ABSTRACT
In the present study, protein complexes emerged in high molecular weight (MW) region (95–170 kDa) were observed in fertilised chicken egg white after 7 days of incubation. Investigations were performed to reveal the possible proteins involved in these complexes. The emerged 10 protein bands from SDS-PAGE gel were confirmed to be ovalbumin through matrix-assisted laser desorption/ionisation time-of-flight tandem mass spectrometry (MALDI-TOF MS/MS) followed by Western blot analysis. To reveal the possible interactions among ovalbumin and other egg white proteins, LC connected to LTQ-Orbitrap mass spectrometer was used. Besides ovalbumin, 11 egg white proteins were identified including ovotransferrin, lysozyme, ovalbumin-related protein X, ovalbumin-related protein Y, hep21, alpha-enolase, ovoinhibitor, and a protein similar to ryanodine receptor 1. These results indicated widespread thermal-induced protein interactions among ovalbumin and other egg white proteins.
Introduction
Chicken egg white provides requisite nutrients and functions as a protective barrier to invading bacteria to the developing embryo. It is also a versatile food for human dietary and major raw material for the food industry. Various proteomic tools have been applied to explore the novel proteins in chicken egg white including using a dual-pressure linear ion trap Orbitrap instrument for the identification of 158 albumen proteins.[Citation1] Moreover, effect of environmental temperature was suggested to be a key factor on the egg white protein changes.[Citation2]
Figure 1. SDS-PAGE of egg white collected from fertilised and unfertilised chicken eggs. A, fertilised fresh eggs (0 day). B, fertilised eggs incubated for 7 days. C, unfertilised fresh eggs (0 day). D, unfertilised eggs after seven days of storage. The MW of marker proteins is shown in kDa left of the lanes. The images shown represent the three independent SDS-PAGE gel replicates of fresh unfertilised and fertilised chicken egg whites, respectively. 88 × 88mm (600 × 600 DPI).
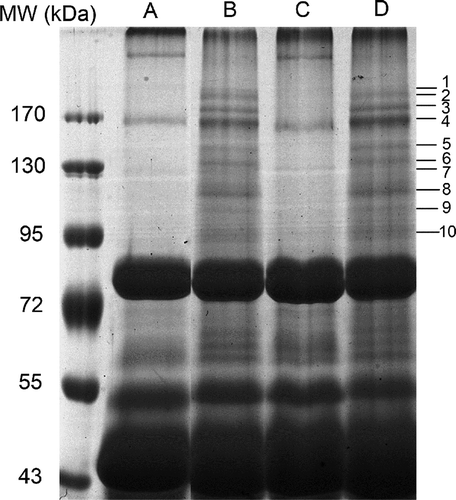
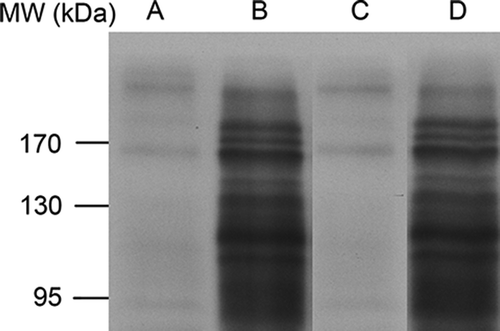
Figure 2. SDS-PAGE of proteins in egg white samples: the Western blot with anti-ovalbumin IgG. A, fertilised fresh eggs (0 day). B, fertilised eggs incubated for seven days. C, unfertilised fresh eggs (0 day). D, unfertilised eggs after seven days of storage. The images shown represent the three independent Western blot gel replicates of fresh unfertilised and fertilised chicken egg whites, respectively. 53 × 32mm (600 × 600 DPI).
