts. Journal of Agricultural and Food Chemistry 1997, 45(6), 2248–2251.
- FAO/WHO. Updating the Principles and Methods of Risk Assessment: MRLs for Pesticides and Veterinary Drugs. Rome, 2006.