Ovalbumin, which is the most abundant protein in egg white, belongs to serine proteinase inhibitor (serpin) superfamily while its inhibitory activity is still controversial.[Citation3,Citation4] It was reported that ovalbumin showed conformational changes to a proteinase inhibitory form under heat conditions.[Citation5] In an early study, ovalbumin was found to remarkably influence the rate of inactivation of lysozyme at temperatures which caused no observable denaturation when the two proteins were heated separately.[Citation6] The sulfhydryl groups of ovalbumin were implicated in the inactivation of lysozyme, probably via the reduction of one or more disulfide bonds.[Citation6] The heat-induced interaction of the egg white lysozyme with N-ovalbumin was also proved to form amyloid-like fibrils.[Citation7] Also, the heat-induced interaction between ovalbumin and ovotransferrin at 65°C was investigated through turbidity development and polyacrylamide gel electrophoresis.[Citation8] It was presumed that ovalbumin interacted with ovotransferrin by the electrostatic attractive force and then formed soluble aggregates through inter-molecular forces such as hydrophobic and disulfide bonds.[Citation8] The binding ability of ovalbumin was proved to be related to the heat-and pH-induced α-to-β structural transformations of ovalbumin.[Citation8]
Previous comparative proteomic analyses had identified numerous ovalbumin spots scattered in a wide range of MW (especially some showed much higher than its theoretical value of 42.9 kDa) on gels under storage and incubation conditions.[Citation2,Citation9,Citation10] Putative ovalbumin complexes including one coupled with RNA-binding protein were detected during the early phase of fertilised egg incubation.[Citation2] Meanwhile, a putative lysozyme-ovalbumin interaction was also suggested to be sensitively regulated by the storage temperature.[Citation10] Furthermore, 12 spots on the two-dimensional electrophoresis (2-DE) gels were identified as ovalbumin exhibiting much higher MW than the theoretical value according to our previous study.[Citation2,Citation10] suggesting that more ovalbumin complexes may exist under the heat-induced conditions. The early embryonic development phase (the first seven days) was focused in this study because the protein exchange between egg white and yolk seldom takes place during the first week of incubation.[Citation10] Thus, the major purpose of this work was to reveal all the candidate proteins that interact with ovalbumin during the early phase of embryonic development.
Materials and methods
Egg white sampling and protein extraction
Fertilised and unfertilised chicken eggs (60 ± 0.5 g for average weight, laid within 24 h) from Lohmann White Single Comb White Leghorn (of the same flock, 40 weeks of age) were collected from the Poultry Research Centre farm of Huazhong Agricultural University. Both fertilised and unfertilised eggs were incubated at 38 ± 0.5°C and 65% relative humidity for seven days in the same incubator. Egg white was collected after seven-day incubation from randomly selected fertilised and unfertilised eggs respectively while fresh eggs (0 day) were considered as control. Protein extraction was carried out according to the method described previously.[Citation10] Ten eggs were used for each sample, and three biological replicates were performed during the following analysis to reduce variations.
SDS-page analysis and Western blot analysis
Egg white protein was diluted in a buffer (10% glycerol, 2% SDS, 0.125 M Tris (pH 6.8)) which loaded on 4–20% premade SDS-polyacrylamide gels (Thermo Scientific) and then was run at 20 mA per gel until the dye front reached the bottom of the gel. Gels were stained with Coomassie Brilliant Blue after electrophoresis or subjected to Western blotting with polyvinylidene difluoride membranes (Millipore) according to the method described before.[Citation11] to confirm the ovalbumin distribution on the gel. Western blot analysis was performed according to Sugimoto et al. using anti-ovalbumin rabbit IgG (Thermo Fisher Scientific) suspended in PBS containing 0.1% BSA, and a negative control was performed by using the anti-ovalbumin rabbit IgG pre-incubated with 100-fold commercial ovalbumin.[Citation11]
LTQ-Orbitrap analysis
The gel slices marked 1 to 10 in were excised for in-gel digestion with trypsin (Promega V5280), and the peptides were cleaned with STAGE tips before mass spectrometric analysis. Peptides obtained from these 10 slices were analysed using MALDI-TOF MS/MS (Ultraflex MALDI-TOF-TOF mass spectrometer Bruker, Karlsruhe, Germany) respectively. The peptides mixture was further analysed by online C18 RP nanoscale LC (Agilent Technologies) connected to LTQ-Orbitrap mass spectrometer (Thermo Electron, Bremen, Germany). Database search with the MS data was performed using MASCOT programme against chicken International Protein Index (IPI) database v3.81. The abundance of identified proteins was estimated by calculating the emPAI.[Citation12] The LTQ-Orbitrap analysis procedures were basically according to the method reported by Mann et al.[Citation1]
Results
A comparative analysis was performed based on the SDS-PAGE gel for the chicken egg albumen prepared after seven days of incubation from the fertilised and unfertilised eggs respectively to those of the corresponding controls (0 day). The emergence of protein bands on the SDS-PAGE gel was detected for the albumen from fertilised eggs after seven-day incubation which mainly focused in the MW range of 95–170 kDa (). Surprisingly, 10 emerged protein bands (numbered from 1 to 10 in ) were all identified as ovalbumin by MALDI-TOF MS/MS respectively (date not shown). Furthermore, both fertilised and unfertilised protein bands subjected to Western blotting with affinity-purified anti-ovalbumin IgG were confirmed to possess ovalbumin or ovalbumin-related proteins (OVAX and OVAY) (). To further detect the other proteins in this high MW range, these emerged protein bands of fertilised egg samples were excised simultaneously for in-gel digestion followed by LC-MS analysis using LTQ Orbitrap mass spectrometer. Twelve egg white proteins were identified including ovalbumin, ovotransferrin, lysozyme C, OVAX, OVAY, hep21 protein, alpha-enolase, ovoinhibitor, RyR1, ovostatin, ovomucin alpha-subunit, and a protein similar to alpha-2-macroglobulin-like-1 (. and Supplementary Table S1).
Table 1. Proteins among the 10 protein bands of fertilised chicken eggs identified by LTQ-Orbitrap analysis.
Discussion
The emergence of protein spots in high MW region of 2-DE has been noticed in our previous study during the early phase of fertilised egg incubatio.[Citation10] and unfertilised egg storage at high temperature.[Citation2] Three adjacent spots representing ovalbumin shared the same MW (82 kDa) and were supposed to be ovalbumin dimer.[Citation10] A protein complex of ovalbumin with an uncharacterised protein similar to poly (rC) binding protein 3 was identified, indicating involvement in pre-mRNA processing and important pathways regulating embryonic development.[Citation10] Moreover, a mixture of lysozyme and ovalbumin was reported to be a hypothetical interaction which was regulated by storage temperature.[Citation2] These putative protein complexes still existed after urea, SDS, and DTT treatments, which indicated the interaction due to covalent bond rather than the hydrophobic interactions or disulfide bonds.[Citation2,Citation10] It is necessary to detect if there are any other egg white proteins that might also interact with ovalbumin to form complexes during the early phase of embryonic development. In the present study, among these candidate proteins which were supposed to interact with ovalbumin, eight of them (including ovotransferrin, lysozyme, OVAX, OVAY, hep21, alpha-enolase, ovoinhibitor, and a protein similar to ryanodine receptor 1) have theoretical MWs less than 95 kDa, which means these proteins may form complex or polymers during the incubation.
Noticeably, unfertilised egg white samples, which were incubated under same conditions, exhibited similar bands distribution (lane D in and ) to those of the fertilised ones (lane B in and ), indicating the independence of the presence of the embryo for the protein interactions. The similar changing patterns in the intensity of the protein bands for fertilised and unfertilised egg white indicated a thermally induced change of ovalbumin in the range of 95–170 kDa during the first week of embryonic development. Studies have been performed to verify that native form of ovalbumin would convert into S-ovalbumin, a thermostable form, during the incubation and storage of fertilised and unfertilised eggs.[Citation11,Citation13] The significant conformational changes of S-ovalbumin may be associated with the protein-protein interaction. The thermally induced altered form of ovalbumin exhibits more hydrophobic exposure and intermolecular interaction, which may contribute to an increasing binding ability to form protein complexes.[Citation14] The emerged ovalbumin complexes in the region from 95 kDa to 170 kDa under thermal conditions in the present study may promote better protein utilisation in the later phase of chicken embryonic development or increase immunomodulating activity to support the developing embryo.
Among the detected proteins, ovotransferrin (OTf) is a 78 kDa antibacterial protein with high abundance in egg white.[Citation15–Citation17] It has been reported that the heat-induced interaction between ovalbumin and ovotransferrin had the inhibiting capacity against coagulation of OTf.[Citation8] In our former comparative study on egg white proteomic alterations under various storage temperatures, one spot representing ovotransferrin BC type with a MW of 118.2 kDa was suggested to be an ovalbumin-OTf complex which showed increasing in abundance when elevating the storage temperature.[Citation2] As OTf is a high heat-sensitive egg white protein,[Citation8] the putative ovalbumin-OTf interaction may increase the stability of OTf under the incubation conditions regarding to its better usage for chicken embryonic development.
Chicken egg white lysozyme (Lysozyme C) is an antimicrobial protein with small calculated MW (16.2 kDa). Lysozyme-ovalbumin interaction has been proved to cause the loss of lysozyme enzymatic activity in thermal conditions.[Citation6] It was also suggested that the inhibition of lysozyme muramidase activity may happen due to the formation of rigid aggregates of ovalbumin and lysozyme rather than the configuration changes of lysozyme itself.[Citation18] It is interesting to find that, in our previous study, the abundance of one spot (experimental MW is 66.1 kDa) representing ovalbumin-lysozyme complex continued to increase during the early incubation period at 38°C in fertilised eggs while remained stable after two days of storage in unfertilised eggs.[Citation2,Citation10] This difference suggests a vital function of ovalbumin-lysozyme interaction to chicken embryonic developing process. However, whether the ovalbumin-lysozyme interaction actually exists during the seven-day storage in the present study and how this hypothetic protein complex affects the albumen antibacterial abilities still needs further studies.
The experiment MW of OVAX and OVAY, which belong to the ovalbumin gene family, are 43.6 kDa and 43.8 kDa, respectively. A large polymorphism of OVAY was demonstrated through 2-DE analysis.[Citation19,Citation20] A previous proteomic study also revealed that OVAY in egg white is the most differential protein between fresh fertilised eggs and unfertilised eggs.[Citation21] Ovalbumin-OVAY complex, as well as OVAX and OVAY complex, were reported in different egg varieties.[Citation22] OVAX was supposed to show antimicrobial activity partly through its heparin-binding site(s) according to a previous study, which suggested that OVAX at least plays a role in egg defense.[Citation23] However, the role of OVAY in chicken eggs remained difficult to define though its protease inhibition activity has been predicted.[Citation24,Citation25] Nevertheless, the interactions between these proteins may play protective barrier roles in the early embryonic development.
Except for these previously reported possible ovalbumin interactions with other egg white proteins, more candidate proteins were identified in the present study. Hep21 is an egg white protein belonging to the uPAR/Ly6 superfamily.[Citation26] No significant changes were observed for hep21 protein during incubation or storage in previous proteomic studies on egg white.[Citation2,Citation9,Citation10] It has been reported that two hep21 protein spots were identified with higher MW (18.1 kDa and 17.1 kDa, respectively) than the theoretical value (10 kDa), suggesting the glycosylation of this protein.[Citation19] It was the first time that hep21 was identified among the 95–170 kDa region during incubation in this study. Forming protein complex through interactions with other identified candidate egg white proteins is a possible explanation. A recent study revealed that hep21 gene expression was regulated by estrogen and participated in the development of chicken oviduct as well as egg production and formation.[Citation27] Thus, hep21 was suggested to be responsible for the development of ovarian carcinogenesis in laying hens.[Citation27] However, the biological function of putative hep21 complex still needs to be verified.
Alpha-enolase has been identified in chicken egg white through LTQ Orbitrap Velos analysis.[Citation1] Alpha-enolase is a wide distributed protein originally described as a key enzyme responsible for the glycolytic pathway.[Citation28] The mammalian alpha-enolase was found to be a main structural component of the eye lens.[Citation29] Alpha-enolase was also identified as one of the major seminal plasma proteins in roosters (Gallus gallus domesticus).[Citation30] Meanwhile, chicken alpha-enolase was also found significantly increased in expression during retinogenesis throughout embryonic development commencing at day 12 to post-hatch.[Citation31] The calculated MW of chicken alpha-enolase is 47.3 kDa. Its appearance in the high MW region () may indicate an interaction with other egg white proteins for further transportation, which putatively play an important role during the chicken retinal maturation.
Ovoinhibitor, as one of the major proteinase inhibitors in egg white, was detected with different levels of glycosylation.[Citation19,Citation32,Citation33] In our previous study, two ovoinhibitor spots were demonstrated with higher experimental MW of 59 kDa and 71 kDa, respectively, than its theoretical value (51.9 kDa), which might be derived from glycosylation.[Citation10] As ovoinhibitor was also identified in this study, forming protein complex might be one of the reasonable explanations for its abundance decrease illustrated in previous study.[Citation10] Since accelerating degradations of ovalbumin, ovotransferrin, and clusterin have been observed in previous comparative proteomic studies.[Citation2,Citation10] the ovalbumin-ovoinhibitor interaction may decrease the inhibitory activity of ovoinhibitor which might contribute to the regulation of proteolytic degradation of egg white proteins. In addition, an ovoinhibitor variant has been found in the chicken pituitary suggesting potentially active as a hormone carrier or playing a role in the control of cell signaling.[Citation34]
Another candidate protein identified in the high MW region is that similar to RYR-1. This protein was not identified as egg white component previously. Nevertheless, studies have been performed on the polymorphisms of Gallus gallus α-RyR (homologous to RyR 1) which could be associated with the meat qualiFty.[Citation35,Citation36] It was also found that heat stress may be a factor that regulates the expression of variants of ryanodine receptors.[Citation36] The theoretical MW of RYR-1 is 565 kDa. However, the protein identified as similar to RYR-1 in this study showed a theoretical MW of 15.8 kDa because the matched sequence is a partial RYR-1. The detected RYR-1 in this study is more possibly a spliced Gallus gallus α-RyR fragment under thermal conditions. In addition, there were three identified proteins (ovostatin, a protein similar to alpha-2-macroglobulin-like-1, and ovomucin alpha-subunit) with high theoretical MW. The identification of these proteins was possibly due to their degradation or existence before incubation, rather than the interactions with other egg white proteins.
Conclusion
In this study, 11 egg white proteins were identified to be involved in the ovalbumin complex under the thermal-induced conditions for seven days. Most putative interactions still need to be validated one by one through protein interaction analyses such as surface plasmon resonance method. Moreover, further investigations on the biological functions of these protein complexes for the chicken embryonic development require to be performed in the future.
LJFP_A_1326056_Supplemental_file.rtf
Download Rich Text Format File (86.8 KB)Funding
This work was financially supported by Science and Technology Program of Wuhan (2016020101010079), the Chinese National Natural Science Funds (Grant No. 31101366), the Special Fund for Agro-scientific Research in the Public Interest (201303084), and the Fundamental Research Funds for the Central Universities (2662017PY096).
Supplemental material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Mann, K.; Mann, M. In-Depth Analysis of the Chicken Egg White Proteome Using an LTQ Orbitrap Velos. Proteome Science 2011, 9, 7.
- Qiu, N.; Ma, M.; Zhao, L.; Liu, W.; Li, Y.; Mine, Y. Comparative Proteomic Analysis of Egg White Proteins under Various Storage Temperatures. Journal of Agricultural and Food Chemistry 2012b, 60, 7746–7753.
- Harvey, D.J.; Wing, D.R.; Kuster, B.; Wilson, I.B. Composition of N-Linked Carbohydrates from Ovalbumin and Co-Purified Glycoproteins. Journal of the American Society for Mass Spectrometry 2000, 11, 564–571.
- Odum, L.;. Trypsin-Inhibitory Activity of Ovalbumin Preparations Is Due to Ovomucoid. Biological Chemistry Hoppe-Seyler 1987, 368, 1603–1606.
- Mellet, P.; Michels, B.; Bieth, J.G. Heat-Induced Conversion of Ovalbumin into a Proteinase Inhibitor. Journal of Biological Chemistry 1996, 271, 30311–30314.
- Cunningham, F.E.; Lineweaver, H. Inactivation of Lysozyme by Native Ovalbumin. Poultry Science 1967, 46, 1471–1477.
- Sugimoto, Y.; Kamada, Y.; Tokunaga, Y.; Shinohara, H.; Matsumoto, M.; Kusakabe, T.; Ohkuri, T.; Ueda, T. Aggregates with Lysozyme and Ovalbumin Show Features of Amyloid-Like Fibrils. Biochemistry and Cell Biology-Biochimie Et Biologie Cellulaire 2011, 89, 533–544.
- Matsudomi, N.; Oka, H.; Sonoda, M. Inhibition against Heat Coagulation of Ovotransferrin by Ovalbumin in Relation to Its Molecular Structure. Food Research International 2002, 35, 821–827.
- Omana, D.A.; Liang, Y.; Kav, N.N.V.; Wu, J. Proteomic Analysis of Egg White Proteins during Storage. Proteomics 2011, 11, 144–153.
- Qiu, N.; Ma, M.; Cai, Z.; Jin, Y.; Huang, X.; Huang, Q.; Sun, S. Proteomic Analysis of Egg White Proteins during the Early Phase of Embryonic Development. Journal Proteomics 2012a, 75, 1895–1905.
- Sugimoto, Y.; Sanuki, S.; Ohsako, S.; Higashimoto, Y.; Kondo, M.; Kurawaki, J.; Ibrahim, H.R.; Aoki, T.; Kusakabe, T.; Koga, K. Ovalbumin in Developing Chicken Eggs Migrates from Egg White to Embryonic Organs while Changing Its Conformation and Thermal Stability. Journal of Biological Chemistry 1999, 274, 11030–11037.
- Ishihama, Y.; Oda, Y.; Tabata, T.; Sato, T.; Nagasu, T.; Rappsilber, J.; Mann, M. Exponentially Modified Protein Abundance Index (Empai) for Estimation of Absolute Protein Amount in Proteomics by the Number of Sequenced Peptides per Protein. Molecular & Cellular Proteomics 2005, 4, 1265–1272.
- Hatta, H.; Nomura, M.; Takahashi, N.; Hirose, M. Thermostabilization of Ovalbumin in a Developing Egg by an Alkalinity-Regulated, Two-Step Process. Bioscience Biotechnology Biochemistry 2001, 65, 2021–2027.
- Hu, H.Y.; Du, H.N. Alpha-To-Beta Structural Transformation of Ovalbumin: Heat and Ph Effects. Journal of Protein Chemistry 2000, 19, 177–183.
- Giansanti, F.; Rossi, P.; Massucci, M.; Botti, D.; Antonini, G.; Valenti, P.; Seganti, L. Antiviral Activity of Ovotransferrin Discloses an Evolutionary Strategy for the Defensive Activities of Lactoferrin. Biochemistry and Cell Biology-Biochimie Et Biologie Cellulaire 2002, 80, 125–130.
- Guérin-Dubiard, C.; Pasco, M.; Hietanen, A.; Quiros Del Bosque, A.; Nau, F.; Croguennec, T. Hen Egg White Fractionation by Ion-Exchange Chromatography. Journal of Chromatography A 2005, 1090, 58–67.
- Mine, Y.; Kovacs-Nolan, J. Biologically Active Hen Egg Components in Human Health and Disease. Journal of Poultry Science 2004, 41, 1–29.
- Stein, P.E.; Leslie, A.G.; Finch, J.T.; Carrell, R.W. Crystal Structure of Uncleaved Ovalbumin at 1.95 A Resolution. Journal of Molecular Biology 1991, 221, 941–959.
- Guerin-Dubiard, C.; Pasco, M.; Molle, D.; Desert, C.; Croguennec, T.; Nau, F. Proteomic Analysis of Hen Egg White. Journal of Agricultural and Food Chemistry 2006, 54, 3901–3910.
- Nau, F.; Pasco, M.; Desert, C.; Molle, D.; Croguennec, T.; Guerin-Dubiard, C. Identification and Characterization of Ovalbumin Gene Y in Hen Egg White. Journal of Agricultural and Food Chemistry 2005, 53, 2158–2163.
- Qiu, N.; Liu, W.; Ma, M.; Zhao, L.; Li, Y. Differences between Fertilized and Unfertilized Chicken Egg White Proteins Revealed by 2-Dimensional Gel Electrophoresis-Based Proteomic Analysis. Poultry Science 2013, 92, 782–786.
- Wang, J.; Liang, Y.; Omana, D.A.; Kav, N.N.; Wu, J. Proteomics Analysis of Egg White Proteins from Different Egg Varieties. Journal of Agricultural and Food Chemistry 2012, 60, 272–282.
- Rehault-Godbert, S.; Labas, V.; Helloin, E.; Herve-Grepinet, V.; Slugocki, C.; Berges, M.; Bourin, M.C.; Brionne, A.; Poirier, J.C.; Gautron, J.; Coste, F.; Nys, Y. Ovalbumin-Related Protein X Is a Heparin-Binding Ov-Serpin Exhibiting Antimicrobial Activities. Journal of Biological Chemistry 2013, 288, 17285–17295.
- Benarafa, C.; Remold-O’Donnell, E. The Ovalbumin Serpins Revisited: Perspective from the Chicken Genome of Clade B Serpin Evolution in Vertebrates. Proceedings of the National Academy of Sciences of the United States of America 2005, 102, 11367–11372.
- Da Silva, M.; Beauclercq, S.; Harichaux, G.; Labas, V.; Guyot, N.; Gautron, J.; Nys, Y.; Rehault-Godbert, S. The Family Secrets of Avian Egg-Specific Ovalbumin and Its Related Proteins Y and X. Biology of Reproduction 2015, 93, 71.
- Nau, F.; Guerin-Dubiard, C.; Desert, C.; Gautron, J.; Bouton, S.; Gribonval, J.; Lagarrigue, S. Cloning and Characterization of HEP21, a New Member of the Upar/Ly6 Protein Superfamily Predominantly Expressed in Hen Egg White. Poultry Science 2003, 82, 242–250.
- Lim, W.; Song, G. Pivotal Roles for Hormonally Regulated Expression of the HEP21 Gene in the Reproductive Tract of Chickens for Oviduct Development and in Ovarian Carcinogenesis. Domestic Animal Endocrinology 2014, 48, 136–144.
- Diaz-Ramos, A.; Roig-Borrellas, A.; Garcia-Melero, A.; Lopez-Alemany, R. Alpha-Enolase, a Multifunctional Protein: Its Role on Pathophysiological Situations. Journal of Biomedicine and Biotechnology 2012, 2012, 1–12.
- Pancholi, V.;. Multifunctional Α-Enolase: Its Role in Diseases. Cellular and Molecular Life Sciences 2001, 58, 902–920.
- Marzoni, M.; Castillo, A.; Sagona, S.; Citti, L.; Rocchiccioli, S.; Romboli, I.; Felicioli, A. A Proteomic Approach to Identify Seminal Plasma Proteins in Roosters (Gallus gallus domesticus). Animal Reproduction Science 2013, 140, 216–223.
- Finnegan, S.; Robson, J.L.; Wylie, M.; Healy, A.; Stitt, A.W.; Curry, W.J. Protein Expression Profiling during Chick Retinal Maturation: A Proteomics-Based Approach. Proteome Science 2008, 6, 34–45.
- Saxena, I.; Tayyab, S. Protein Proteinase Inhibitors from Avian Egg Whites. Cellular and Molecular Life Sciences 1997, 53, 13–23.
- Matsushima, K.I.;. An Undescribed Trypsin Inhibitor in Egg White.American Association for the Advancement of Science. Science 1958, 127, 1178–1179.
- Oubre, C.M.; D’Hondt, E.; Moore, R.W.; Hargis, B.M.; Berghman, L.R. The Chicken Pituitary Expresses an Ovoinhibitor-Like Protein in Subpopulations of Some, but Not All, Hormone-Producing Cell Types. Domestic Animal Endocrinology 2003, 25, 389–397.
- Droval, A.A.; Binneck, E.; Marin, S.R.; Paiao, F.G.; Oba, A.; Nepomuceno, A.L.; Shimokomaki, M. A New Single Nucleotide Polymorphism in the Ryanodine Gene of Chicken Skeletal Muscle. Genetics and Molecular Research 2012, 11, 821–829.
- Strasburg, G.M.; Chiang, W. Pale, Soft, Exudative Turkey-The Role of Ryanodine Receptor Variation in Meat Quality. Poultry Science 2009, 88, 1497–1505.
